Introduction
The proto-oncogene, c-Ski, has been identified as the cellular homologue of v-Ski, that was originally identified as the transforming gene of the avian Sloan-Kettering retroviruses, which transform chicken embryonic fibroblasts, leading to their morphological transformation and anchorage-independent growth (Colmenares & Stavnezer, Reference Colmenares and Stavnezer1989). Transgenic mice expressing the c-Ski gene under the control of a murine sarcoma virus long terminal repeat show large increases in their skeletal muscle mass (Sutrave et al., Reference Sutrave, Kelly and Hughes1990), while c-Ski-deficient mice have defects in their skeletal muscle development (Berk et al., Reference Berk, Desai, Heyman and Colmenares1997). In addition, the mice lacking c-Ski show perinatal lethality due to defects in neurulation and craniofacial patterning as well as skeletal muscle development, and excessive apoptosis has been detected in c-Ski-deficient mouse embryos (Berk et al., Reference Berk, Desai, Heyman and Colmenares1997; Shinagawa et al., Reference Shinagawa, Nomura, Colmenares, Ohira, Nakagawara and Ishii2001), supporting the idea that Ski can act as an anti-apoptotic factor (Soeta et al., Reference Soeta, Suzuki, Suzuki, Naito, Tachi and Tojo2001). It has been also suggested that c-Ski is involved in mediating proliferative effect of 17β-estradiol in uterine epithelial cells (Yamanouchi et al., Reference Yamanouchi, Soeta, Harada, Naito and Tojo1999). Thus, the c-Ski gene product (Ski) has been implicated as having dual roles in both regulating proliferation and differentiation of cells. However, little information is known about the pathways through which Ski exerts its actions.
Ski protein is a nuclear transcription factor that does not bind DNA directly (Nagase et al., Reference Nagase, Mizuguchi, Nomura, Ishizaki, Ueno and Ishii1990; Akiyoshi et al., Reference Akiyoshi, Inoue, Hanai, Kusanagi, Nemoto, Miyazono and Kawabata1999; Kawabata et al., Reference Kawabata, Imamura, Inoue, Hanai, Nishihara, Hanyu, Takase, Ishidou, Udagawa, Oeda, Goto, Yagi, Kato and Miyazono1999). Due to its unique binding properties with multiple factors, Ski could posses various roles in the regulation of both cellular proliferation and differentiation (Nicol & Stavnezer, Reference Nicol and Stavnezer1998). Previous studies have identified c-Ski expression among the tissues (Lyons et al., Reference Lyons, Micales, Herr, Horrigan, Namciu, Shardy and Stavnezer1994), including in the ovary. However, the role of this gene in the ovary remains unknown.
The rat has an incomplete 4–5 day estrous cycle, and their ovaries contain follicles at various stages of development (growing follicles, preovulatory follicles, and atretic follicles) during the estrous cycle. Thus, rat ovaries may be useful to predict the role of Ski protein by immunohistochemical analyses. In addition, several experimental models that represent specific stages of follicular development (Dhanasekaran & Moudgal, Reference Dhanasekaran and Moudgal1989; Shi & Massagué Reference Shi and Massagu2003), and follicular atresia (Boone et al., Reference Boone, Carnegie, Rippstein and Tsang1997) have been well established in the rat. The aim of the present study was to locate the Ski protein expression in ovaries having a single generation of developing follicles in order to find insights into the possible involvement of Ski in follicular development.
Materials and methods
Animals
The adult male and female (8- to 14-week-old) and the immature female (25-day-old) Wistar-Imamichi rats were purchased from the Imamichi Institute of Animal Reproduction (Ibaraki, Japan). Rats were housed under controlled light condition (12 h light: light on 07:00–19:00), and food and water were given ad libitum. All animals received humane care according to the guide for the Care and Use of Animals of Animal Genetic Resources Research Center, National Institute Of Animal Science, RDA (ethics committee approval no. 2013–043).
