Introduction
The tobacco whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) has a wide geographic distribution. As a species, it has a broad host plant range which includes edible and ornamental crops in both field and greenhouses. However, some populations have been shown to have narrow host ranges and a few are monophagous (Bedford et al., Reference Bedford, Briddon, Brown, Rosell and Markham1994). Bemisia tabaci causes damage through direct feeding and as a vector of many different plant viruses (Markham et al., Reference Markham, Bedford, Liu and Pinner1994; Jones, Reference Jones2003). The significant variation in B. tabaci populations led to the identification of a number of biotypes that are currently denoted by the letters A–T and the assumption of a species complex (Bedford et al., Reference Bedford, Briddon, Brown, Rosell and Markham1994; Perring, Reference Perring2001). This was based on studies that found differences between populations in their virus transmission capabilities, host plant ranges and ability to induce phytotoxic responses in certain plant species. Biochemical and molecular markers including allozymes, random amplified polymorphic DNA–polymerase chain reactions (RAPD–PCR), amplified fragment length polymorphisms (AFLP), restriction fragment length polymorphisms (RFLP) and microsatellites have all been used to disclose the genotypic variation of the species and to distinguish and characterize the biotypes (Brown et al., Reference Brown, Perring, Cooper, Bedford and Markham2000; Cervera et al., Reference Cervera, Cabezas, Simón, Martínez-Zapater, Beitia and Cenis2000; Moya et al., Reference Moya, Guirao, Cifuentes, Beitia and Cenis2001; Abdullahi et al., Reference Abdullahi, Atiri, Thottappilly and Winter2004; De Barro, Reference De Barro2005). These techniques also revealed the global spread of the B biotype through the intercontinental trade in ornamental plants (Bedford et al., Reference Bedford, Briddon, Markham, Brown and Rosell1993). In addition, sequences of the mitochondrial cytochrome oxidase I (mtCOI) gene and of the internal transcribed spacer (ITS) have shown that B. tabaci populations are clustered in several well supported groups primarily based on presumed geographic origin at the continental scale (Frohlich, Reference Frohlich, Torres-Jerez, Bedford, Markham and Brown1999; Legg et al., Reference Legg, French, Rogan, Okao-Okuja and Brown2002; Abdullahi et al., Reference Abdullahi, Winter, Atiri and Thottappilly2003; De Barro et al., Reference De Barro, Trueman and Frohlich2005).
The biotype status of B. tabaci has been studied in many countries worldwide (Brown et al., Reference Brown, Coats, Bedford, Markham, Bird and Frohlich1995). In southern Europe and around the Mediterranean Basin the polyphagous B and Q biotypes predominate, although other biotypes that are geographically isolated or monophagous to regional plant species also occur (Guirao et al., Reference Guirao, Beitia and Cenis1997; Moya et al., Reference Moya, Guirao, Cifuentes, Beitia and Cenis2001; Horowitz et al., Reference Horowitz, Denholm, Gorman, Cenis, Kontsedalov and Ishaaya2003; Simón et al., Reference Simón, Cenis, Demichelis, Rapisarda, Caciagli and Bosco2003; Khasdan et al., Reference Khasdan, Levin, Rosner, Morin, Kontsedalov, Maslenin and Horowitz2005; Žanić et al., Reference Žanić, Cenis, Kačić and Katalinić2005). In Greece, non-B biotype B. tabaci were first identified during a survey in Crete in 1992 (Kirk et al., Reference Kirk, Lacey, Roditakis and Brown1993) and in 2000, infections of tomato yellow leaf curl virus (TYLCV, Israeli species), a virus transmitted by B. tabaci, caused substantial crop losses in Crete and southern Peloponnese (Avgelis et al., Reference Avgelis, Roditakis, Dovas, Katis, Vassilakos and Bem2001). Apart from information on variation of insecticide resistance levels (Roditakis et al., Reference Roditakis, Roditakis and Tsagkarakou2005), no other data exist on the diversity of B. tabaci populations in Greece including biotype identification and distribution.
The phenological differences between B. tabaci populations have highlighted the importance of obtaining a genetic identity for them as a prerequisite for an effective and sustainable control of both the pest and its associated plant viruses. The aim of this study was to examine the genetic polymorphism and determine the biotype status of Greek B. tabaci populations by analysing samples from different regions in Greece. Two different approaches were used: sequencing of the mtCOI gene and genotyping using microsatellite markers. Moreover, molecular tests enabling a rapid and convenient method for discriminating between Q and B biotypes were developed and used within this study.
