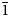Introduction
The mines in the Red Canyon portion of the White Canyon district in south-eastern Utah have yielded many new minerals in recent years. Most of these are from the Blue Lizard mine on the east side of Red Canyon; however, the Green Lizard mine and the Giveaway–Simplot mine, also on the east side of Red Canyon, have yielded new species as well. The vast majority of these new species are uranyl sulfates and most, especially from the Blue Lizard mine, contain Na. None of the new mineral species from the mines on the east side of Red Canyon contain essential Ca or carbonate. The Markey mine, on the west side of Red Canyon, has also proven to be a prolific source of new minerals, having thus far yielded nine, including natromarkeyite and pseudomarkeyite, described herein (Table 1). All of these contain uranyl, most contain carbonate and several contain Ca.
Table 1. New minerals from the Markey mine.

* Cotype locality; also found at the Blue Lizard mine, San Juan County, Utah.
§ Cotype locality; also found at the Green Lizard mine, San Juan County, Utah.
† Cotype locality; also found at the Burro mine, San Miguel County, Colorado.
References: [1] Kampf et al. (Reference Kampf, Olds, Plášil, Marty and Perry2019c); [2] Olds et al. (Reference Olds, Sadergaski, Plášil, Kampf, Burns, Steele, Marty, Carlson and Mills2017a); [3] Kampf et al. (Reference Kampf, Plášil, Kasatkin, Nash and Marty2019d); [4] Kampf et al. (Reference Kampf, Plášil, Kasatkin, Marty and Čejka2018); [5] Kampf et al. (Reference Kampf, Plášil, Olds, Nash, Marty and Belkin2019e); [6] This study; and [7] Kampf et al. (Reference Kampf, Plášil, Nash, Němec and Marty2020)
The new minerals and their names were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA). Natromarkeyite (IMA2018-152, Kampf et al, Reference Kampf, Olds, Plášil, Marty and Burns2019b) is named as a sodium analogue of markeyite, with two Na in place of one Ca in the structure. Pseudomarkeyite (IMA2018-114, Kampf et al., Reference Kampf, Olds, Plášil, Burns and Marty2019a) is named for its similarity to markeyite. The two minerals occur in intimate association and are similar in appearance, composition, Raman spectra and structure. Note that markeyite (/ma:r ′ki: ait/) is named for the locality, the Markey mine. The type specimens for both minerals are deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA. The description of natromarkeyite is based on one holotype and one cotype specimen with catalogue numbers 67487 (holotype) and 67488 (cotype). The description of pseudomarkeyite is based on one holotype specimen, catalogue number 67091, which is also the holotype for markeyite.
Occurrence
Natromarkeyite and pseudomarkeyite were found underground in the Markey mine, Red Canyon, White Canyon District, San Juan County, Utah, USA (37°32′57″N, 110°18′08″W). The Markey mine is located ~1 km southwest of the Blue Lizard mine, on the east-facing side of Red Canyon, ~72 km west of the town of Blanding, Utah, and ~22 km southeast of Good Hope Bay on Lake Powell. The geology of the Markey Mine is quite similar to that of the Blue Lizard mine (Chenoweth, Reference Chenoweth1993; Kampf, et al., Reference Kampf, Plášil, Kasatkin, Marty and Čejka2017), although the secondary mineralogy of the Markey mine is notably richer in carbonate phases. Underground gas measurements collected in 2016 using a hand-held Crowcon Gasman CO2 monitor showed consistently elevated CO2 levels at the Markey mine, averaging ~1000 ppm CO2 with a maximum recorded value of 1600 ppm CO2, levels considerably higher than at the nearby Blue Lizard mine where carbonate mineral species are less abundant. Higher CO2 concentration at the Markey mine may be connected to an abundance of calcite present in the ores, released by the action of acidic waters derived from decaying sulfides.
The following information regarding the history and geology is taken largely from Chenoweth (Reference Chenoweth1993). Jim Rigg of Grand Junction, Colorado began staking claims in Red Canyon in March of 1949. The Markey group of claims, staked by Rigg and others, was purchased by the Anaconda Copper Mining Company on June 1, 1951. After limited exploration and production, the mine closed in 1955. The mine was subsequently acquired from Anaconda by Calvin Black of Blanding, Utah under whose ownership the mine operated from 1960 to 1982 and was a leading producer in the district for nearly that entire period.
The uranium deposits in Red Canyon occur within the Shinarump member of the Upper Triassic Chinle Formation, in channels incised into the reddish-brown siltstones of the underlying Lower Triassic Moenkopi Formation. The Shinarump member consists of medium- to coarse-grained sandstone, conglomeratic sandstone beds and thick siltstone lenses. Ore minerals (uraninite, montroseite and coffinite) were deposited as replacements of wood and other organic material and as disseminations in the enclosing sandstone. Since the mine closed in 1982, oxidation of primary ores in the humid underground environment has produced a variety of secondary minerals, mainly carbonates and sulfates, as efflorescent crusts on the surfaces of mine walls.
Natromarkeyite and pseudomarkeyite are very rare minerals in the secondary mineral assemblage at the Markey mine. They both occur on asphaltum. Natromarkeyite is associated with andersonite, calcite, gypsum and another new calcium uranyl carbonate phase that is currently under study. Pseudomarkeyite is associated with calcite, gypsum, markeyite (Kampf et al., Reference Kampf, Plášil, Kasatkin, Marty and Čejka2017) and natrozippeite.
Physical and optical properties
Natromarkeyite
Natromarkeyite crystals are blades and tablets (Fig. 1) up to ~0.2 mm in maximum dimension, flattened on {001} and elongated on [100]. Crystals exhibit the forms {100}, {010}, {001}, {110}, {101}, {011} and {111} (Fig. 2). No twinning was observed.

