Introduction
Assisted reproductive technologies have provided tools for the study of early embryonic development and for their manipulation in ruminants. A few years ago, in mammals, only mouse embryos were suitable for such manipulations. Nowadays, bovine in vitro embryo production, somatic cell nuclear transfer, embryo sexing and preimplantation diagnosis among other technologies, are readily available for both basic and applied research.
Bovine embryos move to the uterus during the first 3 days after fertilization and become expanded blastocysts around days 7 to 8. In cattle, embryo implantation begins around day 21 post fertilization (Guillomot, Reference Guillomot1995). Bovine blastocysts remain inside the zona pellucida up to day 9 after fertilization growing until approximately 200 μm with a notable expansion of the blastocoele and cell numbers can increase up to 160 cells (Morris et al., Reference Morris, Diskin and Sreenan2000). After hatching from the zona, at day 8–9, embryos expand further and change from spherical shape to a filamentous shape that reaches 150 mm by day 17 when maternal recognition of pregnancy occurs (Betteridge & Flechon Reference Betteridge and Flechon1988; Hue et al., Reference Hue, Renard and Viebahn2001; Maddox-Hyttel et al., Reference Maddox-Hyttel, Alexopoulos, Vajta, Lewis, Rogers, Cann, Callesen, Tveden-Nyborg and Trounson2003). Following this process, bovine embryos expand further to reach a length of around 300 mm by day 21 when implantation begins (Chang, Reference Chang1952; Greenstein et al., Reference Greenstein, Murray and Foley1958; Betteridge & Flechon, Reference Betteridge and Flechon1988; Guillomot, Reference Guillomot1995, Hue et al., Reference Hue, Renard and Viebahn2001; Maddox-Hyttel et al., Reference Maddox-Hyttel, Alexopoulos, Vajta, Lewis, Rogers, Cann, Callesen, Tveden-Nyborg and Trounson2003). Three steps of elongation are usually recognized in bovine: early or spherical, ovoid and filamentous or late (Degrelle et al., Reference Degrelle, Campion, Cabau, Piumi, Reinaud, Richard, Renard and Hue2005).
Elongated blastocysts are very attractive raw material to understand basic embryo and placental development, stem cell fate and embryo–uterine interactions and cross talk. In somatic cell nuclear transfer, there is an aberrant pattern of expression of genes leading to multiple failures in early pregnancy (Daniels et al., Reference Daniels, Hall and Trounson2000, Reference Daniels, Hall, French, Korfiatis and Trounson2001; Hall et al., Reference Hall, Ruddock and French2005; Degrelle et al., Reference Degrelle, Campion, Cabau, Piumi, Reinaud, Richard, Renard and Hue2005). A useful approach to deal with this problem could be to understand the differential gene expression and regulation during the preimplantation stage of embryo development.
The availability of elongated embryos produced by nuclear transfer in cattle is a limiting step towards the understanding of gene expression underlying trophoblast and implantation physiology. The basic flow chart for obtaining elongated bovine embryos would be to transfer day-7 blastocysts into recipient cows and to slaughter them at the desired time point; alternatively elongated embryos can be flushed out non-surgically (Sawai et al., Reference Sawai, Kageyama, Moriyasu, Hirayama, Minamihashi and Onoe2007). The first procedure is expensive while the recovery of embryos by uterine flushing has reduced efficiency.
In order to increase the supply of elongated embryos, as well as to find out a cheaper way to produce elongated cloned embryos, it may be possible to utilize sheep and goats as temporary recipients. To our knowledge, very little has been done in this subject. Rexroad & Pursell (Reference Rexroad and Powell1999) reported the use of sheep ligated oviducts as temporary recipient for the production of elongated bovine embryos. Talbot et al. (Reference Talbot, Powell, Garrett, Edwards and Rexroad2000) transferred cloned bovine day-7 embryos into the uteri of synchronised ewes and found after 7 to 9 days of in vivo development that bovine blastocysts in the sheep uterus resulted in morphologically and karyotypically normal elongation stage bovine blastocysts, suggesting for the first time that in vivo incubation in sheep uterus may be useful for assessment of NT bovine blastocyst developmental competence. To our knowledge no such work has been done using goats as temporary recipients neither to culture cloned embryos in these species.