Materials and methods
Bemisia tabaci samples
Sampling was undertaken within mainland Greece and on the islands of Crete and Santorini between 2002 and 2004 (fig. 1). The origin of the samples and the number of individuals used for each analysis are shown in table 1. Of the 28 B. tabaci samples, nine were collected from non-cultivated plants (mainly Ipomoea sp. and Solanum nigrum) and 19 were collected from cultivated vegetable (eggplant, tomato, cucumber, and melon) or non-food crops (cotton and tobacco). Four samples (GR-KAL, GR-IERE, GR-HER1 and GR-MAL) were from plants in greenhouses and all others were from open environments. In each location, adult whiteflies were collected from several plants within the same field (open environments or greenhouses) and stored until use, at either −80°C or in 70% ethanol. Leaves infested with whitefly puparia were also collected for species identification using the key by Martin et al. (Reference Martin, Mifsud and Rapisarda2000).

Fig. 1. Bemisia tabaci sampling localities in Greece. The codes for each sample are as in table 1.
Table 1. Geographic origin, host plant and date of collection of Bemisia tabaci populations from Greece, and of B and Q biotype reference colonies from different countries.

For each collection different females were used in each of the three diagnostic tests (diagnostic PCR, PCR-RFLP, microsatellite loci BT-t19 or BT-b159).
1 Accession numbers for the mitochondrial cytochrome oxidase I sequences obtained in this study.
2 Loci BT-4, BT-83, BT-b34, BT-b155 and BT-d26 used together with BT-b159 in the study of the population genetic structure.
3 Populations included also in Roditakis et al. (Reference Roditakis, Roditakis and Tsagkarakou2005).
4 In parenthesis, years of rearing before being used in the present work.
N, number of females used in each analysis.
In bold, populations used in the analysis of the genetic structure.
For the development of biotype diagnostic assays, insects from seven B and five Q reference laboratory collections from different geographical origins were included. The biotypes were characterized by esterase profiles using polyacrylamide gel electrophoresis (Byrne & Devonshire, Reference Byrne and Devonshire1993). The original host plants and the countries of collection are shown in table 1.
DNA extraction, PCR amplification and sequencing of the mtCOI
Genomic DNA (gDNA) was extracted from individual whiteflies by placing them in a 1.5 ml tube and grinding with a pestle in 50 μl of ice-cold lysis buffer (100 mm NaCl, 10 mm Tris-HCL, pH 8.0) containing 0.4 mg ml−1 of proteinase K. The extracts were incubated at 55°C for 1 h and at 85°C for 5 min prior to a 5 min centrifugation (10,000 g) to pellet debris. The supernatant was used as the DNA source for the polymerase chain reaction (PCR). The primers C1-J-2195 (5′ TTG ATT TTT TGG TCA TCC AGA AGT 3′, Frohlich et al., Reference Frohlich, Torres-Jerez, Bedford, Markham and Brown1999) and tRNA-1576 (5′ TAT AAA TCT TAA ATT TAC TGC A 3′, present study) were used to amplify an 879 bp fragment of the mtCOI gene. The new primer tRNA-1576 was designed to improve the yield and the quality of the PCR product from an insect conserved region of tRNA-Leu flanking the mtCOI gene. Four microlitres of the gDNA extract were used as the template in a 20 μl reactions containing 0.2 mm dNTPs, 1.5 mm MgCl2, 1.0 μm of each primer, 1 unit Taq polymerase (Minotech) and 1× enzyme buffer (Minotech). The PCR program was carried out on a Perkin-Elmer 9600 with cycling conditions of 93°C for 3 min followed by 35 cycles of 93°C 40 s, 50°C 45 s, 72°C 90 s. The PCR products were purified using the Nucleospin Exctract kit (Macherey-Nagel) and sequenced in both directions using the primers mentioned above. Reactions were performed in a 20 μl reaction volume containing 200 ng of template DNA, 3 pmoles of primer and 2 μl of BigDye Terminator v.3.1 cycle sequencing kit (Applied Biosystems). Both strands were sequenced for all individuals using an MJ BaseStation 100 DNA fragment analyser. Sequence data was analysed using BioEdit v.7.0 software (Hall, Reference Hall1999). The sequences are deposited in the GenBank under the accession numbers DQ365856 to DQ365878 (table 1).
Sequence and phylogenetic analyses
In addition to sequences obtained in the present study, mtCOI sequences of B. tabaci from a range of countries and biotypes were obtained from Genbank (fig. 2) and included in the analyses. Multiple sequence alignment was carried out using the software package Clustal W v.1.7 (Higgins et al., Reference Higgins, Thompson and Gibson1996) and corrected by eye. Phylogenetic inference analyses were conducted using maximum likelihood (ML), neighbour-joining (NJ) and maximum parsimony (MP) methods. Nucleotides were used as discrete, unordered characters. For the ML, the best-fit model of DNA substitution and the parameter estimates used for tree construction were chosen by performing hierarchical likelihood-ratio tests (Huelsenbeck & Crandall, Reference Huelsenbeck and Crandall1997) in Modeltest (v.3.7; Posada & Crandall, Reference Posada and Crandall1998). Heuristic ML searches were performed with 10 replicates of random sequence addition and TBR branch swapping.