Adult animals
Estrous cycles were monitored every day by observation of vaginal smears, and only those animals showing consecutive regular 4-day estrous cycles were used. Ovarian follicles were obtained from 8- to 14-week-old rats at estrus (n = 10–12 animals per time point). Rats were euthanased by decapitation and testis, fallopian tube, uterine wall, uterine cervix and ovary were collected and quickly frozen in liquid nitrogen for RT-PCR or embedded in optimal cutting compound (OCT) compound (Sakura Finetechnical, Tokyo, Japan) for immunohistochemical detection of Ski, proliferating cell nuclear antigen (PCNA) and for TdT-mediated dUTP biotin nick end labelling (TUNEL) respectively. The obtained samples were stored at –80°C until use.
Immature animals
Immature female Wistar-Imamichi hypophysectomized (Hypox) (HPX) rats (at the age of 25 days) were purchased and housed as described above. As shown in Fig. 1, HPX rats were treated subcutaneously with equine chorionic gonadotropin (eCG; 40 IU/rat in 200 μl saline) at the age of 26 days are known to exhibit ovarian follicular development of a single generation of follicles and subsequent follicular atresia due to lack of hypophyseal LH surge (Boone et al., Reference Boone, Carnegie, Rippstein and Tsang1997). Rats were euthanased by cervical dislocation at 0, 1, 2, 3 or 4 days after eCG treatment, and ovaries were excised. The rats were euthanized by cervical dislocation at 0, 1, 2, 3 or 4 days (n = 10–12 animals per time point) after eCG treatment, and ovaries were excised.
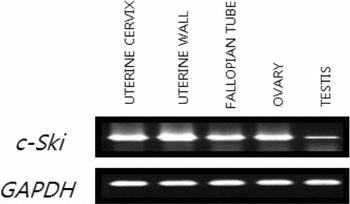
Figure 1 RT-PCR analysis on the expression of c-Ski mRNA in the ovarian follicles of adult female rats on the day of estrous. RT-PCR analysis of c-Ski (upper) and GAPDH (lower) genes in various tissues from adult male and female rats.
After removal of connective tissues, ovaries were weighed, embedded in OCT compound for immunohistochemical detection of Ski, PCNA and for TUNEL, respectively. The obtained samples were stored at –80°C until use.
qPCR (real time PCR)
Granulosa cells were obtained at 0, 1, 2, 3, or 4 days after eCG injection by puncturing the large preovulatory follicles with a 27-gauge needle. The cells were washed with phosphate buffered-saline (PBS) and immediately used for RNA isolation.
Total RNA was isolated using Trizol reagent (Invitrogen), and cDNA was synthesized by SuperScript II (Invitrogen) (200 U/ml) with oligo-dT 16 primer. qPCR was done using with LightCycler (Roche Diagnostics GmbH, Mannheim, Germany) and LightCycler FastStart Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) according to the manufacturer’ instructions. The primer sequences used were as follows: rat c-Ski (forward primer; 5′- CAGCAGA- TCAACTCGGTGTG-3′, reverse primer; 5′-AGGAT- GCCCATGACTTTGAG-3′), rat Arkadia (forward primer; 5′- CGTGAGGAGAACTGCATCAA-3′, reverse primer; 5′-GGATGTGCTAATGCATGCTG-3′), rat Smurf1 (forward primer; 5′-CAGTGAAGGATCT- GCATCTG-3′, reverse primer; 5′-CGATTAGCAA- TGGATTGCAG-3′), rat Smurf2 (forward primer; 5′-CGTGAGGAGAACTGCATCTG-3′, reverse primer; 5′-GGATGTGCTAATGCATGCTG-3′) and rat HPRT (forward primer; 5′-GACCGGTTCTGTCATGTCG-3′, reverse primer; 5′-ACCTGGTTCATCATCACTAATC- AC-3′). Rat-specific primers were designed using PRIMER3 software (available at http://fokker.wi.mit.edu/primer3/), and the specificity for each primer set was confirmed by both electrophoresis of the PCR products and analyzing the melting (dissociation) curve after each qPCR. Twenty microlitres of the reaction solution consisted of 2 μl of the template (appropriate dilution was determined by gene), 10 μl of LightCycler FastStart Thunderbird SYBR qPCR Mix, 1 μl of 10 μm of each primer and 3 μl of diethylpyrocarbonate-treated water. PCR amplification was performed as follows: pre-denature for one cycle at 95°C for 15 min and 45 cycles at 95°C for 15 s, 59°C for 20 s and 72°C for 30 s. Melting curve analysis was performed at 65–95°C with 0.1°C/s temperature transition.