Fig. 1. Natromarkeyite crystals on holotype specimen (#67487); FOV 0.4 mm across.

Fig. 2. Crystal drawing of natromarkeyite; clinographic projection in standard orientation.
Crystals are pale yellow green and transparent with vitreous to pearly lustre. The streak is white. The mineral fluoresces bright bluish white under a 405 nm laser. The Mohs hardness is between 1½ and 2, based upon scratch tests. Crystals are brittle with irregular fracture and three cleavages: perfect on {001}, good on {100} and {010}. At room temperature, the mineral dissolves very slowly in H2O (minutes) and dissolves immediately with effervescence in dilute HCl. The density measured by flotation in a mixture of methylene iodide and toluene is 2.70(2) g cm–3. The calculated density based on the empirical formula and unit-cell parameters obtained from single-crystal X-ray diffraction data is 2.695 g cm–3.
Optically, natromarkeyite is biaxial (–), with α = 1.528(2), β = 1.532(2) and γ = 1.533(2) (measured in white light). The 2V, measured using extinction data collected on a spindle stage and analysed using EXCALIBRW (Gunter et al., Reference Gunter, Bandli, Bloss, Evans, Su and Weaver2004), is 46.5(7)°; the calculated 2V is 53.0°. Dispersion is r > v, weak. The mineral is weakly pleochroic: X = pale green yellow, Y ≈ Z = light green yellow; X < Y ≈ Z. The optical orientation is X = b, Y = a and Z = c. The Gladstone–Dale compatibility index 1 – (K P/K C) for the empirical formula is –0.015, in the superior range (Mandarino, Reference Mandarino2007), using k(UO3) = 0.118, as provided by Mandarino (Reference Mandarino1976).
Pseudomarkeyite
Pseudomarkeyite crystals are tapering blades and tablets (Fig. 3) up to ~1 mm in maximum dimension, flattened on {10![]() $\bar{1}$} and elongated on [010]. Crystals exhibit the forms {10
$\bar{1}$} and elongated on [010]. Crystals exhibit the forms {10![]() $\bar{1}$}, {100}, {010} and {510}; the {100} and {510} forms are based upon observed morphology, but were not measured. Twinning is ubiquitous, by 180° rotation about [101] (Fig. 4).
$\bar{1}$}, {100}, {010} and {510}; the {100} and {510} forms are based upon observed morphology, but were not measured. Twinning is ubiquitous, by 180° rotation about [101] (Fig. 4).

Fig. 3. Pseudomarkeyite (pearly tapering blades) and markeyite (lustrous blades in upper right) with calcite (brown and grey balls) on asphaltum on the holotype specimen (#67091); FOV 3.5 mm across.