Fig. 2. Rooted ML tree (ln L=−4526.92577) showing the phylogenetic relationships of the 16 Greek Bemisia tabaci COI haplotypes; sequences generated in this study are indicated in capitals. Trialeurodes vaporariorum was used as an outgroup. The analysis was based on 772 sites and likelihood-ratio tests indicated that GTR+G model (Rodríguez et al., Reference Rodríguez, Oliver, Marín and Medina1990) fits better the data with base frequencies A=25.7, C=11.6, G=19.0 and T=28.5% and shape parameter=0.3338. Phylogenetic analyses with neighbour joining (NJ) and maximum parsimony (MP) produced trees with similar topologies with regard to the major lineages. Numbers at nodes indicate bootstrap scores after 1000 and 2000 replicates for NJ and MP, respectively; only percentages >75% are indicated and dashes (-) indicate nodes that do not exist in strict consensus NJ and MP bootstrap trees. Abbreviations are as described in table 1.
In NJ, we used PAUP* (v.4.0b10, Swofford, Reference Swofford2002) and the model estimated with Modeltest v.3.7; the confidence of the nodes was assessed by 1000 bootstrap replicates (Felsenstein, Reference Felsenstein1985). MP analysis was also performed with PAUP* (v.4.0b10), with heuristic searches using stepwise addition and performing tree-bisection reconnection (TBR) branch swapping (Swofford et al., Reference Swofford, Olsen, Waddel, Hillis, Hillis, Moritz and Mable1996). Confidence in the nodes was assessed by 2000 bootstrap replicates (Felsenstein, Reference Felsenstein1985) with random addition of taxa.
Diagnostic tests based on the mtCOI sequences
Based on the fixed differences revealed by the comparison of about 90 published mtCOI sequences of known B. tabaci biotypes (Q, B, Ms, A, E, G, C) we developed two diagnostic tests for discrimination of Q and B biotypes.
The first diagnostic assay consists of the amplification by PCR of an 879 bp fragment of mtCOI using the primers tRNA-1576 and C1-J-2195 and subsequent restriction digestion with an endonuclease, cutting in different sites for each biotype. In order to identify restriction enzymes that could be used to discriminate between biotypes, mtCOI sequences were aligned and subsequently examined for restriction recognition sites with a Perl script using the REBASE database (Roberts et al., Reference Roberts, Vincze, Posfai and Macelis2005). A number of enzymes producing restriction fragments that could discriminate between Q and B biotypes were found, for example VspI which has been used by Horowitz et al. (Reference Horowitz, Kontsedalov, Khasdan and Ishaaya2005) and Khasdan et al. (Reference Khasdan, Levin, Rosner, Morin, Kontsedalov, Maslenin and Horowitz2005).
The enzyme AluI, which recognizes and cuts the site AG/CT, was chosen because it can be used to discriminate, not only between Q and B biotypes, but also between Q, B, Ms, A, E, and G biotypes, demonstrated through the comparison of published sequences (the length of the published C biotype sequences does not permit the validation of AluI in their discrimination). AluI recognizes four sites in the Q biotype mtCOI sequences. Two of these sites are also found in the B biotype mtCOI sequences together with a third one found only in the B biotype sequences. Digestion is expected to yield a restriction pattern with four fragments (551 bp, 204 bp, 81 bp and 43 bp) in the B biotype insects and five fragments (307 bp, 229 bp, 204 bp, 124 bp and 15 bp) in the Q biotype insects. There were two exceptions in the published Q biotype mtCOI sequences which are sequences from Israel and Turkey with accession numbers AY518191 and AF342776, respectively, where one of the two Q specific sites is missing. The restriction pattern corresponding to these two sequences is expected to display four fragments (536 bp, 204 bp, 124 bp and 15 bp) and could not be differentiated from the Ms biotype sequences which displayed the same restriction pattern. Restriction digests were performed according to the manufacturer's recommendations.