Immunohistochemical analysis of Ski and PCNA
Frozen tissue sections (5 μm thick) were prepared from the OCT-embedded rat ovary tissues, mounted on glass slides, air-dried and fixed in 4% paraformaldehyde (PFA) in PBS for 20 min, followed by incubation in 0.1% Triton X-100 in PBS for 15 min. After several washes with PBS, endogenous peroxidase activity was inactivated by incubation in 0.3% hydrogen peroxide in Methanol for 30 min. Then the sections were immersed in blocking solution (8% skimmed milk in PBS) for 30 min. Then, the primary rabbit antibody specific for Ski [Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:100 with 5% normal goat serum (NGS) in PBS] or mouse monoclonal antibody specific for PCNA (Santa Cruz Biotechnology; dilution 1:200 with 5% NGS in PBS) was applied and incubated for 60 min, respectively. After several washes with PBS, the sections were incubated with the simple stain MAX-PO (R; for rabbit primary antibody; Nichirei, Tokyo, Japan) or the MAX-PO (M; for mouse primary antibody; Nichirei), which is a horseradish peroxidase conjugated secondary antibody, for 60 min, and then positive signals were developed with 3,3′-diaminobenzidine (DAB; Dojindo, Kumamoto, Japan), respectively. The sections were counterstained with hematoxylin to visualize nuclei.
Determination of apoptotic cells by TUNEL method
Frozen sections of ovaries (5 μm thick) were air-dried and fixed in 4% PFA in PBS for 20 min. To detect the DNA fragmentation, TUNEL was performed using commercial kit (In Situ Cell Detection Kit, POD; Roche, Penzberg, Germany). All experiments were performed according to the manufacturer's instructions. The slides were rinsed with 3% hydrogen peroxide in methanol for 30 min and incubated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. After several washes with PBS, the sections were incubated with TdT and detection buffer conjugated with horseradish peroxidase (Converter-POD) for 60 min at 37°C. Positive signals were developed with DAB, and the sections were counterstained with hematoxylin to visualize nuclei.
Double-staining of Ski- and PCNA-positive cells
For double-staining of Ski- and PCNA-positive cells, the sections were fixed in 4% PFA in PBS for 20 min and followed by incubation in 0.1% Triton X-100 in PBS for 15 min. The sections were incubated in methanol for 30 min. Then, the sections were immersed in blocking solution (8% skimmed milk in PBS) for 30 min. The primary antibody specific for Ski described above (dilution 1:100 with 5% NGS in PBS) was applied and incubated for 90 min. The sections were incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG (H+L) (Invitrogen) for 60 min. The sections were incubated with the primary antibody specific for PCNA described above (dilution 1:200 with 5% NGS in PBS). The sections were incubated with Alexa Fluor 594 conjugated goat anti-mouse IgG (H+L) (Invitrogen) for 60 min. Positive signals were observed under the fluorescence microscope (BX50; Olympus, Tokyo, Japan) equipped with digital camera (DP70; Olympus, Tokyo, Japan).
Statistical analysis
Statistical analyses were conducted using StatView (version J5, Abacus Concepts, Berkley, CA, USA). One-way analysis of variance and Dunnett's test were used to determine differences between eCG-treated and control groups. Differences were considered statistically significant at P < 0.05.
Results
Localization of c-Ski mRNA in the follicles in adult female rats
To analyze the tissue distributions of c-Ski gene in the rat, RT-PCR analysis was performed. As shown in Fig. 1, c-Ski gene was expressed in all the tissues examined. The c-Ski gene was expressed in all the tissues examined, which was in agreement with the previous findings made in mice by Lyons et al. (Reference Lyons, Micales, Herr, Horrigan, Namciu, Shardy and Stavnezer1994) and in equine by Yamanouchi et al. (Reference Yamanouchi, Kano, Soeta, Hasegawa, Ishida, Mukoyama, Tojo and Tachi1997). The ovary was one of the tissues that intensively expressed the c-Ski gene.