Goats have an estrus cycle length similar to cattle cycle and longer to that of ewes (Cox & Alfaro, Reference Cox and Alfaro2007) and the apposition of the elongated conceptus to the uterine endometrium starts at day 18 after fertilization, similar to cattle (days 19–21; Guillomot, Reference Guillomot1995), although later than in sheep (day 15; Guillomot, Reference Guillomot1995). For all these reasons, we decided to use both sheep and goats as recipients of bovine cloned embryos. We evaluated the development to the elongated status, their morphology and length, presence of embryonic disc as well as the expression of several important genes for the embryonic development. Homologous (bovine embryos transferred to cattle) was also included. The main aim of our study was to find a relatively simple and cheaper alternative to conventional systems for the production of elongated cloned embryos, for gene and protein expression studies. From this point of view, the incubation of expanded blastocysts in goats and sheep uteri allowed for the production of elongated cloned embryos that grow at lower rate and have similar, but not identical pattern of gene expression when compared with embryos obtained from homologous uteri.
Materials and Methods
Nuclear transfer
Somatic cell nuclear transfer embryos were produced by the hand-made cloning method (Peura & Vajta, Reference Peura and Vajta2003) with minor modifications made in our laboratory (Rodríguez et al., Reference Rodríguez, Navarrete, Tovar, Cox and Castro2008).
Preparation of recipient cytoplasts
Immature intrafollicular oocytes were aspirated from bovine ovaries and matured in vitro for 21 h in M199 supplemented with FSH (0.01 U/ml), LH (0.01 U/ml), 17β-estradiol (1 μg/ml) and EGF (Heber Biotec 10 ng/ml) at 39 °C in a 5% CO2 in air atmosphere. At this time, oocytes were treated with demecolcine (0.4 μg/ml) to induce protrusion of the meiotic cones and were released from the zona pellucida by pronase digestion. Enucleation was achieved by bisecting off the meiotic cones at 23 h after beginning of maturation. All reagents except otherwise stated were purchased from Sigma Chemical Company.
Nuclear transfer, reprogramming and activation
Donor fibroblast cell line was isolated from the ear of adult Japanese Black Cattle (Wagyu). A single cell was placed over each enucleated half oocyte and attached to it by brief incubation with phytohaemagglutinin (1 mg/ml). The cytoplasmic volume of a normal oocyte was reconstituted by adding a second enucleated half oocyte; the triplet was fused together by means of a single DC current pulse of 1 kV/cm. The reconstructed embryo was reprogrammed for 2 h, activation was induced by 7% ethanol (5 min) followed by 5 h incubation in cycloheximide (10 μg/ml) and cytochalasin B (5 μg/μl).
Embryo culture, transfer and collection at elongated stage
Embryo culture
Cloned embryos were cultured individually in SOFaaci (SOF supplemented with 0.4 mM sodium pyruvate, 0.2 mM l-glutamine, 1× final concentration of each essential and non-essential amino acids, 0.5 mg/ml myo-inositol, 0.1 mg/ml citric acid, 2% fetal calf serum, 0.3% essential fatty acid-free BSA, 10 ng/ml recombinant hEGF and 0.05 mg/ml gentamycin) in an atmosphere of 5.5% O2; 5% CO2 and 89.5% N2, 100% humidity at 39 °C, using the well-on-the-well system (Vajta et al., Reference Vajta, Peura, Holm, Paldi, Greve and Trounson2000a). At day 7, embryos were graded and transferred non-surgically into naturally cycling day-7 heifers (day 0 = day of standing oestrus) or surgically into synchronized sheep and goat on day 7 after induced oestrus.
Animals
Creole and hybrids (Saanen × Creole) goats and Suffolk and Merino sheep maintained under the surveillance of a health and nutritional programme were used in the study. During the day, animals were maintained in paddocks and by night they were housed on dry bed considering individual requirement for spacing, ventilation and free access to fresh water. The females were sexually mature, 2 to 5 years old and clinically sound. Nulliparous heifers of Overo Colorado breed, with an active and recorded oestrus activity were selected for standing estrus by visual inspection and were used as recipients.
Preparation of recipients
Experiments were conducted during the last two-thirds of the winter period (July, August) in the Southern hemisphere (latitude 36.60 South), which coincides with the breeding season for ewes and goats. Ewes and goats were synchronized by the hygienic insertion of progesterone-releasing devises (CIDR-G®, Pfizer) for 7 days followed by the administration of PGF2α analogue (0.1 mg i.m. of cloprostenol, Estrumate®, Drag Pharma Invetec) and eCG (300 IU i.m. Folligon®, Intervet) 24 h before release of the devise. The estrous detection was carried out by the observation of estrous behavior of the flock, for periods of 30 min each 12 h. A female was considered to be in estrus when she stood to be mounted by teasers.
Embryos
Cloned embryos were generated in four separate replicates using adult ear fibroblasts at passage 7. Day-7 expanded grade I blastocysts were bulk transferred to recipient animals. All the blastocysts produced in replicate 1, were transferred to three goats; those of replicate 2 were transferred to three ewes. Blastocysts produced in replicates 3 and 4 were transferred to three and three recipient heifers respectively. A summary of these and other relevant data about the source and replicates of experimental groups of transferred cloned embryos is presented in Table 1.