Fig. 4. Crystal drawing of pseudomarkeyite twin; clinographic projection in non-standard orientation, b vertical.
Crystals are pale green yellow and transparent with vitreous to pearly lustre. The streak is white. The mineral fluoresces bright bluish white under a 405 nm laser. The Mohs hardness is ~1, based upon scratch tests. Crystals are brittle with stepped fracture. Cleavage is perfect and very easy on {10![]() $\bar{1}$}, good on {010} and fair on {100}. Pseudomarkeyite loses birefringence (presumably due to decomposition), but does not dissolve in room-temperature H2O; it dissolves immediately with effervescence in dilute HCl. The density measured by flotation in a mixture of methylene iodide and toluene is 2.88(2) g cm–3. The calculated density based on the empirical formula and unit-cell parameters obtained from single-crystal X-ray diffraction data is 2.877 g cm–3.
$\bar{1}$}, good on {010} and fair on {100}. Pseudomarkeyite loses birefringence (presumably due to decomposition), but does not dissolve in room-temperature H2O; it dissolves immediately with effervescence in dilute HCl. The density measured by flotation in a mixture of methylene iodide and toluene is 2.88(2) g cm–3. The calculated density based on the empirical formula and unit-cell parameters obtained from single-crystal X-ray diffraction data is 2.877 g cm–3.
Optically, pseudomarkeyite is biaxial (–), with α = 1.549(2), β = 1.553(2) and γ = 1.557(2) (measured in white light). The 2V measured directly on a spindle stage is 88(2)°; the calculated 2V is 89.8°. No dispersion was observed and the mineral is nonpleochroic. The optical orientation is Y = b, Z ^ a = 30° in obtuse β (X ≈⊥ {10![]() $\bar{1}$}). The Gladstone–Dale compatibility index 1 – (K P/K C) for the empirical formula is –0.024, in the excellent range (Mandarino, Reference Mandarino2007), using k(UO3) = 0.118, as provided by Mandarino (Reference Mandarino1976).
$\bar{1}$}). The Gladstone–Dale compatibility index 1 – (K P/K C) for the empirical formula is –0.024, in the excellent range (Mandarino, Reference Mandarino2007), using k(UO3) = 0.118, as provided by Mandarino (Reference Mandarino1976).
Raman spectroscopy
Raman spectroscopy was conducted on a Horiba XploRA PLUS using a 532 nm diode laser. The Raman spectra of natromarkeyite, pseudomarkeyite and markeyite are very similar. The spectra are compared in Fig. 5 and the bands are listed in Table 2 with their likely band assignments.

Fig. 5. The Raman spectra of markeyite, natromarkeyite and pseudomarkeyite.
Table 2. Raman bands and mode assignments for markeyite, natromarkeyite and pseudomarkeyite.

