Introduction
Estrus synchronization is one of the most important basic steps required for embryo transfer, transgenic animal preparation, and animal cloning production. Synchronizing embryonic development to the blastocyst stage with uterine differentiation, which takes place to produce the receptive state, is crucial for successful implantation, and therefore for pregnancy outcome (Wang & Dey, Reference Wang and Dey2006). Although many potential factors leading to infertility have been resolved, the early implantation rate of in vitro produced embryos is still much lower than in vivo.
In mice, the principal steroid hormones that direct uterine receptivity are ovary estrogen and progesterone. Progesterone is essential for implantation and pregnancy maintenance in all mammals, whereas the requirement for ovary estrogen is species specific. In addition, it is well known that ovary estrogen and progesterone are essential for implantation in mice (Wang & Dey, Reference Wang and Dey2006), which indicates that they play an important role in the differentiation to the receptive state of the uterus (Ma et al., Reference Ma, Song, Das, Paria and Dey2003). The actions of the sex steroid, estrogen, have been hypothesized to be mediated through the transcriptional regulation of target genes (Halachmi et al., Reference Halachmi, Marden, Martin, MacKay, Abbondanza and Brown1994). These effects mainly occur when estrogen binds to its specific nuclear receptors, the complex then either binds to response elements featured in various genes or modifies transcription through protein–protein interactions (Naglera et al., Reference Naglera, Cavileera, Verduccic, Schultzd, Hooke and Haytonf2012). Studies on the effect of selective deletion of nuclear receptors have provided evidence for their isoform-specific functions in uterine biology and implantation. Estrogen receptor (ER)α−/− murine uteri are hypoplastic and unable to support implantation, whereas ERβ−/− uteri retain biological functions that maintain normal implantation (Lim & Wang, Reference Lim and Wang2010; Dey et al., Reference Dey, Lim, Das, Reese, Paria, Daikoku and Wang2004), which indicates that ERα plays an important role in mouse early implantation. Meanwhile, the uterine biological role of progesterone is primarily executed by its specific nuclear receptor, progesterone receptor (PR), which plays an important part in pregnancy establishment, maintenance, blastocyst implantation and stroma cells’ decidualization (Halachmi et al., Reference Halachmi, Marden, Martin, MacKay, Abbondanza and Brown1994). Mice lacking PR also show many defects in ovary and uterine functions, leading to female infertility (Dey et al., Reference Dey, Lim, Das, Reese, Paria, Daikoku and Wang2004; Wang & Dey, Reference Wang and Dey2006; Lim & Wang, Reference Lim and Wang2010).
Pregnant mares' serum gonadotrophin (PMSG), a common hormone for inducing estrus, is a gonadotropic hormone produced in the chorion of pregnant mares. It plays an important role in follicular development, ovulation and luteinization, and is commonly used in concert with progestogen to induce ovulation in livestock prior to artificial insemination. However, PMSG has a longer circulatory half-life (40–125 h), and the remnant PMSG can usually impact follicular maturation and ovulation to induce large anovulatory follicles that continuously secrete estrogen, which may cause high estrogen levels in peripheral plasma. Consequently, the premature degradation of corpus luteum will have a negative function on further embryonic development (Lessey et al., Reference Lessey, Palomino, Apparao, Young and Lininger2006), which finally causes implantation failure. Anti-PMSG serum can be specifically combined with PMSG, to form an antigen–antibody complex, which reduces the negative effect of PMSG and maintains the secretion of estrogen at normal levels (Alexiadis et al., Reference Alexiadis, Eriksson, Jamieson, Davis, Drummond, Chu, Clyne, Muscat and Fuller2011). Immunohistochemical (IHC) staining assays have become the standard method for measuring ERα and PR levels (Lessey et al., Reference Lessey, Palomino, Apparao, Young and Lininger2006). Therefore, the immunolocalization of ERα and PR in PMSG and anti-PMSG-treated mouse ovaries, oviducts and uterus was initiated for a further understanding of the potential function of gonadal steroids during the estrous cycle.
Materials and methods
Animals and tissue collection
All mice used in this investigation were housed in barrier facilities according to Beijing Laboratory Animal Management Committee guidelines for the use of laboratory animals. Adult CD-1 female mice were obtained from the laboratory animal center of Peking University [SYXK (Jing) 2011–0039] at 5 weeks of age. Mice were housed in a temperature-controlled room with proper darkness–light cycles, fed with a regular diet, and maintained under the care of the Laboratory Animal Unit, Beijing University of Agriculture, China. The mice were killed by cervical dislocation. In total, 40 mice (body weight, 60–65 g) were randomly assigned to either a control or treatment group (Fig. 1). After the last steroid injections, the uteri, ovaries and fallopian tubes were immediately fixed and embedded in paraffin for immunohistochemical staining or frozen, stored, and processed together for western blot analysis.