Table 1 Summary of relevant data about the source of the embryos and experimental replicates.

Embryo transfers
Embryo transfer was done 7 days after the beginning of estrous. Recipients were fastened by 24 h and they were anaesthetized by a combination of ketamine (5 mg/kg; Ketostop®, Drag Pharma Invetec) and xylazine (0.02 mg/kg; Xylavet®, Agroland). The uterus was exteriorized through a midline incision, about 2–3 cm using an Allis forceps guided by laparoscopic observation (Cox & Alfaro, Reference Cox and Alfaro2007). Embryos were carefully suspended in a small volume of SOF medium and were released in the uterine horn adjacent to the corpus luteum, by the introduction of a tomcat catheter through a puncture made by a blunt needle. A new CIDR-G was inserted to each recipient for exogenous progesterone supplementation and maintained for another 10 days until embryo collection. Heifers were transferred non-surgically according to conventional procedures published elsewhere (de Armas et al., Reference de Armas, Solano, Riego, Pupo, Aguilar, Ramos, Aguirre, de la Fuente and Castro1994).
Collection of elongated embryos
At day 10 after transfer, recipients (goats and ewes) were submitted to midventral laparotomy to expose the uteri, under general anesthesia. A small pulled plastic tip coupled to a 60-ml syringe was introduced to the tip of one uterine horn and Dulbecco's medium was flushed through and collected at the other open extreme (fimbrial end) into a 50 ml conical tube (Falcon, BD Labware).
Cattle
Ten days after embryo transfer, heifers were slaughtered in a local abattoir, their uteri collected, placed into individual zip lock bags with warm PBS and transported to the laboratory. Uteri were flushed essentially as described for goats and ewes, except that forceps were put at the cervical channel to avoid vaginal drainage of medium. General procedures were based on the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes and approved by the Faculty of Veterinary Medicine Committee in Care and Use of Research Animals.
Processing of the embryos
After decantation, supernatant was disposed and embryos were searched under stereomicroscope at ×10 magnification. All elongated embryos were washed several times in warm PBS to eliminate blood and/or remaining tissue. Embryos were measured under a stereomicroscope, with a graduated eyepiece, scored for the presence of embryonic disc (ED), pooled or not and placed in 1000 μl of RNALater (Ambion Inc.) and left overnight at 4 °C, in an 2-ml Eppendorf tube and placed at −80 °C until use. For details on the composition of the pools, please refer to Table 2. Morphologically not ovoid embryos recovered from ewes and goats, with a length of at least 46.0 mm (according to Tveden-Nybert et al., Reference Tveden-Nyborg, Peura, Hartwich, Walker and Maddox-Hyttel2005) were classified as late elongated, shorter embryos were scored as early elongating.
Table 2 Summary of gene expression in elongated embryos and in somatic cells used for cloning.
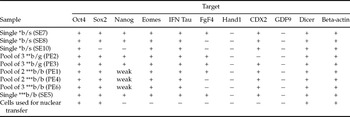
All embryos were day 17 and were cloned from the same cell line and passage.
SE, single embryo; PE, pool of embryos; numbers following SE or PE are for reference and do not indicate number of embryos.
*b/s, bovine embryo in sheep uterus; ** b/g, bovine embryo in goat uterus; *** b/b, bovine embryo in bovine uterus.
Gene expression analysis
The elongated embryos were thawed on ice inside the RNALater solution and 1 ml of sterile ice-cold PBS was added to each tube and centrifuged at 8000 g on a cold centrifuge. The supernatant was discarded and the elongated embryos were transferred with sterile forceps to a new tube and subjected to lysis and to total RNA extraction using miRVAna kit (Ambion Inc.) according to manufacturer's instructions. Total RNA was quantified using NanoDrop ND 1000 (Wilmington, DE, USA) and the quality checked with 2100 Bioanalyser microchips (Agilent).
cDNA synthesis and RT-PCR
One μg of total RNA from elongated embryos was used for first strand cDNA synthesis in a 20 μl reaction, using M-MuLV (Fermentas). Previous to RT, samples were treated with 1 unit (in 10 μl reaction) of RNase-free DNase (Fermentas) for 30 min in order to eliminate DNA contamination from the extraction. For RT, we used the complete reaction of the DNase treatment, plus 30 ng/μl of random primers, 10mM each dNTP, DEPC water, 4 μl first-strand 5× buffer and 200 U/ml of the RT enzyme. The settings for the RT reaction were: 25 °C for 10 min, 42 °C for 1 h and 10 min at 70 °C. For each sample a complete reaction but without the reverse transcriptase (RT–) was performed.