The broad multiple bands in the 3700–3100 cm–1 range are attributed to ν O–H stretching vibrations of structurally non-equivalent/symmetrically distinct hydrogen-bonded H2O groups. According to the correlation given by Libowitzky (Reference Libowitzky1999), this corresponds to approximate O–H⋅⋅⋅O hydrogen bond-lengths between 3.2 and 2.7 Å, which is consistent with what we report in the structure determinations. The broad bands in the 2800–2300 cm–1 range in the markeyite and pseudomarkeyite spectra were originally interpreted as corresponding to strong (short) hydrogen bonds (Kampf et al., Reference Kampf, Plášil, Kasatkin, Marty and Čejka2018); however, no such bonds appear to exist in the structures of markeyite, natromarkeyite or pseudomarkeyite. We now think that this is a spectral artefact because we have also observed it in spectra of anhydrous minerals and we have recorded other spectra for markeyite that do not exhibit this feature. It is also possible that a small amount of asphaltum adhering to the crystals caused the bands in this region.
The weak bands between 1439 and 1358 cm–1 can be attributed to the ν3 (CO3)2– antisymmetric stretching vibrations of the (CO3)2– units. Medium to strong multiple bands between 1094 and 1067 cm–1 are connected with the ν1 (CO3)2– symmetric stretching vibrations of several structurally non-equivalent carbonate units (Koglin et al., Reference Koglin, Schenk and Schwochau1979; Anderson et al., Reference Anderson, Chieh, Irish and Tong1980; Čejka, Reference Čejka, Burns and Finch1999, Reference Čejka2005).
A weak band near 900 cm–1 may be due to the ν2 (δ) (CO3)2– bending vibrations or to the ν3 (UO2)2+ antisymmetric stretching vibration; an overlap/coincidence of these two bands is possible. The very strong bands centred at 825, 829 and 826 cm–1 for markeyite, natromarkeyite and pseudomarkeyite are due to the ν1 (UO2)2+ symmetric stretching vibrations and provide inferred U–O bond lengths around 1.78–1.79 Å (Bartlett and Cooney, Reference Bartlett and Cooney1989). The ν2 (δ) (CO3)2– bending vibration may coincide with this band.
Several weak to strong bands between 773 and 688 cm–1 are assigned to the doubly degenerate ν4 (δ) (CO3)2– bending vibrations. The medium broad band in the spectra centred near 240 cm–1 is assigned to the split doubly degenerate ν2 (δ) (UO2)2+ bending vibrations. Bands between 189 and 127 cm–1 are due to lattice modes (Koglin et al., Reference Koglin, Schenk and Schwochau1979; Anderson et al., Reference Anderson, Chieh, Irish and Tong1980; Čejka, Reference Čejka, Burns and Finch1999, Reference Čejka2005).
Chemical composition
Chemical analyses (five for natromarkeyite and eight for pseudomarkeyite) were performed using a JEOL JXA-8230 electron microprobe operated in wavelength dispersive mode at 15 kV and 1 nA, with a 10 μm beam diameter. Matrix effects were accounted for using the PAP correction routine (Pouchou and Pichoir, Reference Pouchou, Pichoir, Heinrich and Newbury1991). For natromarkeyite, a time-dependent intensity correction was applied to Na. H2O and CO2 were not determined directly because of extreme paucity of material. The H2O and CO2 contents were calculated by stoichiometry in accord with the crystal structure determinations: based on 4 U, 13 C and 74 O atoms per formula unit (apfu) for natromarkeyite and 4 U, 12 C and 65 O apfu for pseudomarkeyite. The high analytical total for natromarkeyite is presumably due to the loss of loosely bound H2O under vacuum resulting in higher concentrations for the remaining constituents than are to be expected for the fully hydrated phase. Analytical data are given in Table 3. No other elements with atomic numbers higher than 8 were above the detection limits.
Table 3. Chemical composition (in wt.%) for natromarkeyite and pseudomarkeyite.