Figure 1 Model of the experimental design. Based on different treatments, 40 mice (body weight, 60–65 g) were divided into four groups: (1) PMSG group: five female mice were injected s.c. with 10 IU PMSG; (2) AP-36 h group: five female mice were injected s.c. with 10 IU PMSG, then injected s.c. with 10 IU anti-PMSG after 36 h; (3) AP-42 h group: five female mice were injected s.c. with 10 IU PMSG, then injected s.c. with 10 IU anti-PMSG after 42 h. All the animals were killed by cervical dislocation at 48 h after the first injection; and (4) AP-48 h group: five female mice were injected s.c. with 10 IU PMSG, then injected s.c. with 10 IU anti-PMSG after 48 h, the animals were killed by cervical dislocation after 52 h.
Immunohistochemical staining
Harvested tissues were cut into 5-μm paraffin sections using a slicer (RM2235, Leica Microsystems, Wetzlar, Germany). Paraffin sections were dewaxed and dehydrated, then rinsed in distilled water and treated with 3% (v/v) hydrogen peroxide for 30 min at room temperature. Non-specific binding was blocked in 4% normal bovine serum albumin for 1 h at 37°C. Sections were incubated with an ERα antibody (1:00; Epitomics, Burlingame, CA, USA) or PR antibody (1:00; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After washing with PBS three times, sections were incubated with biotinylated rabbit anti-goat IgG (1:200; Vector Laboratories, Burlingame, CA, USA), followed by incubation in SABC for 40 min at 37°C. 3,3′-Diaminobenzidine (DAB) reagents were used to develop colour, which was stopped when a light brown colour was visible under the microscope. Nuclei were counterstained with hematoxylin for 15 min. PBS instead of primary antibodies was used as a negative control. Sections were dehydrated in gradient alcohol, cleared in xylene and mounted with neutral gum.
Immunohistochemical evaluation
Slides were examined and photographed using a light microscope (BX60; Olympus Optical Co., Ltd, Tokyo, Japan) attached to a digital camera (DP50; Olympus) connected to a Hewlett Packard computer with Image-Pro Plus software (Media Cybernetics, Silver Springs, MD, USA). Slides immunostained for ERα or PR were analyzed using the Image-Pro Plus system. Slides were scanned using ×4 objective magnification, and a field at objective magnification ×20 was randomly chosen. Ten sections were chosen for analysis in each group, at least four representative areas were chosen for analysis from tissue biopsies and then the number of nuclei present within the ERα or PR-stained area (brown) was manually counted, to compute the ratio of nuclei per positive area. We also measured the average absorbance of nuclei per ERα- or PR-positive area according to the shade depth.
Hormone measurements
Serum was separated from all blood samples and frozen in aliquots at ‒20°C until used for subsequent centralized analysis. Serum levels of estrogen, were determined using an automated multi-analysis system with chemiluminescence detection (ACS-180; Bayer Diagnostics, Puteaux, France). For estrogen, functional sensitivity was 15 pg/ml.
Protein extraction and western blot analysis
Tissues were thawed and homogenized in Triton lysis buffer (50 mM Tris–HCl [pH 8.0], 150 mM NaCl, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 1% (v/v) Triton X-100, and 10% (v/v) glycerol) supplemented with 1 mM dithiothreitol (DTT), 10 μg/ml leupeptin, 0.3 U/ml aprotinin, 1 mM PMSF, 1 mM sodium orthovanadate, 50 mM sodium fluoride, and 25 mM P-glycerophosphate. Extracts were clarified by centrifugation at 12,000 rpm for 10 min, and protein concentrations were determined by the method of Bradford using Bio-Rad dye reagent (Bio-Rad, Richmond, CA, USA) and BSA as standard. Soluble protein were fractionated in a 10% SDS-PAGE reducing gel, and then blotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 1% BSA (w/v) for 1 h. The antibodies used were ERα (1:1000; Epitomics, Burlingame, CA, USA) or PR antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After three washes, the membrane was incubated for 1 h with horseradish peroxidase-linked anti-rabbit IgG (Cell Signaling Technology, Beverly, MA, USA), and proteins were visualized using an enhanced chemiluminescence detection system (Invitrogen). β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the loading control.
Statistical analysis
The specific hypotheses posed were tested by Student's t-test. A comparison among different time points for ERα or PR was performed using one-way analysis of variance (ANOVA) with SPSS11.0 statistical analysis software. A P-value <0.05 was considered to be statistically significant.