For PCR, 1 μl of the RT reaction was used in a block qualitative PCR assay. Conditions for PCR were: 1× final concentration of enzyme buffer, 10 mM each dNTP, 10 pmol of each primer (forward and reverse) and 0.5 U of HotStart Immolase DNA polymerase (Bioline, Lukenwalde, Germany) in 25 μl of reaction. For primer sequencing and specific PCR conditions for each target gene, please refer to Table 3.
Table 3 Oligonucleotides and PCR conditions used in the experiments for gene expression in elongated embryos.

q-PCR
Selected genes were also analysed by quantitative real-time PCR. All the primers used in q-PCR runs were validated earlier in separate experiments. Standard curves were generated using serial 10-fold dilutions of purified and sequenced products of the expected size obtained by conventional block PCRs. Ten dilutions were made for each gene, starting quantities were 18.5 pg for IFN-tau; 16.6 pg for FGF4 and 8.5 pg for Nanog. The correlation coefficients were 0.967, 0.998 and 1.0 respectively. The dilutions and the samples were ran in triplicates in a 10 μl reaction consisting of 5 μl 2× SensiMix dT (Quantace, Berlin, Germany), 0.2 μl of 50× SYBRGreen (Quantace) on a StepOnePlus™ Real-Time PCR (Applied Biosystems). A melting curve experiment was carried out on each run, the CP was calculated with built-in software and all the runs were normalized against the relative content of beta-actin as reference. Relative quantity of a given target was calculated by extrapolating values with the respective standard curve after normalization with an internal calibrator (beta-actin) and was expressed in N-fold difference.
Results
We produced 67 grade I cloned expanded blastocysts (day 7) in four separate experiments. The overall rate of blastocysts formation was 64.5%. A total of 58 blastocysts were bulk transferred to recipient females. The details of the embryo transfers are provided in Table 4.
Table 4 Development of cloned bovine blastocysts transferred to heterologous (sheep and goats) or to homologous (cattle) at day 7 during winter and recovered at day 17.

Heterologous transfer of bovine cloned embryos to ewes
Bovine zona-free cloned blastocysts were able to continue their growth after transfer into sheep uteri. We collected 10 out of 11 (90.9%) blastocysts from the three recipient sheep. All recovered embryos had undergone extensive morphological changes. Half of the embryos (n = 5) developed into late or filamentous stage, while the other half reached the early phase of elongation although at the final steps of it as judged by their length and morphology. The average length of the elongated embryos was 54.66 mm ± 28.04 and four out of 10 (40%) showed a clearly visible embryonic disc (Table 4, Fig. 1a).

Figure 1 Stereomicroscopic photography of cloned bovine elongated embryos recovered at day 17 of development from sheep uterus (a); goat uterus (b, c); or cattle uterus (d). Magnification ×10 for (a), (c), (d) and ×40 for (b). Actual sizes for these embryos were: 83 mm (a), 34 mm (b), 39 mm (c) and 156 mm (d).
Heterologous transfer of bovine cloned embryos to goats
Seventeen cloned expanded bovine blastocysts were transferred to three goats, two of which yielded 11 elongated blastocysts (64.7%), their mean length was similar to that of sheep; 48.4 mm ± 11.67, embryonic disc was present in 7/11 (63.6%). Six out of the 11 recovered embryos were in the late phase of elongation. Clear embryonic discs were present, indicating morphological and functional changes of the blastocysts. (Fig. 1b, c).
Homologous transfer to recipient cattle
In order to have a homologous control of blastocyst development, we collect 19 out of 30 (63.3%) elongated embryos from three heifers, the other half did not yield elongated embryos. In two independent experiments we transferred 30 day-7 cloned blastocysts to six recipient heifers. Eleven of the 19 recovered blastocysts were scored as late phase elongated (approximated length between 71 and 180 mm) and eight as early (less elongated; length between 20 to 70 mm). The average length of embryos was 91. 8 mm ± 45.8 (range from 25 to 183 mm). The embryonic disc was visually found in 12 out of 19 (63.2%) of the recovered elongated embryos. When grouped into categories according to their phases of development (early or late) their lengths in mm were: 46.2 ± 14.8 and 125 ± 26.3 and the presence of embryonic disc was seen in nine out of 11 (81.8%) and three out of eight (37.5%) embryos respectively. A representative picture for late elongated embryo is shown in Fig. 1d.