* Based on the structure.
bdl: below detection limit; S.D. standard deviation.
The empirical formula for natromarkeyite (calculated on the basis of 74 O apfu) is Na2.01Ca7.97Mg0.03Cu0.05U4.00C13.00O74.00H53.89 or Na2.01Ca7.97Mg0.03Cu0.05(UO2)4(CO3)13(H2O)24⋅3H2O (–0.11 H). The ideal formula is Na2Ca8(UO2)4(CO3)13(H2O)24⋅3H2O. The empirical formula for pseudomarkeyite (calculated on the basis of 65 O apfu) is Ca7.95U4.00C12.00O65.00H42.11, or Ca7.95(UO2)4(CO3)12(H2O)18⋅3H2O (+0.10 H). The ideal formula is Ca8(UO2)4(CO3)12⋅21H2O.
X-ray crystallography and structure refinement
Powder X–ray diffraction studies were done using a Rigaku R–Axis Rapid II curved imaging plate microdiffractometer, with monochromatised MoKα radiation (λ = 0.71075 Å). A Gandolfi-like motion on the φ and ω axes was used to randomise the samples and observed d values and intensities were derived by profile fitting using JADE 2010 software (Materials Data, Inc.). The powder data are presented in Supplementary Tables S1 and S2 for natromarkeyite and pseudomarkeyite, respectively. These tables, along with the crystallographic information files, have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
The single-crystal structure data were collected at room temperature using the same diffractometer and radiation noted above. The selection of a crystal of natromarkeyite was straightforward because, despite their rarity, the natromarkeyite crystals are untwinned and of good quality for single-crystal study. Unfortunately, pseudomarkeyite crystals are invariably twinned (by rotation on [101]) and exhibit high mosaicity. These problems, coupled with the close spacing of reflections due to the large a and b cell parameters, made the integration of reflections problematic. The best data were obtained from a twinned crystal with one larger twin component; however, it proved impossible to adequately separate the reflections from the smaller component during integration. In addition, relatively weak diffraction only provided data to 40°2θ.
For each mineral, single-crystal data were processed using the Rigaku CrystalClear software package and an empirical (multi–scan) absorption correction was applied using the ABSCOR program (Higashi, Reference Higashi2001) in the CrystalClear software suite. The structures were solved by direct methods using SIR2011 (Burla et al., Reference Burla, Caliandro, Camalli, Carrozzini, Cascarano, Giacovazzo, Mallamo, Mazzone, Polidori and Spagna2012). SHELXL-2016 (Sheldrick, Reference Sheldrick2015) was used for the structure refinements.
For natromarkeyite, all non-hydrogen atoms were successfully refined with anisotropic displacement parameters. Difference-Fourier synthesis located the hydrogen atom positions related to OW1 to OW7; however, no geometrically reasonable H positions could be found for OW8 to OW11. This could be because of disorder for these H2O groups. The located H-atom positions were refined with soft restraints of 0.82(2) Å on the O–H distances and 1.30(2) Å on the H–H distances and with the U eq of each H set to 1.2 times that of its donor O atom. Hydrogen bonds for OW8 to OW11 are proposed based upon O–O distances and geometries. It should be further noted that, because of the presence of considerable unresolved residual electron density, the assignment of H atom positions related to OW1 to OW7 was difficult and some anomalies suggest that several assignments are questionable: (1) The distance between the H1B and H2B sites is unusually short (1.47 Å), so that at least one of these sites may be incorrect; (2) the OW7–H7A⋅⋅⋅OW8 hydrogen bond, with a bond strength of 0.21 valence units, seems unlikely because OW8 would be highly oversaturated in bond strength; and (3) at least one of the three hydrogen bonds received by OW10 is probably incorrect because it is otherwise very oversaturated.
For pseudomarkeyite, all atoms were located and successfully refined at full occupancies to provide a very reasonable structure model; however, because of the aforementioned problems, only the Ca and U atoms could be refined with anisotropic displacement parameters and hydrogen atom sites could not be located. While the general structure is clearly correct, the aforementioned twinning and high mosaicity led to some bond-length anomalies, which we consider refinement artefacts. In particular, some bond lengths are significantly shorter than normal: C5–O13 (1.19 Å), C7–O18 (1.18 Å) and U3–O24 (1.72 Å).
Data collection and refinement details are given in Table 4. Atom coordinates and displacement parameters are given in Tables 5 and 6 for natromarkeyite and pseudomarkeyite, respectively. Selected bond distances are in Tables 7 and 8 for natromarkeyite and pseudomarkeyite, respectively. Bond-valence analyses are in Tables 9 and 10 for natromarkeyite and pseudomarkeyite, respectively. Note that the bond-valence analysis for pseudomarkeyite does not include hydrogen-bond contributions because of the difficulty in proposing an unambiguous hydrogen-bonding scheme for such a complex structure without hydrogen atom positions.
Table 4. Data collection and structure refinement details for natromarkeyite and pseudomarkeyite.*

*R int = Σ|F o2–F o2(mean)|/Σ[F o2]. GoF = S = {Σ[w(F o2–F c2)2]/(n–p)}½. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o2–F c2)2]/Σ[w(F o2)2]}½; w = 1/[σ2(F o2)+(aP)2+bP] where P is [2F c2+Max(F o2,0)]/3; for natromarkeyite, a is 0.0201 and b is 9.22; for pseudomarkeyite, a is 0.1226 and b is 100.5513.
Table 5. Atom coordinates, displacement parameters (Å2) and site occupancies for natromarkeyite.

Table 6. Atom coordinates and displacement parameters (Å2) for pseudomarkeyite.

Table 7. Selected bond distances (Å) and angles (°) for natromarkeyite.

* The H3B site is split into two symmetrically related, half-occupied sites, each of which bonds to an O5 atom.
Table 8. Selected bond distances (Å) for pseudomarkeyite.

