Introduction
The chemical 2,4-D is a selective herbicide developed in the 1940s for broadleaf weed control in crops, noncropland, pastures, rangelands, and turf (Peterson Reference Peterson1967; Peterson et al. Reference Peterson, McMaster, Riechers, Kelston and Shalman2016). In the United States, two-thirds of 2,4-D is used in agricultural settings; the remaining third is applied to nonagricultural landscapes (USEPA 2005). Use in lawn and garden market sectors, as well as industrial, commercial, and government sectors makes 2,4-D the most frequently used herbicide in nonagricultural markets (USEPA 2011). In its acid form, 2,4-D is only slightly water soluble; as such, it is commonly formulated as water-soluble amine salts or emulsifiable ester formulations (Peterson et al. Reference Peterson, McMaster, Riechers, Kelston and Shalman2016; Ross and Lembi Reference Ross and Lembi1999).
2,4-D is classified as a weak acid herbicide (Senseman Reference Senseman2007). Weak acid herbicides are unique because water-soluble formulations can exist as a protonated molecule with no charge or as a deprotonated molecule that is dissociated and has an anionic charge (Klein Reference Klein2012). Weak acid herbicides have a dissociation constant (pKa) equal to the pH value at which half the molecules are protonated and the other half are deprotonated. The pKa of 2,4-D is 2.64 (Bekbölet et al. Reference Bekbölet, Yenigün and Yücel1999). Spray water with a pH below the pKa of 2,4-D allows the herbicide molecule to remain primarily in the salt formulation; however, the 2,4-D molecule becomes deprotonated and 2,4-D exists primarily as an anion when spray water–solution pH increases above the pKa (Tan and Crabtree Reference Tan and Crabtree1994). The pH of spray-carrier water generally ranges from 6.0 to 8.5 (Hem Reference Hem1985). Because this range of water pH is well above the pKa of 2,4-D, water soluble 2,4-D formulations dissociate in the spray solution and interact with divalent cations.
Water hardness, commonly reported in equivalents of calcium carbonate (CaCO3), is the measure of dissolved minerals or cations in water (Boyd Reference Boyd2015). Calcium and magnesium are the major divalent cations present in surface waters and groundwater with iron and manganese also present (Boyd Reference Boyd2015). As such, calcium, magnesium, manganese, and iron are the divalent cations that commonly cause hard-water antagonism when present in sprays with weak acid herbicides (Nalewaja and Matysiak Reference Nalewaja and Matysiak1991; Patton et al. Reference Patton, Weisenberger and Johnson2016; Roskamp et al. Reference Roskamp, Chahal and Johnson2013; Thelen et al. Reference Thelen, Jackson and Penner1995). Divalent cations could interact with the carboxyl groups of dissociated 2,4-D molecules to form less readily absorbed salts, similar to the known association of calcium cation with glyphosate carboxyl groups (Thelen et al. Reference Thelen, Jackson and Penner1995), resulting in reduced weed control.
Water can be classified into four levels of hardness: soft (0–50 mg CaCO3 L−1), moderately hard (50–150 mg CaCO3 L−1), hard (150–300 mg CaCO3 L−1), and very hard (>300 mg CaCO3 L−1) (Boyd Reference Boyd2015). Most research on hard-water antagonism of weak acid herbicides was conducted with glyphosate-salt formulations in the presence of divalent cations (Nalewaja et al. Reference Nalewaja, Woznica and Manthey1990; Nalewaja and Matysiak Reference Nalewaja and Matysiak1991, Reference Nalewaja and Matysiak1993; Zollinger et al. Reference Zollinger, Nalewaja, Peterson and Young2010). Hard-water levels as low as 50 mg Ca L−1 (125 mg CaCO3 L−1) antagonize glyphosate on common wheat (Triticum aestivum L.) (Shea and Tupy Reference Shea and Tupy1984). Glyphosate efficacy on yellow nutsedge (Cyperus esculentus L.) was increased when AMS was added to water with 200 mg Ca L−1 (499 mg CaCO3 L−1) (Costa and Appleby Reference Costa and Appleby1986). Water sources used by herbicide applicators include groundwater wells, surface water, and municipal water (Whitford et al. Reference Whitford, Penner, Johnson, Bledsoe, Wagoner, Garr, Wise, Obermeyer and Blessing2009), with hardness varying considerably among sources and locations. Extension publications commonly advise including water conditioners when using a weak acid herbicide in spray water that exceeds 150 mg CaCO3 L−1 hardness (Tharp and Sigler Reference Tharp and Sigler2013; Whitford et al. Reference Whitford, Penner, Johnson, Bledsoe, Wagoner, Garr, Wise, Obermeyer and Blessing2009).