Results
Expression of ERα and PR in murine ovaries, oviducts and uterus
We found an even distribution of ERα and PR in the ovaries, oviducts and uterus of the different groups of mice by immunostaining and immunoblotting (Figs. 2–4). ERα and PR were present in the nucleus of granulosa, theca and interstitial cells. A moderate intensity of ERα and PR immunoreactivity was detected in granulosa and theca cells of small preantral follicles, however, the intensity of ERα was higher in granulosa cells. Furthermore, a localized expression of ERα and PR was evident in the lamina propria and epithelium mucosae of the oviduct. In the uterus, ERα and PR immunoreactivity markedly localized to glandular epithelium and luminal epithelium cells.

Figure 2 Roles of ERα and PR on PMSG and anti-PMSG induction in the ovary of female mouse (n = 5). Detection of ERα (A1) and PR (A2) in different groups by immunohistochemistry. 1. PMSG group, 2. AP-36 h group, 3. AP-42 h group, 4. AP-48 h group. Scale bar, 200 μm. Positive characteristics are shown by arrowheads. FA: Follicular antrum, CO: Cumulus oophorus, PO: Primary oocyte, SG: Stratum granulosum. The expression intensity and average absorbance of ERα (B1, C1) and PR (B2, C2) in the ovarian follicles (x ± s). (D) Western blot showing expression of ERα or PR after different treatments. Data are expressed as least square mean ± SEM. Values with different superscripts are significantly different, P < 0.05.

Figure 3 Role of ERα and PR on PMSG and anti-PMSG induction in the oviduct. Detection of ERα (A1) and PR (A2) in different groups by immunohistochemistry. (1) PMSG group; (2) AP-36 h group; (3) AP-42 h group; (4) AP-48 h group. Scale bar, 200 μm. Positive characteristics are indicated by arrowheads. LP: Lamina propria, EM: Epithelium mucosae, CML: Circular muscle layer. Comparison of intensity and average absorbance of ERα (B1, C1) and PR (B2, C2) by immunohistochemistry in the oviduct after different treatments. (D) Western blot showing expression of ERα or PR in different groups. Data are expressed as least square mean ± SEM. Values with different superscripts are significantly different, P < 0.05.

Figure 4 Role of ERα and PR on PMSG and anti-PMSG induction in the uterus. Detection of ERα (A1) and PR (A2) in different groups by immunohistochemistry. (1) PMSG group; (2) AP-36 h group; (3) AP-42 h group; (4) AP-48 h group. Scale bar, 200 μm. Positive characteristics are indicated by arrowheads. GE: Glandular epithelium, LE: Luminal epithelium, S: Endometrial stroma. The intensity and average absorbance of ERα (B1, C1) and PR (B2, C2) in the endometrial stroma, luminal epithelium and glandular epithelium of uterus (x ± s). (D) Western blot showing expression of ERα or PR after different treatments. Data are expressed as least square mean ± SEM. Values with different superscripts are significantly different, P < 0.05.
Expression of ERα in the different treatment groups
In primordial follicles, there were no significant differences between the PMSG and anti-PMSG (AP) groups (P < 0.05); however, the average absorbance in the PMSG group was significantly higher than in the AP-36 h and AP-42 h groups (P < 0.05). The positive rate in primary follicles of the AP-42 h group was significantly lower than others (P < 0.05), while in the secondary follicles of the PMSG group, the ERα positive rate was significantly higher than in the other groups (P < 0.05). In Graafian follicles, the ERα positive rates in AP-42 h and AP-48 h were significantly lower than in the PMSG group. In the oviduct, the positive rate and average absorbance levels of ERα in the PMSG group were significantly lower than in all other groups (P < 0.05), and the average absorbance in the AP-42 h group was highest among all groups. In the uterus, the positive rate of the AP-36 h group in the luminal epithelium was significantly lower than other groups (P < 0.05), meanwhile, the positive rate in the PMSG group in the glandular epithelium was highest among all the groups (P < 0.05), and lowest in the AP-48 h group (P < 0.05). Meanwhile, in the stroma, staining in the AP-36 h group was significantly lower than in the PMSG group (P < 0.05), and the average absorbance in the AP-36 h group was significantly lower than in the AP-42 h and AP-48 h groups (P < 0.05; Figs. 2, 3 and 4(B1, C1)). The downregulation of ERα expression by anti-PMSG in ovary and uterus was determined by western blotting (Figs. 2, 3 and 4(D)).