Table 9. Bond valence analysis for natromarkeyite. Values are expressed in valence units.*

* Multiplicity is indicated by ×↓→. Bond-valence parameters from Gagné and Hawthorne (Reference Gagné and Hawthorne2015); hydrogen-bond strengths based on O–O bond lengths from Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988). Donated hydrogen-bond strengths are shown as negative values. There was no obvious hydrogen-bond receptor for one H atom of the OW3 and the OW5 group; assuming that these H atoms donate multiple weak hydrogen bonds, a donated bond strength of –0.20 (shown in italics) is assigned to OW3 and OW5.
Table 10. Bond valence analysis for pseudomarkeyite. Values are expressed in valence units.*

* Multiplicity is indicated by ×2↓. Bond valence parameters from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). Hydrogen-bond contributions are not included.
Description and discussion of the structures
The structures of markeyite, natromarkeyite and pseudomarkeyite are constructed of similar components. The U sites in the structures are surrounded by eight O atoms forming a squat UO8 hexagonal bipyramid. These bipyramids are each chelated by three CO3 groups, forming a uranyl tricarbonate cluster (UTC) of formula [(UO2)(CO3)3]4– (Fig. 6). As noted by Burns (Reference Burns2005), UTCs are especially common structural features in uranyl carbonates that crystallise from alkaline solutions.

Fig. 6. The uranyl tricarbonate cluster (UTC) of formula [(UO2)(CO3)3]4– consisting of a squat UO8 bipyramid and three CO3 groups sharing alternating UO8 equatorial edges.
Natromarkeyite
Two independent U sites (U1 and U2) in the structure of natromarkeyite centre two UO8 hexagonal bipyramids, which combine with CO3 groups centred by four independent C sites (C1, C2, C3 and C4) to form two different UTCs. As was noted in the structure of markeyite, there is an additional CO3 group (centred by C5) that is not chelated to a UO8 hexagonal bipyramid. The C5O3 group is half-occupied, shares an O15–O15 edge with an equivalent C5O3 group and is completed by a half-occupied O16 site. Due to steric limitations, only one of the adjacent C5 sites can be occupied at the same time. Another half-occupied O site (OW7) is located 1.28 Å from the O16 site and cannot be occupied when the O16 site is occupied. There are two different Ca–O polyhedra in the structure. Ca1 bonds to seven fully occupied O sites, Ca2 bonds to seven fully occupied and two half-occupied O sites for a total effective coordination of eight. The Na1 occupies a position equivalent to the Ca3 site in the markeyite structure, while the Na2 site has no counterpart in the markeyite structure. Na1 is octahedrally coordinated to five fully occupied O sites and one O site (OW11) with a refined occupancy of 0.96 that can be considered essentially fully occupied. Na2 bonds to five fully occupied O sites forming a Na2O5 trigonal bipyramid.
The two Ca–O polyhedra share edges and corners with the UTCs in very different ways. The Ca1O7 polyhedra share edges with U1 and U2 bipyramids and corners with C1O3 and C2O3 triangles in different UTCs. Pairs of Ca2O8 polyhedra share an edge to form a dimer, which is linked to a second dimer through the half-occupied C5O3 triangles and OW7 sites. The group of four Ca2O8 polyhedra (with the C5O3 triangle at its centre) is linked to two U1 UTCs by edge sharing between Ca2O8 polyhedra and C1O3 triangles. The Na1O6 octahedra share corners with two C3O3 and two C4O3 triangles, each belonging to independent UTC units. The Na2O5 trigonal bipyramids share their apical vertices (O11) with apical (uranyl) vertices of two symmetrically related U1 hexagonal bipyramids. The linkages between the Ca and Na polyhedra and the UTCs form thick corrugated heteropolyhedral layers parallel to {010} (Fig. 7) and these layers link to one another and to interlayer H2O groups (OW10) only via hydrogen bonds (Fig. 8). This explains the perfect {010} cleavage.

Fig. 7. The heteropolyhedral layers in markeyite, natromarkeyite and pseudomarkeyite. The polyhedra are labelled and for natromarkeyite the H atoms are shown as small white spheres. The unit cells are outlined with dashed lines.