Before 2016, to our knowledge, there was no published research on the water hardness level causing 2,4-D antagonism, although 2,4-D hard water antagonism was demonstrated (Nalewaja and Matysiak Reference Nalewaja and Matysiak1993; Nalewaja et al. Reference Nalewaja, Woznica and Manthey1990; Patton et al. Reference Patton, Weisenberger and Johnson2016; Roskamp et al. Reference Roskamp, Chahal and Johnson2013). Devkota and Johnson (Reference Devkota and Johnson2016) tested the influence of water hardness level on efficacy of an experimental 2,4-D choline salt formulation for control of great ragweed (Ambrosia trifida L.), horseweed, and Palmer amaranth (Amaranthus palmeri S. Watson). For all three weed species, a linear decrease in 2,4-D choline efficacy was reported as water hardness increased from 0 to 1,000 mg L−1. However, their research also documented weed control decreased 19% to 25% in the presence of 10.2 g L−1 AMS with increased water hardness, indicating AMS did not sufficiently overcome hard-water antagonism.
The most widely used salt formulation of 2,4-D is the dimethylamine salt (Peterson et al. Reference Peterson, McMaster, Riechers, Kelston and Shalman2016). Despite evidence of hard water antagonizing 2,4-D salt formulations (Devkota and Johnson Reference Devkota and Johnson2016; Nalewaja and Matysiak Reference Nalewaja and Matysiak1993; Nalewaja et al. Reference Nalewaja, Woznica and Manthey1990; Patton et al. Reference Patton, Weisenberger and Johnson2016; Roskamp et al. Reference Roskamp, Chahal and Johnson2013), 2,4-D herbicide labels do not include instructions or precautions about mixing with hard water (Patton et al. Reference Patton, Weisenberger and Johnson2016). Only one phenoxy herbicide label—2,4-DB; 4-(2,4-dichlorophenoxy)butanoic acid—cautions against the use of the herbicide when water hardness exceeds 500 mg CaCO3 L−1 (Anonymous 2009). Warnings of hard-water antagonism are common on glyphosate, glufosinate, and sethoxydim labels (Patton et al. Reference Patton, Weisenberger and Johnson2016). As such, additional research on the influence of water hardness levels and the utility of AMS to alleviate antagonism is needed on commonly used, commercially available 2,4-D formulations.
Once mixed, some pesticides become less effective when they are stored and not applied shortly after mixing (Whitford et al. Reference Whitford, Penner, Johnson, Bledsoe, Wagoner, Garr, Wise, Obermeyer and Blessing2009). This is particularly true of carbamate and organophosphate fungicides and insecticides, which are subject to alkaline hydrolysis (Etō Reference Etō1974; Kuhr and Dorough Reference Kuhr and Dorough1976). In previous studies, researchers found no differences in control with premixtures of glyphosate and dicamba 24 h after mixing (Devkota et al. Reference Devkota, Whitford and Johnson2016) or glufosinate and glyphosate when spray solutions were stored up to 7 d after mixing (Stewart et al. Reference Stewart, Nurse, Cowbrough and Sikkema2009). Furthermore, glyphosate efficacy was not reduced in hard water when spray solutions were stored up to 7 d after mixing with or without AMS (Mahoney et al. Reference Mahoney, Nurse and Sikkema2014). Spray mixture storage time is variable because some applicators mix immediately before applications and others mix ahead of time or save unused mixtures for later applications. Previous research on 2,4-D antagonism was conducted by applying the herbicide shortly after mixing (Devokota and Johnson Reference Devkota and Johnson2016; Patton et al Reference Patton, Weisenberger and Johnson2016; Roskamp et al. Reference Roskamp, Chahal and Johnson2013). The impact of spray solution storage time on 2,4-D dimethylamine efficacy is unknown.