Analysis of expression of genes involved in embryonic development in all the species
To assess if bovine cloned embryos that developed in heterologous environment were able to express early embryonic genes, we used a qualitative PCR approach linked to reverse transcription of extracted total RNA. A set of 10 genes was studied. The embryos recovered were pooled or treated individually as shown in Table 2. In total three, six and eight embryos were analysed from goats, ewes and cattle respectively (Table 2). The selection of target genes was based on their presence in early embryonic development in cattle. Oct-4, Nanog and Sox2 are totipotency markers of the embryo proper derived lineages and FGF-4 is essential for trophoblast growth, Eomes, Cdx2, IFN-tau are markers of trophoblastic development, GDF9 is a negative control as it is expressed only in oocytes, while Dicer is an enzyme involved in miRNA processing and has not been previously studied in bovine embryos. β-actin is a housekeeping gene.
Oct4, Eomes, IFN-tau and Cdx2 were expressed in all the samples assayed. Dicer was also expressed in all embryos tested, what constitutes a new finding. We were interested in Dicer expression as we have profiled the pattern of miRNA expression in bovine cloned and IVP embryos (unpublished) and hypothesized that Dicer expression could be altered in cloned embryos, from our data it does not seems to be thecase.
In Fig. 2, we summarized the results of qualitative RT-PCR gene expression in the elongated embryos that developed in sheep and goats and their comparison with bovine embryos in the homologous environment. A summary of oligonucleotides, melting temperatures and cycling conditions for each target gene is provided in Table 3. The results of gene expression in embryos as single or in pools are shown in Table 2.

Figure 2 Agarose gel electrophoresis (2%) and ethidium bromide staining of PCR products for the genes included in the study. MW = molecular weight markers, the more intense bands in the MW corresponding to 250 and 500 base pairs are indicated on the left side. SE means single embryo, PE means pool of embryos. For details about the composition of the pools, please refer to Table 3. RT– stands for representative reactions for each gene without addition of the enzyme reverse transcriptase. Target genes are listed on the right side of the figure.
Most of the genes studied showed, qualitatively, a similar pattern of expression in all embryos despite of incubating them in a heterologous (caprine or ovine uteri) or homologous (bovine uteri) environments. We did not quantified the expression levels of Sox2, however as judged by band intensity and after comparison with β-actin expression, it seems like that Sox2 gene is only barely detected in all the embryos that elongated in the ovine uteri (Fig. 2). FGF-4, Nanog and IFN-tau showed a clear differential expression among embryos. As judged by qualitative RT-PCR, in cows, the FGF-4 gene was expressed only in one single embryo (SE5, Fig. 2) and in one pool of embryos (PE1), but not in pools PE4 and PE6. Likewise in sheep, FGF-4 expression was detected only in a single embryo (Fig. 2; SE8), but not in the other two embryos. Both pools of embryos derived from goats (PE2 and PE3) did express FGF-4.
Nanog was weakly expressed in all but one sample assayed in bovine (PE6) and in two out of the three embryos recovered from sheep. Both pools of embryos derived from goats (PE2 and PE3) did express FGF-4. IFN-tau was clearly more expressed in embryos obtained from bovine uteri than from both sheep and goats, although all the assayed samples expressed this gene. All embryos expressed β-actin and Dicer in similar amounts. As expected, GDF9 was not expressed in any of the embryos analysed. A RT– control was included in each PCR and no band was obtained, neither in the template minus reaction (not shown).
In order to quantify the actual levels of expression of some genes, we conducted a q-PCR study for FGF-4, Nanog, IFN-tau and β-actin for internal normalization. The figures of the real-time PCRs matched exactly with the findings of the block PCRs (data not shown). We further decided to analyse the results of the q-PCR by pooling the data according to the species in which the embryos were incubated rather than on individual basis, in order to avoid the bias of information of pooled versus individual embryos (Fig. 3). FGF-4, Nanog and IFN-tau were differentially expressed among samples. IFN-tau was clearly expressed at higher levels in embryos collected from cattle (Fig. 3a), while Nanog and FGF-4 were more expressed in goats (Fig. 3b, c). Nanog was only very weakly expressed in bovine embryos transferred to bovine uteri, or was not expressed at all; however it was expressed in all embryos recovered from goats and in two out of three individual embryos coming from ewes. Notably, the same embryo from goats also did not express Nanog, Sox2 and very weakly FGF-4. FGF-4 also showed a variable pattern of expression. It was detected by RT-PCR in 33%, 100% and 50% of the elongated embryos recovered from sheep, goats and cattle respectively.

Figure 3 Quantification of PCR amplification of selected genes expressed in elongated embryos. Each bar represents the average of quantification of at least three biological replicates of the same target gene pooled according to the species in which they were incubated. (a) IFN-tau, (b) Nanog and (c) FGF-4. Values on Y axis are expressed in N-fold differences after normalization with beta-actin.