Overlapping of Ski-positive and PCNA-positive cells
In order to determine if Ski-positive granulosa cells of the follicles are overlapped with PCNA-positive cells and are exclusive to PCNA-positive cells as was observed in follicles, double-staining experiments were performed. As shown in Fig. 2, proliferating PCNA-positive cells were not co-localized with Ski-positive cells. Conversely, Ski was present in TUNEL-positive cells. These results suggest that Ski protein expressed in granulosa cells of atretic follicles may have an apoptosis-related function in these cells.

Figure 2 Immunofluorescent double-staining of Ski and PCNA in the follicles of adult female rat. Note that Ski-positive cells (green) and PCNA-positive cells (red) are not overlapped. GC, granulosa cells; TC, theca cells; AN, follicular antrum. Scale bar = 200 μm.
Localization of Ski protein in the follicles of single generation in eCG-primed immature hypophysectomized rats
To examine if the correlations between Ski expression and PCNA expression as well as those between Ski expression and TUNEL staining are also observed in this immature rat model, immunohistochemical and TUNEL analyses were performed in the ovary having follicles of single generation. As shown in Fig. 3 A, on day 0, some follicles exhibited positive staining of either PCNA or TUNEL in granulosa cells but they were exclusive. In early proliferating granulosa cells so-called early antral follicles, no Ski-positive and TUNEL-positive granulosa cells were observed while they were mostly positive for PCNA. As shown in Fig. 3 A, on day 2 after eCG administration, more follicles with PCNA-positive granulosa cells became evident indicating the increased number of growing follicles but still no Ski-positive and TUNEL-positive granulosa cells were observed in late antral follicles. However, as shown in Fig. 3 A, on day 4 after eCG administration, a numerous number of follicles with TUNEL-positive granulosa cells appeared indicating that these follicles are undergoing to atresia, and the granulosa cells in these atretic follicles were positive for Ski and negative for PCNA. Thus, these results confirm that Ski protein is expressed in granulosa cells undergoing to apoptosis. Quantitative analysis revealed that the proportion of Ski-positive cells at 96 h or later after eCG injection was significantly higher than that of the previous time points (before 96 h) (Fig. 3 B). Since follicular atresia in this rat model is expected to occur at around 96 h, this result suggests that the number of Ski-positive cells increases after atresia.

Figure 3 (A) Immunohistochemical analyses of ovarian sections from eCG-primed immature hypophysectomized rats. Panels (Aa, Ab and Ac) are adjacent sections showing the Ski, TUNEL and PCNA signals, respectively, 0 days after eCG treatment. Panels (Ad, Ae and Af) are adjacent sections showing the Ski, TUNEL and PCNA signals, respectively, 2 days after eCG treatment. Panels (Ag, Ah and Ai) are adjacent sections showing the Ski, TUNEL and PCNA signals, respectively, 4 days after eCG treatment. Arrowheads indicate positive signals for PCNA (Ac, Af), Ski (Ag) and TUNEL (Ah) in granulosa cells. Scale bars = 400 μm. (B) Quantitative analyses of Ski-positive cells. The data are expressed as proportions of Ski-positive cells. The data are means ± standard error (SE) (n = 6). *P < 0.01 versus eCG day 0.
Analysis of c-Ski mRNA expression in granulosa cells obtained from eCG-primed immature rats
To examine this possibility, granulosa cells were obtained from eCG-primed rats before ovulation and their expression of c-Ski mRNA was determined by qPCR. Unexpectedly, c-Ski mRNA was present in granulosa cells during ovarian folliculogenesis, and its expression level was unchanged after eCG injection (Fig. 4 A). These results indicate that c-Ski mRNA expression in granulosa cells is not regulated during ovarian folliculogenesis.