The second diagnostic assay consists of a bidirectional PCR amplification of mtCOI fragments with four primers used in each PCR reaction. The two outer primers are those described above, they are not biotype specific and yield a ‘control’ fragment of 879 bp. The two inner primers LQ (5′ AAG GGG CCT GAA TTT ATT G 3′) and RB (5′ CTA CTT TGG GTG GAA TAA AGT CT 3′) amplify fragments if the individual being tested belongs to the Q or B biotypes, respectively. These primers point in opposite directions and give differently sized fragments in combination with the outer primers; 310 bp for the LQ/C1-J-2195 primers and 609 bp for the RB/tRNA1576 primers. However, from published sequences it is noted that in addition to the B biotype the primer RB also amplifies the non-European biotype, Ms, (Delatte et al., Reference Delatte, Reynaud, Granier, Thornary, Lett, Goldbach and Peterschmitt2005) and therefore would not be discriminatory when these biotypes coexist. The primers LQ and RB are designed based on published sequences and they do not amplify A, C, E, and G biotypes. Although the amplification of a single 879 bp fragment is an indication that an individual belongs to one of these four biotypes, the lack of amplification may be due to a reaction failure. In order to discriminate between these two situations it is useful to obtain additional information from the RFLP–PCR test before the precise identity of an individual is investigated by sequencing.
Six to eleven individuals from each of the laboratory collections of known biotypes were subjected either to the PCR–RFLP assay or to the four primer PCR before testing field-collected whiteflies. For both diagnostic assays, the mtCOI amplicons were separated by electrophoresis on a 2% agarose gel containing ethidium bromide. A 100 bp DNA ladder was used as a marker to determine fragment sizes.
Microsatellite markers
In addition to the ten microsatellite loci that had been previously isolated by Tsagkarakou & Roditakis (Reference Tsagkarakou and Roditakis2003), two more loci (BT-t19 and BT-e49) were described and used in the present study. These microsatellites were isolated from libraries enriched for the motifs AC and AAG, respectively, following the protocol described in Tsagkarakou & Roditakis (Reference Tsagkarakou and Roditakis2003). The characteristics of these microsatellites are shown in table 2. Bemisia tabaci is a haplo-diploid species in which males are haploid and result from unfertilized eggs, therefore all genotyping was done using adult, diploid females. PCR reactions were performed as described in Tsagkarakou & Roditakis (Reference Tsagkarakou and Roditakis2003) and products were run on an MJ BaseStation 100 DNA fragment analyser. Allele size was determined by comparing the mobility of the PCR products to that of the GeneScan 400HD size standard (Applied Biosystems).
Table 2. Characteristics of the two new microsatellite loci in Bemisia tabaci. The genotyping results of BT-t19 and BT-e49 microsatellite loci for whiteflies coming respectively from nine and six Greek B. tabaci populations are presented.

T a, PCR annealing temperature; N ind, number of Q biotype whiteflies from Greece genotyped; N A, number of alleles; letters (f, t) before the sequence of the forward primers indicate respectively fluorescent labels 6-FAM and TET.
The examination of possible discriminative loci was performed by genotyping insects from laboratory collections of well characterized Q and B biotype whiteflies (table 1). During a first screening step, the genotype of five females for each of two Q and three B reference samples was defined for the 12 microsatellite loci either described here (BT-t19 and BT-e49), or characterized previously (BT-4, BT-83, BT-b34, BT-b159, BT-b155, BT-d26, BT-b53, BT-b103, BT-b69 and BT-b55) by Tsagkarakou & Roditakis (Reference Tsagkarakou and Roditakis2003). At least 15 individuals per sample (six Q and seven B samples) were used to confirm the existence of diagnostic alleles in the loci BT-t19 and BT-b159, which had been selected after the initial screening.
Population data analysis
Six of the 28 samples were subjected to microsatellite analysis. Out of the ten microsatellites characterized previously (Tsagkarakou & Roditakis, Reference Tsagkarakou and Roditakis2003), the six most polymorphic and easily interpretable (BT-4, BT-83, BT-b34, BT-b159, BT-b155 and BT-d26) were used to investigate the genotypic variability of B. tabaci coming from different localities in Crete (table 1) and to analyse the distribution of the genetic variation within and among populations.
Conformity of genotype frequencies to Hardy-Weinberg (H-W) proportions, genotypic linkage disequilibrium and population differentiation were tested using Genepop v.3.4 software (Raymond & Rousset, Reference Raymond and Rousset1995a). Genotypic associations between loci within each population and departure from H-W equilibrium at each locus were tested using the exact test procedures described by Raymond & Rousset (Reference Raymond and Rousset1995b). F IS estimates were computed according to Weir & Cockerham (Reference Weir and Cockerham1984), and heterozygote deficits were tested with a score test as described by Rousset & Raymond (Reference Rousset and Raymond1995). The genetic structure was analysed by estimating F ST parameters (Weir & Cockerham, Reference Weir and Cockerham1984) and by testing for differences in genotype frequencies between populations based on an exact test (Raymond & Rousset, Reference Raymond and Rousset1995b). The sequential Bonferroni correction was applied for the linkage disequilibrium tests and for the H-W equilibrium tests (one per locus) performed on each sample. Overall significance of several independent tests was calculated using Fisher's combined probability test (Fisher, Reference Fisher1970).