Discussion
Elongation of bovine embryos in heterologous and homologous temporary recipients
First attempts to culture bovine embryos in heterologous recipients were made more than 20 years ago. Sirard et al. (Reference Sirard, Lambert, Ménard and Bedoya1985) showed that recently fertilized cow oocytes can be cultured in rabbit oviduct, recovered as blastocysts, transferred to cattle and resulted in pregnancies. Eyestone et al. (Reference Eyestone, Leibfried-Rutledge, Northey, Gilligan and First1987) and Bondioli et al. (Reference Bondioli, Beiry, Hill, Jones, De Mayo, First and Haseltine1991) used ligated sheep oviduct for the culture of 1–2-cell bovine embryos to the blastocyst stage, similarly, Ellington et al. (Reference Ellington, Farrell, Simkin, Foote, Goldman and McGrath1990) used ligated rabbit oviduct for transfer of cattle morulae, in all cases, transferable grade I expanded blastocysts were recovered after the appropriate experimental time points. However, in the two former cases, embryos were embedded in agar and in the later, oviducts were ligated and both approaches were therefore technically challenging and demanding. Other methods include the use of complete (isolated) mouse oviducts as hosts for bovine embryos. The recovered blastocysts were used to measure gene expression of the bovine embryos (Rizos et al., Reference Rizos, Pintado, de la Fuente, Lonergan and Gutiérrez-Adán2007). With the advancement of in vitro culture systems and the definition of nutrients required for adequate embryonic development to the blastocysts stage, the transfer of bovine embryos to temporary recipients was discarded (Bavister, Reference Bavister2002). Moreover the experiments cited above were aimed to produce expanded blastocysts. The goal of our work was to produce elongated blastocysts for gene expression analysis.
Few data however are available about heterologous transfer of bovine blastocysts to temporary recipients in order to produce elongated embryos. Rexroad & Powell (Reference Rexroad and Powell1999) successfully recovered elongated bovine embryos transferred to sheep uteri. Their recovery rate was 78% for elongated blastocysts and 68% of the recovered embryos showed embryonic disc. In addition the average length of day-16 blastocysts in sheep was similar to that of cattle on day 14. Our data are very similar to those of Rexroad & Powell for both recovery and percentage of embryonic disc. The length of the bovine embryos produced in our sheep system were however higher than theirs (55.0 vs 1.0 mm). In spite of not being directly comparable, as they recovered the embryos at day 16, the difference in length is very significant. In a previous experiment, we recovered five out of six (83.3%) bovine cloned embryos at day 14 of development (day 7 after transfer) from sheep uteri. The mean length of the recovered ovoid embryos was 2.94 ± 0.7 mm (data not shown). Whether this can be related to a better developmental potential of our embryos is not clear, however it seems unlikely as Rexroad & Pursell (Reference Rexroad and Powell1999) used in vivo and in vitro produced embryos, while we used cloned embryos. Our data also compare favourably with those of George et al., Reference George, Daniaux, Genicot, Verhaeghe, Lambert and Donnay2008 who reported a 48.5% of recovery of bovine blastocyst transferred to temporary ovine recipients and a mean length of 3.2 mm for fresh embryos and 1.7 mm for frozen embryos. Although authors flushed back the embryos at day 13 and we did at day 17, the recovery rate was higher in our hands and the length of our day-14 embryos collected from sheep (data not shown) were similar and compared with both. In our experiments, cloned embryos obtained from bovine uterus almost double in size those from small ruminants.
Talbot et al. (Reference Talbot, Powell, Garrett, Edwards and Rexroad2000) transferred cloned bovine day-7 embryos into the uteri of synchronised ewes and found after 7 to 9 days of in vivo development that bovine blastocysts in the sheep uterus resulted in morphologically and karyotypically normal elongation stage bovine blastocysts. In this paper, authors suggested for the first time, that in vivo incubation in the sheep uterus may be useful for assessment of NT bovine blastocyst developmental competence. Our findings corroborate their prediction. Other groups had used sheep oviduct as intermediate recipients to study maternal–fetal interaction in bovine peri-implantation development (Hoffert et al., Reference Hoffert, Batchelder, Bertolini, Moyer, Famula, anderson and Anderson2005). To our knowledge no such study had been conducted using goats as intermediate recipients.