Figure 4 Expression of c-Ski mRNA in granulosa cells from eCG-primed rats before ovulation. qPCR analyses of c-Ski (A), Arkadia (B), Smurf1 (C), and Smurf2 (D). Relative expression levels to HPRT were calculated and graphed. The data are means ± standard error (SE) (n = 5–8).
Analysis of Smurf1, Smurf2 and Arkadia mRNA expression in granulosa cells obtained from eCG/hCG-primed immature rats
The observation that c-Ski mRNA is expressed in granulosa cells during follicular development, while Ski protein is absent and c-Ski mRNA level was unchanged during ovarian folliculogenesis was unexpected. This raises the possibility that the amount of Ski protein is regulated at the translational, not transcriptional, level during follicular development. Recently, Nagano et al. (Reference Nagano, Koinuma, Miyazawa and Miyazono2010) reported that knockdown of Arkadia, an E3 ubiquitin ligases, abrogated TGF-β-induced degradation of Ski protein, suggesting that Arkadia is responsible for the degradation of Ski protein. Therefore, the author next examined the possible involvement of Arkadia in the regulation of Ski protein during follicular development. The expression level of Arkadia, Smurf1, and Smurf2 mRNA during follicular development was assessed by qPCR. As shown in Fig. 4 B–D, the levels of E3 ubiquitin ligases (Arkadia, Smurf1, and Smurf2) mRNA expression were unchanged during follicular development, suggesting that contribution of E3 ubiquitin ligases to the changes in Ski protein level may be little if any.
Discussion
The presence of Ski has been demonstrated in a variety of tissues (Lyons et al., Reference Lyons, Micales, Herr, Horrigan, Namciu, Shardy and Stavnezer1994; Yamanouchi et al., Reference Yamanouchi, Kano, Soeta, Hasegawa, Ishida, Mukoyama, Tojo and Tachi1997), as was shown in this study, and is suggested to play multiple roles in a variety of cell types (Colmenares & Stavnezer, Reference Colmenares and Stavnezer1989; Ambrose et al., Reference Ambrose, Bottazzi and Goodenow1995). For example, Ski is expressed in proliferating myoblast (Soeta et al., Reference Soeta, Suzuki, Suzuki, Naito, Tachi and Tojo2001) and uterine epithelial cells (Yamanouchi et al., Reference Yamanouchi, Soeta, Harada, Naito and Tojo1999). In the uterus, endometrial c-Ski gene expression has been induced by estrogen treatment, which is known to induce proliferation of uterine epithelial cells (Yamanouchi et al., Reference Yamanouchi, Soeta, Harada, Naito and Tojo1999), and progesterone treatment eliminated estrogen-induced c-Ski expression (Yamanouchi et al., Reference Yamanouchi, Soeta, Naito and Tojo2000). In addition, c-Ski is known to induce myogenic differentiation of quail embryonic cells (Colmenares & Stavnezer, Reference Colmenares and Stavnezer1989). Thus, most studies to date have indicated that Ski mediates cell proliferation and differentiation (Liu et al., Reference Liu, Sun, Weinberg and Lodish2001; Luo, Reference Luo2003; Medrano, Reference Medrano2003). Actually, the initial observation showing distinct expression of Ski in granulosa cells in the follicles lead the author to predict that Ski might be expressed in growing follicles. However later experiments revealed that this was not the case, and Ski was unexpectedly found to be expressed in apoptotic granulosa cells rather than proliferating granulosa cells in the ovary.