The objectives of this experiment reported here were to (1) determine the water hardness level needed to antagonize 2,4-D dimethylamine, (2) determine if the inclusion of AMS alleviates hard-water antagonism at the tested hardness levels, and (3) determine if spray solution storage time influences hard water antagonism. By determining the optimum mix conditions (i.e., water hardness, adjuvant inclusion, and storage time), applicators will be equipped with information to maximize 2,4-D efficacy.
Materials and Methods
Effects of Water Hardness and Adjuvant Inclusion on Weed Control
Experiment 1 was conducted in the Purdue University Horticulture greenhouses in West Lafayette, IN (40.421°N, 86.914°W) to determine the influence of water hardness level on the efficacy of 2,4-D dimethylamine. Dandelion, a common weed in turf and right-of-ways (Beck and Patton Reference Beck and Patton2015; Van Wychen Reference Van Wychen2016), and horseweed, a troublesome weed among terrestrial crop and noncrop areas (Van Wychen Reference Van Wychen2016), were used to bioassay the effects of water hardness and adjuvant treatments on 2,4-D dimethylamine efficacy.
Weed seed planted in the greenhouse was collected from Lafayette, IN. Weed species were planted in 54 × 28 × 6-cm flats (Hummert International, Earth City, MO 63045) containing potting soil (Fafard growing mix; Sun-Gro Horticulture, Agawam, MA 01001). After germination and emergence, a single seedling of each species was transplanted into 3.8-cm–diameter cone-tainers (Ray Leach SC-10 Super Cell Cone-tainers; Stuewe and Sons, Tangent, OR 97389) containing a 1:2:1 mixture of potting soil, sand, and a Whitaker silt loam soil (fine-loamy, mixed, active, mesic Aeric Endoaqualf). After transplanting, plants were irrigated with municipal tap water as needed and fertilized biweekly with two water-soluble fertilizers (3:1 mixture of 15-2.2-12.5, and 21-2.2-16.6 [N-P-K], respectively; The Scotts Co., Marysville, OH 43040) to provide the following (in mg L−1): 200 N, 26 P, 163 K, 50 Ca, 20 Mg, 1.0 Fe, 0.5 each Mn and Zn, 0.24 each Cu and B, and 0.1 Mo. Seventy-six percent of the nitrogen provided was in the nitrate form.
The experiment was conducted in the greenhouse starting in March 2015 and repeated in June 2015. Each run was arranged as a randomized complete block design with five blocks. After the herbicide application, day/night temperatures and photosynthetically active radiation were measured continuously (hourly) with a mini-weather station (WatchDog 2475 Plant Growth Station; Spectrum Technologies, Plainfield, IL 60585). Temperatures averaged 24.6 C, with an average daily light integral of 29.0 mol m−2 d−1 during the first experimental run, and 23.9 C, with an average daily light integral of 30.1 mol m−2 d−1 when repeated.
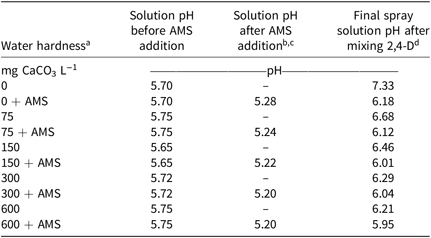
2,4-D dimethylamine (2,4-D Amine 4; Albaugh Inc., Ankeny, IA 50021) was applied at 1.60 kg ae ha−1 (the maximum single application rate to ornamental turf) in water with the following five levels of hardness: distilled water (dH2O), dH2O with 75 mg CaCO3 L−1 equivalents (110.9 mg CaCl2 2[H2O] L−1 [calcium chloride dihydrate]; Fisher Scientific, Fair Lawn, NJ 07410); dH2O with 150 mg CaCO3 L−1 equivalents (221.8 mg CaCl2 2[H2O] L−1); dH2O with 300 mg CaCO3 L−1 equivalents (443.5 mg CaCl2 2[H2O] L−1); and dH2O with 600 mg CaCO3 L−1 equivalents (887.1 mg CaCl2 2[H2O] L−1). At each hardness level, adjuvant treatments including with and without AMS (APF S-Sul Sprayable Ammonium Sulfate 99.5%; American Plant Food Corporation, Galena Park, TX 77547) at 20 g L−1 (2% w/w) were tested to determine the influence of an adjuvant on herbicide efficacy. A rate of 2% w/w AMS was used because this is a commonly recommended rate to prevent hard-water antagonism of glyphosate (Anonymous, 2004). All solutions were prepared in dH2O. Measurements were taken at each step of mixing to determine solution pH (B40PCID sympHony Benchtop Multi Parameter Meter; VWR International, Inc., Radnor, PA 19087) (Table 1).