We showed in this paper that transfer of bovine blastocysts into the uteri of both sheep and goats resulted in elongation of most of the embryos and that the majority of them (55%; 11/20) developed an embryonic disk. The length of the elongated blastocysts that we obtained in goats was similar to that obtained in ewes and both were lower than the length of cattle embryos in cattle uterus. It is well known that embryo–maternal interaction influences embryo development in mammals and the possibility of the embryo to survive to term. There are also limitations in the capability of ruminant's uteri to carry pregnancies (Lopez-Gatius & Hunter, Reference López-Gatius and Hunter2005). Therefore it is tempting to suggest that the differences in the growth rate of cattle embryos seen between cattle and small ruminants are related to the capacity of the uterus of these species to support their development. However, it seems unlikely that the uterus of the small ruminants does not provide adequate nutrients for sustaining the growth of the embryos to a similar degree as their cattle counterpart, on the contrary, data from Eyestone et al. (Reference Eyestone, Leibfried-Rutledge, Northey, Gilligan and First1987), Rexroad & Powell (Reference Rexroad and Powell1999) and of Talbot et al. (Reference Talbot, Powell, Garrett, Edwards and Rexroad2000) showed that embryos indeed can proceed further in development in surrogate mothers.
We found in our experiments, that large embryos could be found in both sheep (four were larger than 7 cm) and goats (two embryos were in the edge of 7 cm), probably indicating that under certain conditions, embryo development could closely mirror the growth performance in homologous uteri. The differences observed in the length of the embryos recovered from cattle and sheep/goats are not surprising as elongation seems to vary significantly depending on the embryo quality. Ranges from 1.1 to 282 mm for in vivo and from 1.0 to 122 mm for in vitro produced embryos had been reported earlier (Vajta et al., Reference Vajta, Hyttel and Trounson2000b; Bertolini et al., Reference Bertolini, Beam, Shim, Bertolini, Moyer, Famula and Anderson2002).
As mentioned above, the generation of elongated bovine embryos in homologous uteri is an expensive, labour and time-consuming task. Nonetheless elongated bovine embryos had been produced both in vivo and in vitro. Conventional production of elongated embryos in vivo followed by non-surgical flushing or flushing from collected uteri produced similar results to ours (Degrelle et al., Reference Degrelle, Campion, Cabau, Piumi, Reinaud, Richard, Renard and Hue2005; Arnold et al., Reference Arnold, Bordignon, Lefebvre, Murphy and Smith2006; Sawai et al., Reference Sawai, Kageyama, Moriyasu, Hirayama, Minamihashi and Onoe2007; Rodríguez et al., Reference Rodríguez, Navarrete, Tovar, Cox and Castro2008). A second approach implies the in vitro culture of expanded blastocysts in agarose matrices (Vajta et al., Reference Vajta, Hyttel and Trounson2000b; Maddox-Hyttel, Reference Maddox-Hyttel, Alexopoulos, Vajta, Lewis, Rogers, Cann, Callesen, Tveden-Nyborg and Trounson2003; Brandao et al., Reference Brandao, Maddox-Hyttel, Løvendahl, Rumpf, Stringfellow and Callesen2004). In the later approach although marked elongation and initial steps of differentiation was achieved, the embryos were not able to elongate beyond day 15, most likely as the result of the suboptimal culture environment. It is becoming clear that early elongation relies on cell divisions, growth and remodelling (Hue et al., Reference Hue, Degrelle, Campion and Renard2007) which might not be provided by the in vitro systems utilized.
From the data showed here, it seems that embryonic development continues in the temporary heterologous uteri albeit at lower rate than in homologous recipient as judged by the smaller length of the elongated embryos obtained from sheep and goats.
Gene expression of developmentally important genes in the elongated embryos
Based on qualitative RT-PCR, we found an obvious enhancement of band intensity for IFN-tau expression in bovine embryos coming from bovine uterus. This finding was further verified using q-PCR and agrees with the accepted view that larger embryos produce a bigger amount of IFN-tau (Robinson et al., Reference Robinson, Fray, Wathes, Lamming and Mann2006). One possibility is that factors available for regulating transcription of this gene are more readily available in the homologous rather than in the heterologous environment. Robinson et al. (Reference Robinson, Fray, Wathes, Lamming and Mann2006) showed that the amount of IFN-tau protein is proportional to the length of the elongated blastocysts in cattle; however, they were not able to find differences in mRNA level for this gene. In apparent contradiction with these findings, Bertolini et al. (Reference Bertolini, Beam, Shim, Bertolini, Moyer, Famula and Anderson2002) reported a positive correlation between trophoblast length and IFN-tau mRNA levels on day 16. However findings of Bertolini and co-workers might be due to an increased cell number in the trophoblast rather than an actual increase in mRNA transcripts at the individual cell level. It has been shown previously that the levels of secretion of IFN-tau differ notably among blastocysts when studied individually (Kubisch et al. Reference Kubisch, Larson and Roberts1998).