In addition to F-statistics that rely on a predefined population organization, a model-based method developed by Pritchard et al. (Reference Pritchard, Stephens and Donnelly2000) was used to identify clusters of individuals. This method, implemented in the software STRUCTURE, is a Bayesian approach which allows us to identify the number of different subpopulations (K) and to estimate the ancestry of the sampled individuals on the basis of their genotypes. We used a burn-in of 50,000 Markov Chain Monte Carlo (MCMC), a run length of 100,000 MCMC and a model allowing for admixture and correlated allele frequencies. Log-likelihood estimates were calculated for K=1 to 9 with six replicates of each. The modal value of ΔK, a quantity based on the second order rate of change with respect to K of the likelihood function was used also to detect the number of clusters according to Evanno et al. (Reference Evanno, Regnaut and Goudet2005). Finally, the DISTRUCT program was used for the graphical display of structure results (Rosenberg, Reference Rosenberg2002).
Results
Biotype diagnostic assays
Diagnostic assays based on the mtCOI sequence
The biotype discrimination has been investigated through (i) the enzymatic digestion of mtCOI PCR-fragment or (ii) the amplification of biotype specific mtCOI fragments. Both assays yielded the expected fragment sizes when using insects from B or Q biotype reference collections.
Digestion with AluI yielded three distinct restriction patterns differing by the number and/or size of the fragments: (i) a restriction pattern with three fragments (approximately 550 bp 200 bp and 80 bp) in all four B biotype laboratory collections tested (NL-AAL, EG-EGY-1, US-GRB and US-McK); (ii) a restriction pattern with four fragments (approximately 300 bp, 230 bp 200 bp, and 120 bp) in three of the Q biotype collections tested (ES-SP1, ES-SP2 and PT-POR); and (iii) a restriction pattern with three fragments (approximately 530 bp, 200 bp and 120 bp) in the Q biotype IL-HC colony. Fragments of 43 bp and 15 bp in B and Q biotypes respectively were not detected as they are too small to be visualized by electrophoresis in a routine agarose gel. The third pattern is consistent with the absence of one ‘Q’ biotype restriction site revealed by the sequencing of the mtCOI of the IL-HC colony. This ‘Q’ site is lacking also from the mtCOI sequence of CY-CHL Q colony, and in the published ‘Q’ sequences from Israel and Turkey with accession numbers AY518191 and AF342776, respectively.
Insects from the eight Greek populations (table 1), in which this diagnostic test was applied, displayed the same restriction pattern with four fragments as the Q biotype reference colonies (ES-SP1, ES-SP2 and PT-POR).
The bidirectional PCR amplification of the mtCOI using four primers yielded fragments of approximately 880 bp (the control fragment) and 610 bp (the B biotype specific fragment) when insects of the seven B biotypes were used, and fragments of 880 bp and 310 bp (the Q biotype specific fragment) in the case of Q biotype insects (from Cyprus, Israel, Portugal and Spain). Seven to twelve insects from each of 26 field populations from Greece (table 1) were examined with this assay and all found to belong to the Q biotype.
Diagnostic microsatellite loci
The genotyping of five Q and seven B reference biotypes showed that two (BT-t19 and BT-b159) out of the 12 microsatellite loci have alleles that are diagnostic for these two biotypes. Alleles 177 and 273 of the BT-t19 and BT-b159 loci, respectively, were fixed (frequency 100%) in all seven B biotype insects examined (115 females). The absence of these alleles from the five Q biotypes (83 females) suggests that they may be diagnostic for the B biotype. In the Q biotypes, five alleles (193, 195, 197, 199 and 201) and six alleles (275, 279, 281, 283, 285 and 287) segregated at the loci BT-t19 and BT-b159, respectively. In the field samples (open environments or greenhouses) at locus BT-t19, the Greek insects (50 females from nine populations) shared in part the same alleles (alleles 187, 195, 197, 199 and 201) as the Q biotype reference insects but lacked allele 177 found only in B biotype collections. Similarly, at the locus BT-b159 the Greek whiteflies (275 females from 18 populations) had eight alleles (275, 279, 281, 283, 285, 287, 289 and 299) of which six were found in reference Q biotypes but lacked allele 273 (specific to B biotype).
Based on the comparison of the allele frequencies, both microsatellite loci indicated that the B biotype was not present in the Greek samples.
Mitochondrial COI sequence
The sequence of a mitochondrial mtCOI fragment (∼760 bp) was determined in 16 B. tabaci individuals collected in Greece. Comparisons of Greek B. tabaci sequences revealed only five variable positions, all of which involved synonymous substitutions (table 3). Eleven of the 16 individuals showed an identical mtCOI sequence.