Furthermore, to examine whether Ski is expressed in TUNEL-positive granulosa cells, but not in PCNA-positive granulosa cells, in the immature rat model, immunohistochemical and TUNEL analyses were also performed on ovaries having a single generation of follicles. Gonadotropin is an important survival factor for developing follicles in escaping atresia and reaching the preovulatory follicle stage (Byskov, Reference Byskov and Jones1978; Greenwald & Terranova, Reference Greenwald, Terranova, Knobil and Neill1988; Hirshfield et al., Reference Hirshfield, Flickinger and Ben-Rafael1988; Byskov, Reference Byskov, Midgley and Sadler1979). Moreover, preovulatory follicles that are not exposed to an LH surge during the appropriate period undergo atresia (Nagano et al., Reference Nagano, Koinuma, Miyazawa and Miyazono2010; Zhao et al., Reference Zhao, Taverne, Weijden, Bevers and Hurk2001). Immature hypophysectomized rats primed with eCG are known to exhibit development of a single generation of follicles and subsequent follicular atresia due to lack of an LH surge (Xiao et al., Reference Xiao, Robertson and Findlay1992). In the present study, 2 days after eCG treatment, the great majority of the follicles was positive for PCNA immunoreactivity although they did not express the Ski protein. Four days after eCG treatment, however, most of the follicles were positive for TUNEL staining, and they also expressed Ski signals. These observations also support the involvement of Ski in follicular atresia.
In relation to apoptosis, Ski has been implicated to have a role as anti-apoptotic factor. For examples, mice deficient for c-Ski show excessive apoptosis (Berk et al., Reference Berk, Desai, Heyman and Colmenares1997; Shinagawa et al., Reference Shinagawa, Nomura, Colmenares, Ohira, Nakagawara and Ishii2001) and overexpression of antisense c-Ski in L6 myoblasts causes apoptosis of the cells (Soeta et al., Reference Soeta, Suzuki, Suzuki, Naito, Tachi and Tojo2001). Thus, the present notion that Ski may be involved in apoptosis proposes a new concept regarding the Ski function. Ski, as a nuclear protein, has been shown to be associated with a variety of other cellular proteins (Akiyoshi et al., Reference Akiyoshi, Inoue, Hanai, Kusanagi, Nemoto, Miyazono and Kawabata1999; Wu et al., Reference Wu, Krawitz, Chai, Li, Zhang, Luo and Shi2002), and it is believed that such a unique property of Ski enables it to express multiple functions (Colmenares & Stavnezer, Reference Colmenares and Stavnezer1989; Ambrose et al., Reference Ambrose, Bottazzi and Goodenow1995; Yamanouchi et al., Reference Yamanouchi, Soeta, Naito and Tojo2000). In this regard, the search for novel Ski-interacting proteins in granulosa cells would be of interest to further clarify the uncovered function of Ski. Further studies are still required to reveal the molecular mechanisms regulating Ski protein levels and activity in the ovary.
Many apoptosis-related genes have been discovered in various tissues and organs including the ovary. It has been reported that p53 up-regulates several apoptosis-related proteins, such as Bax and Bcl-2 (Miyashita et al., Reference Miyashita, Kitada, Krajewski, Horne, Delia and Reed1995), cyclin G (Okamoto & Beach, Reference Okamoto and Beach1994), p21 (El-Diery et al., Reference El-Diery, Tokino, Velculescu, Levy, Parsons, Trent, Lin, Mercer, Kinzler and Vogelstein1993), IGFBP-3 (Buckbinder et al., Reference Buckbinder, Talbott, Valesco-Miguel, Takenaka, Faha, Seizinger and Kley1995), Gadd45 (Kastan et al., Reference Kastan, Zhan, El-Deiry, Carrier, Jacks, Walsh, Plunkett, Vogelstein and Fornace1992), mdm-2 (Barak et al., Reference Barak, Juven, Haffner and Oren1993), and Fas (Muller et al., Reference Muller, Strand, Hug, Heinemann, Walczak, Hofmann, Stremmel, Krammer and Galle1997). Although the precise role of Ski in mediating apoptosis of granulosa cells in the ovary is unknown so far, Ski may induce some downstream apoptosis-related gene in these cells during apoptosis. The mechanism underlying the Ski-induced apoptosis of granulosa cells, if present, is currently unknown. Ski, as a nuclear protein, has been shown to be associated with a variety of other cellular proteins(Akiyoshi et al., Reference Akiyoshi, Inoue, Hanai, Kusanagi, Nemoto, Miyazono and Kawabata1999; Wu et al., Reference Wu, Krawitz, Chai, Li, Zhang, Luo and Shi2002), and it is believed that such a unique property of Ski enables it to express multiple functions (Colmenares & Stavnezer, Reference Colmenares and Stavnezer1989; Ambrose et al., Reference Ambrose, Bottazzi and Goodenow1995; Yamanouchi et al., Reference Yamanouchi, Soeta, Naito and Tojo2000). In this regard, the search for novel Ski-interacting proteins in granulosa cells would be of interest to further clarify the uncovered function of Ski.