Expression of PR in the different treatment groups
In the ovary, on average, we observed that the positive rate of PR in primordial follicles in the AP-42 h and AP-48 h groups was significantly higher than that in the AP-36 h and PMSG groups (P < 0.05), the average absorbance in the AP-48 h group was the highest among all groups (P < 0.05); while in primary follicles, the positive rate of PR in the AP-42 h and AP-48 h groups was higher than in the PMSG group (P < 0.05). In secondary follicles, we detected that the positive rate of PR in the AP-36 h group was lowest among all groups (P < 0.05), and in the AP-48 h group it was significantly higher than in the PMSG group (P < 0.05), furthermore the average absorbance in the PMSG group was significantly lower than in the other groups. Meanwhile, in the Graafian follicle, the positive rate in the AP-42 h and AP-48 h groups was significantly higher than others (P < 0.05), and the average absorbance in the PMSG group was lowest among all groups (P < 0.05). The upregulation of PR expression in response to anti-PMSG treatment in the ovary was determined by western blotting (Fig. 2(D)). In the oviduct, we identified that the positive rate in the PMSG group was significantly lower than in the AP-42 h group (P < 0.05), and the average absorbance in the PMSG group was lowest among all groups (P < 0.05). In the uterus, the positive cells throughout the stroma and luminal epithelium were identified, demonstrating that the PMSG group had the lowest positivity rate among all groups (P < 0.05), furthermore the average absorbance in the AP-42 h and AP-48 h groups was significantly higher than in the PMSG and AP-36 h groups in stroma (P < 0.05). In the glandular epithelium, the positive rate and average absorbance in the AP-42 h group was significantly higher than that observed in other groups (P < 0.05; Figs. 2, 3 and 4(B2, C2)). Western blot showed that PR activation was increased when affected by anti-PMSG in the oviduct and uterus (Figs. 3 and 4(D)).
Serum estradiol level in the different treatment groups
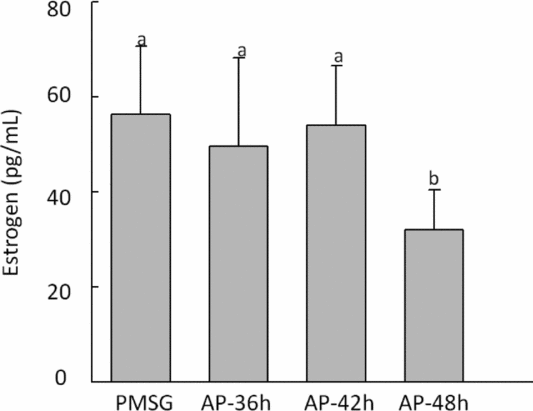
Serum estradiol was detected in different groups after the last injection, data distribution of the levels is shown in Fig. 5, data distribution in the AP-48 h group was significantly lower than that observed in all groups (P < 0.05).

Figure 5 Serum estradiol (E2) levels were measured in different treatment groups. Statistically significant differences (P < 0.05) are indicated by different superscripts. Values are the means ± standard error of the mean (SEM) of three independent experiments.
Discussion
Progesterone and estrogen are the primary female hormones that play major roles in the mammalian estrous cycle, and the ovary is their main source. Estrogen is produced in granulosa cells from androgenic precursors derived from the theca, and progesterone is mainly secreted by the corpus luteum, preparing the body for pregnancy in the event that the released egg is fertilized (Dehdashti et al., Reference Dehdashti, Mortimer, Trinkaus, Naughton, Ellis, Katzenellenbogen, Welch and Siegel2009; Linden et al., Reference Linden, Kurland, Peterson, Schubert, Gralow and Specht2011). Studies on selective deletions of ERα and/or PR in mice have provided evidence for their isoform-specific functions in uterine biology and implantation (Linden et al., Reference Linden, Stekhova, Link, Gralow, Livingston and Ellis2006). Estrogen can stimulate follicular development in the ovary via ER, and transform the progesterone-primed mouse uterus into the receptive state. Superovulation with PMSG induces a second wave of follicles after ovulation, causing high concentrations of oestradiol in the peripheral blood during early embryonic development, which appears to be because of the long half-life of PMSG (about 5 days). Anti-PMSG has a high degree of specificity, it quickly offsets the action of PMSG, reduces the concentration of PMSG in the blood plasma, and improves the situation. Using anti-PMSG, we showed that ERα expression in primordial follicles was not significantly different among the groups, probably because in the initial stages of folliculogenesis, a functional estrogen receptor is not yet formed (Vitt et al., Reference Vitt, Hayashi, Klein and Hsueh2000). In the secondary follicles, the positive rate of ERα in the PMSG group was significantly higher than others. Also, in the Graafian follicles, the positive rate of ERα in AP-42 h and AP-48 h groups was significantly lower than in the PMSG group. While the ovary is considered to be a PMSG target tissue, the PMSG receptor expression may differ between developmental stages, and treatment with anti-PMSG could reduce the ERα expression in follicles. Differential expression patterns of PR protein in different periods of follicle development were observed: the positive rate in the AP-42 h and AP-48 h groups was significantly higher than in the PMSG group, and the average absorbance in the PMSG group was lower than in the other groups in secondary and Graafian follicles, demonstrating that anti-PMSG affected the distribution of PR, and that treatment with anti-PMSG could improve the level of progesterone. This observation coupled with previous findings on the expression of PR in ovary suggests that these tissues must be progesterone targets. Indeed, PMSG can affect follicle maturation and ovulation, resulting in large follicles and continuous secretion of estrogen, which inhibits the effects of progesterone, and anti-PMSG can eliminate this negative action.