Table 3. Variable nucleotide sites among mitochondrial cytochrome oxydase I sequences of samples from Greece.
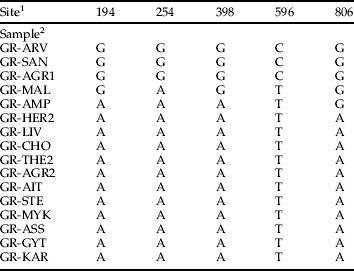
1 The base numbers refer to the position in the sequence of the 879 bp fragment of the mitochondrial cytochrome oxidase I amplified with the primers tRNA 1576 and C1-J-2195.
2 Abbreviations of the collection sites are as in Table 1.
The sequences of the Greek whiteflies were then compared with seven sequences from reference B and Q biotypes obtained in this study as well as 32 sequences available in Genbank, and selected to enable comparisons of approximately 770 bp region. For the phylogenetic analyses, a data set of 56 combined sequences, including outgroup, were used. Finally, 772 sites were examined and there were 370 variable COI sites, of which 234 were parsimony informative. All analyses (NJ, MP, and ML) were congruent regarding the different biotypes, differing only in the branching order of these biotypes, i.e. the relationships between B, Q and Ms biotypes. Likelihood-ratio tests indicated that GTR+G (Rodríguez et al., Reference Rodríguez, Oliver, Marín and Medina1990) model with base frequencies A=25.7, C=11.6, G=19.0, T=28.5; rate matrix=1.5505/8.8713/1.0615/2.7134/15.3217 and shape parameter=0.3338, showed a significantly better fit than the other less complicated models. Equally weighted parsimony analysis of the parsimony-informative characters produced six most-parsimonious trees with a length of 784 steps. Maximum likelihood analysis under the GTR+G model for the data set resulted in a topology (ln L=−4526.92577). The phylo-genetic analyses showed that individuals from Greece clustered together with the sequences of reference Q biotypes and with other Q sequences from GenBank. Within this cluster, there is indication of two subclusters: one comprising sequences from Greece, Spain and Morocco and another with sequences from Turkey, Israel and Cyprus.
A second sister group was formed by individuals of the recently identified genetic type Ms, which is indigenous to the islands of the south-west Indian Ocean (Delatte et al., Reference Delatte, Reynaud, Granier, Thornary, Lett, Goldbach and Peterschmitt2005). B biotype was branched out of the Q/Ms group in the ML analysis although both NJ and MP analyses supported the clustering of B and Q biotypes with high bootstrap support (99 and 100, respectively). All the remaining sequences formed three clusters previously reported to correspond to their African, Asian and American origins (Frohlich et al., Reference Frohlich, Torres-Jerez, Bedford, Markham and Brown1999; De Barro et al., Reference De Barro, Driver, Trueman and Curran2000; Legg et al., Reference Legg, French, Rogan, Okao-Okuja and Brown2002) except one individual from Italy belonging to the host specific T biotype that is genetically related to the Indian clade (Simón et al., Reference Simón, Cenis, Demichelis, Rapisarda, Caciagli and Bosco2003).
Analysis of the microsatellite polymorphism
In total, 8, 11, 6, 8, 9 and 11 alleles were identified at the loci BT-4, BT-83, BT-b34, BT-b159, BT-b155 and BT-d26, respectively, in the six populations studied.
Non-random genotypic associations (P<0.05) were found between BT-83 and BT-b34, and between BT-d26 and BT-b155 loci in a single sample (GR-KOL). This indicates that across samples the six loci carried independent information.
Significant (P<0.05) deviations from H-W expectations, always associated with heterozygote deficits, were observed at the BT-b34 (populations GR-IERE and GR-KOL), BT-83 (populations GR-IERE, GR-KOL, and GR-HER2), BT-b155 (populations GR-KOL and GR-ARH) and BT-d26 (populations GR-HER2 and GR-ARH) loci.
Genetic differentiation was analysed by comparing genotypic distributions and computing F ST estimates. The overall differentiation among samples was highly significant (P<10−5) and corresponded to an F ST of 0.260. Significant differentiation (P<0.01) was found in each pairwise comparison of samples across all loci with F ST estimates ranging from 0.437 to 0.025 (table 4). The higher F ST values were found between GR-MAL and each of the other populations and the lower among samples collected on Ipomoea plants (GR-KOL, GR-HER2, GR-ARH) populations or between GR-AFO and GR-IERE.
Table 4. F ST estimates for six Bemisia tabaci populations collected in Crete.
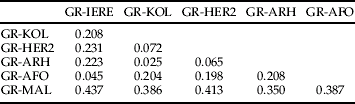
Genotype differentiation is significant (P<0.01) for all pairwise comparisons.