Fig. 8. The structures of markeyite, natromarkeyite and pseudomarkeyite viewed down [010] with heteropolyhedral layers horizontal. The polyhedra are labelled, the O atoms of the isolated H2O groups are shown as large white spheres and H atoms for natromarkeyite are shown as small white spheres. The unit cells are outlined with dashed lines.
As noted above, the Na2 site in the natromarkeyite structure has no counterpart in the markeyite structure. The OW3 and OW8 groups, which are coordinated to Na2 in natromarkeyite, are interlayer H2O sites in markeyite. Additionally, there is a partially occupied OW12 site bonded to Ca3 in the markeyite structure, which has no counterpart in the natromarkeyite structure. Summing the H2O site occupancies in the two structures provides 27.30 H2O pfu (ideally 27) for natromarkeyite and 28.25 H2O pfu (ideally 28) for markeyite. Expressing the structurally bound H2O groups (those bonded to cations) separately from the isolated H2O groups (those linked only via hydrogen bonds), results in the ideal formulas Ca9(UO2)4(CO3)13(H2O)22⋅6H2O for markeyite and Na2Ca8(UO2)4(CO3)13(H2O)24⋅3H2O for natromarkeyite. In spite of their minor structural differences, markeyite and natromarkeyite are considered essentially isostructural.
Pseudomarkeyite
The three U sites (U1, U2 and U3) in the structure of pseudomarkeyite centre three UO8 hexagonal bipyramids, which coordinate to CO3 groups centred by seven independent C sites (C1 to C7) to form three different UTCs. There is no additional non-UTC CO3 group in the pseudomarkeyite structure. Five Ca–O polyhedra share edges and corners with the UTCs. The UTCs and Ca–O polyhedra form thick corrugated heteropolyhedral layers parallel to {10![]() $\bar{1}$}. These layers are very similar to the layers in markeyite (Fig. 6), but differ in several respects. The most obvious difference is that the pseudomarkeyite layer is missing the distinctive grouping of four Ca2 polyhedra (and C5 triangle) found in the markeyite layer. Viewed down the b axis (Fig. 8), another important difference in the structures is evident. In the markeyite structure, the heteropolyhedral layers link to one another and to interlayer H2O groups only via hydrogen bonds; however, in the pseudomarkeyite structure, the layers are linked through edge and corner links to Ca polyhedra.
$\bar{1}$}. These layers are very similar to the layers in markeyite (Fig. 6), but differ in several respects. The most obvious difference is that the pseudomarkeyite layer is missing the distinctive grouping of four Ca2 polyhedra (and C5 triangle) found in the markeyite layer. Viewed down the b axis (Fig. 8), another important difference in the structures is evident. In the markeyite structure, the heteropolyhedral layers link to one another and to interlayer H2O groups only via hydrogen bonds; however, in the pseudomarkeyite structure, the layers are linked through edge and corner links to Ca polyhedra.
Another interesting contrast relates to the edges shared between UO8 bipyramids and Ca polyhedra. Note that in any UTC, the UO8 bipyramid has three equatorial edges that are not shared with CO3 groups. In the markeyite and natromarkeyite structures, each of the two UO8 bipyramids shares two of these equatorial edges with Ca polyhedra, the third equatorial edge being unshared. In the pseudomarkeyite structure, the U1 and U3 bipyramids share all three available non-CO3 equatorial edges with Ca polyhedra, while the U2 bipyramid shares only two such edges.
Comparison with other structures
Lussier et al. (Reference Lussier, Lopez and Burns2016) provide a review of inorganic uranyl compounds that includes specifics of the structures of uranyl carbonate minerals. Their review shows the UTC structural unit to be a prominent feature in the majority of uranyl carbonate minerals. In these structures, the UTCs are linked by various combinations of counter cations and hydrogen-bonding networks as 0-dimensional clusters. These are usually incorporated into heteropolyhedral sheets, such as in the minerals liebigite (Mereiter, Reference Mereiter1982), grimselite (Plášil et al., Reference Plášil, Fejfarová, Skála, Škoda, Meisser, Hloušek, Císařová, Dušek, Veselovský, Čejka, Sejkora and Ondruš2012) and línekite (Plášil et al., Reference Plášil, Čejka, Sejkora, Hloušek, Škoda, Novák, Dušek, Císařová, Němec and Ederová2017). There are also complex clusters, such as those in ewingite (Olds et al., Reference Olds, Plášil, Kampf, Simonetti, Sadergaski, Chen and Burns2017b) and paddlewheelite (Olds et al., Reference Olds, Plášil, Kampf, Dal Bo and Burns2018), which combine UTCs with other building units, such as the trimers of uranyl polyhedra in ewingite and the square–pyramidal copper polyhedra in paddlewheelite. Less commonly, the structural units in uranyl carbonate minerals are infinite sheets of uranyl polyhedra and carbonate triangles, such as in rutherfordine (Finch et al., Reference Finch, Cooper, Hawthorne and Ewing1999), fontanite (Hughes and Burns, Reference Hughes and Burns2003), sharpite (Plášil, Reference Plášil2018) and meyrowitzite (Kampf et al., Reference Kampf, Plášil, Olds, Nash, Marty and Belkin2019e), and sheets containing also lanthanide-centred polyhedra, such as in bijvoetite-(Y) and kamotoite-(Y) (Plášil and Petříček, Reference Plášil and Petříček2017).
The uranyl carbonate mineral with which the structures of markeyite, natromarkeyite and pseudomarkeyite are most similar is liebigite, Ca2(UO2)(CO3)3⋅11H2O (Mereiter, Reference Mereiter1982). All four structures contain the same structural components and the same types of polyhedral linkages. In all four, the Ca–O polyhedra link the UTCs forming thick corrugated heteropolyhedral layers. In the structure of liebigite, as in those of markeyite, natromarkeyite and natromarkeyite, these layers link to one another and to interlayer H2O groups only via hydrogen bonds; however, the topology of the layer in liebigite (Fig. 9) is quite different from those in markeyite, natromarkeyite and pseudomarkeyite.