Estrogen can maintain the fallopian tube in the proliferative phase, increase the number of epithelial cells in mitosis, and thicken the intima and cilia. During ovulation, estrogen can increase the activity of smooth muscles in the fallopian tube isthmus to send the fertilized egg into the uterus. We noticed that the positive rate of ERα in the PMSG group was significantly lower than in all the other groups; the average absorbance in the AP-42 h group was the highest among all groups. While the positive rate of PR in the AP-42 h group was significantly higher than in the PMSG group, the average absorbance in the anti-PMSG treatment groups was significantly higher than in the PMSG group. In mouse, following fertilization in the oviduct, the embryo undergoes several rounds of mitotic cell division, ultimately forming a morula. Before the late morula stage, higher concentrations of estrogen and progesterone coordinate oviductal motility for a normal journey of the embryo into the uterus, which is conducive for embryo entry into the uterine lumen where it transforms into a blastocyst (Wang et al., Reference Wang, Guo, Wang, Kingsley, Marnett, Das, DuBois and Dey2004).
Uterine sensitivity with respect to implantation is divided into pre-receptive, receptive and non-receptive (refractory) phases, and uterine receptivity occurs only for a limited period during pregnancy or pseudopregnancy. A reciprocal interaction between the blastocyst and receptive uterus is essential for implantation. Estrogen can transform the progesterone-primed uterus to the receptive state, then activate the blastocyst and initiate the implantation process. Next, the receptive uterus spontaneously transforms into the non-receptive stage. The events are regulated by co-ordinated effects of estrogen and progesterone (Palstra et al., Reference Palstra, Schnabel, Nieveen and Spaink2010). Early pregnancy loss in females is often induced because of defects that occur before, during or immediately after implantation, but higher estrogen causes the uterus to maintain a non-receptive status (Simón et al., Reference Simón, Velasco, Valbuena, Peinado, Moreno, Remohí and Pellicer1998; Ng et al., Reference Ng, Lau, Yeung and Ho2001; Das et al., Reference Das, Wang, Paria, Damm, Abraham, Klagsbrun, Andrews and Dey1994). Herein, we examined ERα expression in three different cell types in the uterus. In the luminal epithelium, the positive rate in the AP-36 h group was lower than in the other groups, whereas in the glandular epithelium, ERα levels in the PMSG group were higher than in the other groups, our investigation indicated that anti-PMSG could profoundly neutralize the function of PMSG and regulate the distribution of estrogen, especially after 36 h. Notably, however, in the stroma and glandular epithelium, the positive rate of PR in the AP-42 h group was significantly higher than in the other groups. The physiological functions of PR are not only regulated by progesterone, but also by coordinated effects of estrogen (Miura et al., Reference Miura, Higashino and Miura2007). In the uterus, a higher level of progesterone and a lower estrogen level are better for embryo implantation. Tightly regulated signalling is important for uterine and embryonic functions, such as local immunomodulation for acceptance of the ‘semi-allogenic’ embryo in the uterus, zygotic gene activation, blastocyst expansion, cell polarity, and embryonic compaction for morula to blastocyst transformation.
Conversely, the uterus becomes receptive for implantation only for a limited period during pregnancy (Ng et al., Reference Ng, Lau, Yeung and Ho2001). The ovaries, oviducts and uterus are sensitive to the changes in progesterone and estrogen, which are critical for the embryo-implantation process. During the estrous cycle, progesterone can suppress ERs. Therefore, ERα expression can reflect progesterone levels and its receptor changes in the organism. Anti-PMSG can regulate the dynamic changes in estrogen and progesterone, and initiate a new reproductive cycle by stimulating oogonial proliferation and subsequently maintaining a better state of hormones for early implantation.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant no. 31272526) and The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (grant no. PXM2013_014207_000067).