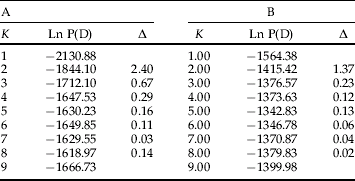
The differentiation between samples was further supported by the results obtained with STRUCTURE software: the lnP(D) increased from K=1 to K=5 and reached a plateau for K>5. (table 5). The smallest value of K that captures the major structure in the data is 2 which is supported by the use of the ΔK as predictor of the real number of clusters. Most individuals (144/159) were clearly assigned to one of the two clusters (Q>90). (fig. 3). In the first cluster only the 28 individuals from the sample GR-MAL are included and in the second one, all the individuals coming from the other collection sites. Few individuals however (15/159) were identified as having admixed ancestry. When we run STRUCTURE after removing the first cluster (i.e. GR-MAL) from the data set, the second group was further divided to two subclusters. One of these subdivisions included only whiteflies collected on Ipomoea plants. Although most of the individuals (113/131) were assigned to one of the two subdivisions (107 individuals with Q>0.91, 6 individuals with 0.91>Q>0.81), 18 individuals were categorized as having admixed ancestry.

Fig. 3. Model-based ancestry of each individual of the six sampled populations of Bemisia tabaci from Crete: (a) the clustering outcome for all samples (K=2), (b) the clustering outcome without the sampled population GR-MAL (K=2).
Table 5. Posterior probability Ln P(D) and ΔK for the maximun numbers of populations K; (A) for all (159) individuals belonging to the six sampled populations (GR-KOL, GR-HER2, GR-ARH, GR-AFO, GR-IERE and GR-MAL) and (B) without the GR-MAL sample (28 individuals).
Discussion
Bemisia tabaci polymorphism in Greece was studied by investigating mtCOI gene sequences and microsatellite loci genotype data of whiteflies from different localities. Few polymorphic sites were detected in the mtCOI sequences of Greek B. tabaci insects (table 3). Comparison of the mtCOI sequences obtained in the present work with sequences from reference biotypes generated in the present study or obtained from Genbank, identified only the Q biotype within Greek samples, which clustered together with other sequences of the Mediterranean/Asia Minor/Africa race (De Barro et al., Reference De Barro, Trueman and Frohlich2005). Inside this region, Greek whiteflies seem to group together with other Q biotype B. tabaci from Spain, Portugal and Morocco, and were loosely separated from a group that included Q biotype sequences from more eastern Mediterranean countries (Turkey, Cyprus and Israel). Whether this separation reflects a phylogeographical structure within the Q biotype or not should be investigated by sequencing additional Q samples from the different localities.
Currently, Q and B biotypes are the most frequently reported from the Mediterranean countries so our research on developing biotype diagnostic assays focused on the discrimination between Q, B and non-Q/non-B biotypes. The three diagnostic assays developed in the present study (four primer PCR, PCR–RFLP and diagnostic microsatellite loci BT-t19 and BT-b159) have been validated with the use of a number of individuals belonging to B and Q biotype reference collections from several countries. Of these three, the four primer PCR was found to be the simplest assay enabling discrimination between Q and B biotypes with a single PCR reaction, however, with the limitation of the absence of Ms individuals as described in detail in the materials and methods section. If non-B/non-Q biotype individuals are suspected within a sample, then the PCR amplification of mtCOI, followed by digestion with AluI restriction enzyme should help towards characterizing them before identifying the biotype by sequencing. In the two diagnostic microsatellite loci, the size differences between the B specific alleles and the alleles found in the Q biotype populations are too small to allow the separation of the fragments on an agarose gel and therefore their utilization at routine biotype screenings is not possible. However, when these two microsatellite loci are included in the population genetic studies of B. tabaci they allow the direct classification of an individual to the B or the Q biotype. Molecular tools which are diagnostic between Q and B biotypes have also been developed and used recently in population studies in Israel by Khasdan et al. (Reference Khasdan, Levin, Rosner, Morin, Kontsedalov, Maslenin and Horowitz2005).
Application of the diagnostic tests enabled the absence of the B biotype and presence of the Q biotype to be confirmed in all 28 Greek populations. This is contrary to the already published studies which showed that within most of the Mediterranean countries, Q and B biotypes can occur together and in some cases even at the same sampling site (Guirao et al., Reference Guirao, Beitia and Cenis1997). In 2000, the B. tabaci-transmitted geminivirus TYLCV caused substantial crop losses in Crete and southern Peloponnese. This virus was identified as the Israeli species by Avgelis et al. (Reference Avgelis, Roditakis, Dovas, Katis, Vassilakos and Bem2001). Although the appearance of the B biotype of B. tabaci has often been correlated with the emergence of whitefly-transmitted geminiviruses (Moriones & Navas-Castillo, Reference Moriones and Navas-Castillo2000; Brown, Reference Brown2000 and references therein), it was not known if this was the case in Greece. Had the sudden outbreak of TYLCV been associated with an introduction of the B biotype or were indigenous populations responsible?