Fig. 9. Heteropolyhedral layer in the structure of liebigite viewed down [010]. The unit cell is outlined with dashed lines.
Structural complexity
We examined the structural complexity of markeyite, natromarkeyite and pseudomarkeyite, as well as that of the related mineral liebigite. As developed by Krivovichev (Reference Krivovichev2012, Reference Krivovichev2013, Reference Krivovichev2014, Reference Krivovichev2018), structural complexity can be quantified as the total structural information content, I G,total. As available structure determinations of liebigite, markeyite and pseudomarkeyite lack H atom positions, the approximate calculations of the fictive H atoms contribution to the total structure complexity were undertaken. An overview of the structural and chemical complexity measures of the four minerals, calculated using the program TOPOS (Blatov et al., Reference Blatov, Shevchenko and Proserpio2014), is given in Table 11.
Table 11. Information measures for selected uranyl carbonates.

v = number of atoms in the reduced unit cell.
I G = structural information content.
I chem = chemical information content normalised by Z.
Both structurally and chemically, the least complex is liebigite, which represents a ‘relatively simple’ layered structure. The structures of markeyite, natromarkeyite and pseudomarkeyite are significantly more complex, owing to the additional linkages (e.g. the Ca2–C5O3–C5O3–Ca2 linkages in markeyite). These three structures are nearly as complex as the suite of complex uranyl carbonate structures based upon ‘paddle-wheel’ units, which have I G,total >2000 bits/unit cell: braunerite, K2Ca(UO2)(CO3)3⋅6H2O (Plášil et al., Reference Plášil, Mereiter, Kampf, Hloušek, Škoda, Čejka, Němec and Ederová2016), línekite, K2Ca3[(UO2)(CO3)3]2⋅7H2O (Plášil et al., Reference Plášil, Čejka, Sejkora, Hloušek, Škoda, Novák, Dušek, Císařová, Němec and Ederová2017) and paddlewheelite, MgCa5Cu2(UO2)4(CO3)12⋅33H2O (Olds et al., Reference Olds, Plášil, Kampf, Dal Bo and Burns2018). The most structurally complex uranyl carbonate mineral, ewingite, Mg8Ca8(UO2)24(CO3)30O4(OH)12⋅180H2O (Olds et al., Reference Olds, Plášil, Kampf, Simonetti, Sadergaski, Chen and Burns2017b), is also the most structurally complex mineral known.
Given the similarities in the structures of markeyite, natromarkeyite and pseudomarkeyite, it is not surprising that their structural complexities are very similar. The chemical complexity (I chem, norm.) of natromarkeyite is somewhat higher due to incorporation of an additional cation in the structure. These minerals certainly form under similar conditions, although natromarkeyite is found in in assemblages more enriched in Na, typically including the mineral andersonite.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.59
Acknowledgements
Reviewers Fernando Cámara and Igor V. Pekov are thanked for their constructive comments on the manuscript. A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County. This research was also financially supported by the Czech Science Foundation (project 20-11949S to J.P.).