Several studies have indicated that c-Ski transcripts are ubiquitously expressed in many tissues and mRNA levels are relatively constant during the cell cycle, myogenic differentiation and embryogenesis (Grimes et al., Reference Grimes, Ambrose and Goodenow1993; Ambrose et al., Reference Ambrose, Bottazzi and Goodenow1995). Thus, the author speculated that regulated ubiquitin-proteasome (Nagano et al., Reference Nagano, Mavrakis, Lee, Fujii, Koinuma, Sase, Yuki, Isogaya, Saitoh, Imamura, Episkopou, Miyazono and Miyazawa2007; Saitoh et al., Reference Saitoh, Imamura, Episkopou, Miyazono and Miyazawa2007; Yoshiko et al., Reference Yoshiko, Oizumi, Hasegawa, Minamizaki, Tanne, Maeda and Aubin2010), proteolysis of Ski is one of the important ways to control its activity during folliculogenesis of granulosa cells. For this reason, the author hypothesized that Arkadia may be one of the candidates that regulates Ski protein in granulosa cells. Unfortunately, the result suggested the involvement of Arkadia was unlikely. However, other several ubiquitin ligases, including Smad ubiquitin regulatory factor, Smurf1, Smurf2, and the APC/Cdh1 complex, could still be the candidates to degradate Ski protein in granulosa cell (Kavsak et al., Reference Kavsak, Rasmussen, Causing, Bonni, Zhu, Thomsen and Wrana2000; Nagano et al., Reference Nagano, Mavrakis, Lee, Fujii, Koinuma, Sase, Yuki, Isogaya, Saitoh, Imamura, Episkopou, Miyazono and Miyazawa2007; Saitoh et al., Reference Saitoh, Imamura, Episkopou, Miyazono and Miyazawa2007).
PAL31 (proliferation-associated leucine-rich protein) is a nuclear protein expressed by various cell types (Sun et al., Reference Sun, Hattori, Mutai, Toyoshima, Kimura, Tanaka and Shiota2001). Analysis of PAL31 mRNA in the adult tissues revealed that it is expressed at a high level in the spleen, testis, thymus and ovary but at a low level in the adult brain (Mutai et al., Reference Mutai, Toyoshima, Sun, Hattori, Tanaka and Shiota2000). PAL31 has been shown to be required for cell cycle progression (Sun et al., Reference Sun, Hattori, Mutai, Toyoshima, Kimura, Tanaka and Shiota2001) and co-localized with PCNA in neural progenitor cells in rat brain and PC12 cells (Mutai et al., Reference Mutai, Toyoshima, Sun, Hattori, Tanaka and Shiota2000). PAL31 also has been shown to function as a caspase-3 inhibitor(Sun et al., Reference Sun, Kimura, Hattori, Tanaka, Matsuyama and Shiota2006). These results indicate that PAL31 acts not only in cell cycle progression but also as a cell survival factor. This was quite contrast to the possible function of Ski in granulosa cells as suggested in this study, and lead the author to suspect possible relation of Ski to PAL31 during follicular development and atresia in the ovary. This issue would be further studied in the future.
In conclusion, although the exact roles of Ski and the regulatory mechanism of its expression are currently unknown, the present study demonstrated that high levels of Ski are expressed in atretic follicles, but not in healthy follicles. Ski may be involved in inducing apoptosis of granulosa cells and may play a key role in follicular selection. Further studies are needed to clarify its function, interaction, and correlation with other molecules in rat ovaries.
Acknowledgements
This work was carried out with the support from the Agenda Program (No. PJ009418022014) of the National Institute of Animal Science, Rural Development Administration, Republic of Korea.
Competing interests
The authors have declared no conflict of interest.