Although the present study has identified all of the collected Greek whiteflies as Q biotypes it cannot exclude the presence of B or other biotypes within Greece. However, considering the number of whiteflies studied here (608 insects from 25 localities) we would have expected to have found evidence of other biotypes, even at low numbers, if they are present. We found that the 14 populations from Crete collected in 2002 (two years after the outbreak of TYLCV) were all Q biotypes. Together with confirmation of non-B biotype samples from Crete in 1992 and 1993 (Kirk et al., Reference Kirk, Lacey, Roditakis and Brown1993) it is possible that at least in Crete, the B biotype may never have been introduced. Either that or the selection pressures including agricultural practices within Crete rapidly suppressed the spread and establishment of any introduced B biotypes. When multiple biotypes are found together, their distributions are known to be dynamic, for example, the B biotype appears to have been supplanted by the Q biotype in southern Spain (Moya et al., Reference Moya, Guirao, Cifuentes, Beitia and Cenis2001) and Israel (Khasdan et al., Reference Khasdan, Levin, Rosner, Morin, Kontsedalov, Maslenin and Horowitz2005). Horowitz et al. (Reference Horowitz, Kontsedalov, Khasdan and Ishaaya2005) and Khasdan et al. (Reference Khasdan, Levin, Rosner, Morin, Kontsedalov, Maslenin and Horowitz2005) have suggested that B biotypes may possess a survival advantage over Q biotypes under untreated conditions, and that the predominance of Q biotypes in areas of intense insecticide use may be favoured by the differential development of resistance against contemporary products such as neonicotinoids and pyriproxyfen. Compared to B biotypes, the more rapid development of resistance in Q biotype populations is in accordance with their higher levels of polymorphism demonstrated by RAPD patterns (Moya et al., Reference Moya, Guirao, Cifuentes, Beitia and Cenis2001) and microsatellite markers (A. Tsagkarakou, unpublished data). As no information is available on the biotype status in Greece before the first application of such insecticides, it is not known if the development of resistances to several insecticide classes has affected B. tabaci biotype distributions. However, in addition to the populations GR-MAL and GR-IERE from Crete that are resistant to pyrethroids and neonicotinoids, the insecticide-susceptible GR-EPI from Crete (Roditakis et al., Reference Roditakis, Roditakis and Tsagkarakou2005) was also identified as Q biotype.
The diagnostic tests developed and used in this study should facilitate a periodical screening of the biotype status within Greece, and help the detection of possible associations between biotype identity and insecticide resistance. In addition, recording temporal and spatial biotype distributions may further our understanding of the epidemiology of viruses vectored by B. tabaci. This might for example, help to explain the recent appearance of the Sardinia species of TYLCV in Greece from samples collected from Peloponnese and Crete (A.D. Avgelis & N.I. Katis, personal communication).
In contrast to the relative homogeneity between the mtCOI sequences of the Greek B. tabaci samples, a high genetic differentiation was detected between populations belonging to the Q biotype using the microsatellite markers. This was even found at a regional geographic level on the island of Crete. In a genetic survey of B. tabaci populations from a broad geographic distribution within the Asia-Pacific region, De Barro (Reference De Barro2005) found a strong geographic structure with a lack of gene flow between populations that could not be explained by physical barriers alone. Although only six samples were used in the exploratory work presented here, gene flow was shown to be low between populations of the same biotype that are separated by just a few kilometres (e.g. populations GR-HER2 and GR-ARH). Furthermore, the detection of the uppermost hierarchical level of structure using a Bayesian approach disclosed that the individuals included in this study clustered into at least two groups based on their genotypes. The way in which the geographic distance, the type of the habitat and the host plant species may affect the genetic structure of the Q biotype B. tabaci is currently under investigation using samples from different localities of Greece. The results presented here demonstrate the ability of microsatellite markers as valuable tool for studying the genetic structure of B. tabaci populations and in doing so, for disclosing important information on the dynamics of insecticide resistance and the epidemiology of the associated viruses.
Acknowledgements
The authors wish to thank Cila Antoniou and Panagiotis Kasapidis for helpful comments on the phylogenetic and population structure analyses, N. Roditakis and M. Nomikou for kindly providing samples GR-MAL, GR-EPI, GR-THE2 and GR-KAR, as well as colleagues who assisted in collecting samples. This work was funded in part by the prefecture of Lassithi, Crete and by a National Agricultural Research Foundation–British Council partnership in Natural Resources.