Quantitative PCR for Nanog showed that its expression was weaker in embryos derived from cattle and stronger in those from ewes. The somatic cells from which the elongated embryos were generated also did not express Nanog, what implies that this gene was not always adequately reprogrammed to an embryonic pattern of expression during somatic cell nuclear transfer. This aberrant pattern of expression of Nanog gene is a new finding for elongated embryos. Interestingly, the pattern of Nanog expression in temporary recipients differed from that of cattle. We do not have an explanation for this and we reasoned that due to the limited sample number assayed, this event probably reflects a stochastic distribution of the event rather than a species-mediated phenomenon.
Variegated expression of FGF4 described here is in agreement with a recent report from Amarnath et al. (Reference Amarnath, Li, Kato and Tsunoda2007), who found FGF-4 to be expressed in 67% of IVF blastocysts and in 44% of nuclear transfer day-7 blastocysts. Others have reported expression of the FGF-4 gene during elongation in cattle embryos (Degrelle et al., Reference Degrelle, Campion, Cabau, Piumi, Reinaud, Richard, Renard and Hue2005) but no data were shown on individual variations, thus our data for expression of FGF-4 gene in elongated cloned embryos are new.
Other potential factor affecting the pattern of expression of the genes assayed could be the lack of zona pellucida during all the manipulations. The role of the zona pellucida in maintaining a given pattern of gene expression is unknown, recently it was reported that mouse embryos from which zona pellucida was removed shortly after fertilization showed a significant reduction in the DNA methylation level at the 2-cell and 4-cell stages, but no differences were found at pronuclear, morula and blastocyst stages, as judged by protein analysis (Ribas et al., Reference Ribas, Taylor, McCorquodale, Maurício, Sousa and Wilmut2006). Interestingly in that paper, no differences were found regarding the onset of transcription of Nanog and FGF-4 genes. Whether the removal of the zona for nuclear transfer affects the general pattern of expression of developmentally important genes is not known, although as judged from the comparison of our findings with earlier reports (for conventional cloning and elongated embryos as well as for normal in vivo development) it seems not to be the case at least for most of the genes studied here.
When the results of q-PCR gene expression in the elongated embryos were pooled together according to the species in which the original blastocysts were incubated, we observed a differential pattern among species. IFN-tau expression was higher in cattle, while FGF-4 and Nanog was higher in goats; sheep-derived embryos expressed all the three genes at intermediate levels between cattle and goats. The amount of samples tested precludes probably a statistically relevant analysis of these findings and even though we do not have a clear explanation for this, neither can we affirm that this is a species-specific pattern, it is noteworthy to mention and further research is needed to clarify the observed phenomenon.
Very few data concerning embryonic gene expression after hand-made cloning had been published (Hall et al., Reference Hall, Ruddock and French2005) particularly insufficient is the literature on elongated bovine cloned embryos generated by HMC. This situation makes difficult the comparison with similar studies and with conventional cloning. This study provides novel information regarding the expression pattern of Eomes, Sox2, IFN-tau, CDX2, GDF9 and Dicer in bovine cloned embryos created by hand-made cloning. Also, our data on the deregulation of Nanog expression are an interesting finding not previously reported for elongated bovine cloned embryos.
In summary, we established a model for producing elongated bovine cloned embryos in sheep and goats as temporary recipients. Elongated embryos are produced at acceptable rates in terms of numbers, growth rates and preliminary information on gene expression as to make the model repeatable and attractive. In addition, we described for the first time the patterns of expression of developmentally important genes in elongated embryos produced in heterologous (sheep and goat) and homologous (cattle) surrogate mothers. However, more information is required to assess the potential usefulness of the model for research on physiology of preimplantation embryos and embryo–maternal interactions. A more detailed study using microarray technology with subsequent validation via q-PCR is in progress in order to compare more accurately and at the massive screening level the preliminary findings reported here. As drawback, the average length of the elongated embryos recovered from sheep and goats’ uteri are smaller than those recovered from cattle, which can have an effect on the study of cell proliferation.
Acknowledgements
Authors are grateful to R. Einspanier (FUB Berlin for laboratory space and support), to O. Skewes and X. Letelier for providing animals (cows), to P. Bustamante for help and support and to Carnes Ñuble Chillán, Chile for providing ovaries as well as to Heribelt Tobar for technical assistance. This work was partially supported by grant FIA PIC-2005–1-P-097 from the Ministry of Agriculture of Chile and by a fellowship from the German Academic Exchange Agency (DAAD, for R.LL.)
Conflict of interest statement
Authors declare no conflict of interest whatsoever regarding this manuscript.