Table 1. Solution pH as influenced by hardness, AMS, and 2,4-D additions at each stage of mixing in the first experiment.
a Solutions of 75, 150, 300, and 600 ppm CaCO3 equivalents were created by dissolving 110.9, 221.8, 443.5, or 887.1 mg CaCl2 2[H2O] L−1, respectively.
b Abbreviation: AMS, ammonium sulfate.
c AMS was added at a concentration of 20 g L−1.
d 2,4-D dimethylamine was mixed at a concentration of 4.3 ml L−1 to apply 1.60 kg ae ha−1 at a spray volume of 815 L ha−1.
Treatments were applied within 2 min of mixing to 5- to 12-cm–diameter dandelion rosettes and to 4- to 7-cm–diameter horseweed rosettes. Plant size varied within each experiment but not within each block. Applications were made on March 14, 2015, in experimental run 1 and June 1, 2015, in experimental run 2. When mixing treatments, calcium solutions were added first, followed by adjuvant treatments, which prevented the effect of cations on the herbicide (McMullan Reference McMullan2000), followed by the herbicide. After AMS and herbicide were added, bottles were agitated for 5 s before spraying. Treatments were applied using compressed air in a track spray chamber (Generation III Research Sprayer; DeVries Manufacturing, Hollandale, MN 56045) calibrated to deliver 815 L ha−1 using a TeeJet® 8004EVS nozzle (TeeJet Technologies; Spraying Systems Co., Wheaton, IL 60187) at 275 kPa. Plants were not irrigated for 24 h after spray application.
Weed epinasty was assessed 2 wk after application (WAA) on a 0% to 100% scale, where 0% was no epinasty and 100% represented complete epinasty, with all leaves exhibiting symptoms including twisting or bending of stems and curling of leaves. Visual estimates of percentage weed control were recorded at 4 WAA on a scale of 0 (no control) to 100 (complete plant death). Digital images were taken of the weeds 4 WAA using a camera and light box and analyzed for percentage green coverage using ImageJ (version 1.48; National Institutes of Health, Bethesda, MD 20892) (Schneider et al. Reference Schneider, Rasband and Eliceiri2012) as described by Patton et al. (Reference Patton, Weisenberger and Schortgen2018). At 4 WAA, plants were destructively harvested, roots were washed free of soil, and plants were dissected into aboveground shoot tissue and belowground root tissue and then placed into a forced-air dryer at 60 C for 3 d before dry weights were measured. Leaf fresh weight was measured before drying. A nontreated control for both adjuvant treatments (0 and 20 g L−1) was included.
ANOVA was conducted using PROC GLIMMIX in SAS, version 9.4 (SAS Institute Inc., Cary, NC 27513). There were no significant treatment by experimental run interactions, so data were analyzed across experimental runs. When a significant water hardness level by adjuvant interaction existed (P ≤ 0.05), data were subject to nonlinear regression and analysis using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA 92037). The relationship between treatments with and without AMS at various water hardness levels was best fit with the following second-order polynomial (quadratic) equation (Equation 1):
where y represents the measurement (i.e. fresh weight) or rating (i.e. control), b 0 represents the y-intercept, b 1 and b 2 represent regression coefficients, and x represents the water hardness in CaCO3 equivalents. The 95% confidence intervals (CIs) were fit to the quadratic curves. Antagonism was deemed to occur when the CIs of the adjuvant treatments no longer overlapped (Figure 1A) and when mean separation using the Tukey honest significant difference test (α = 0.05) indicated a treatment was different than the 0 CaCO3 treatment without AMS.
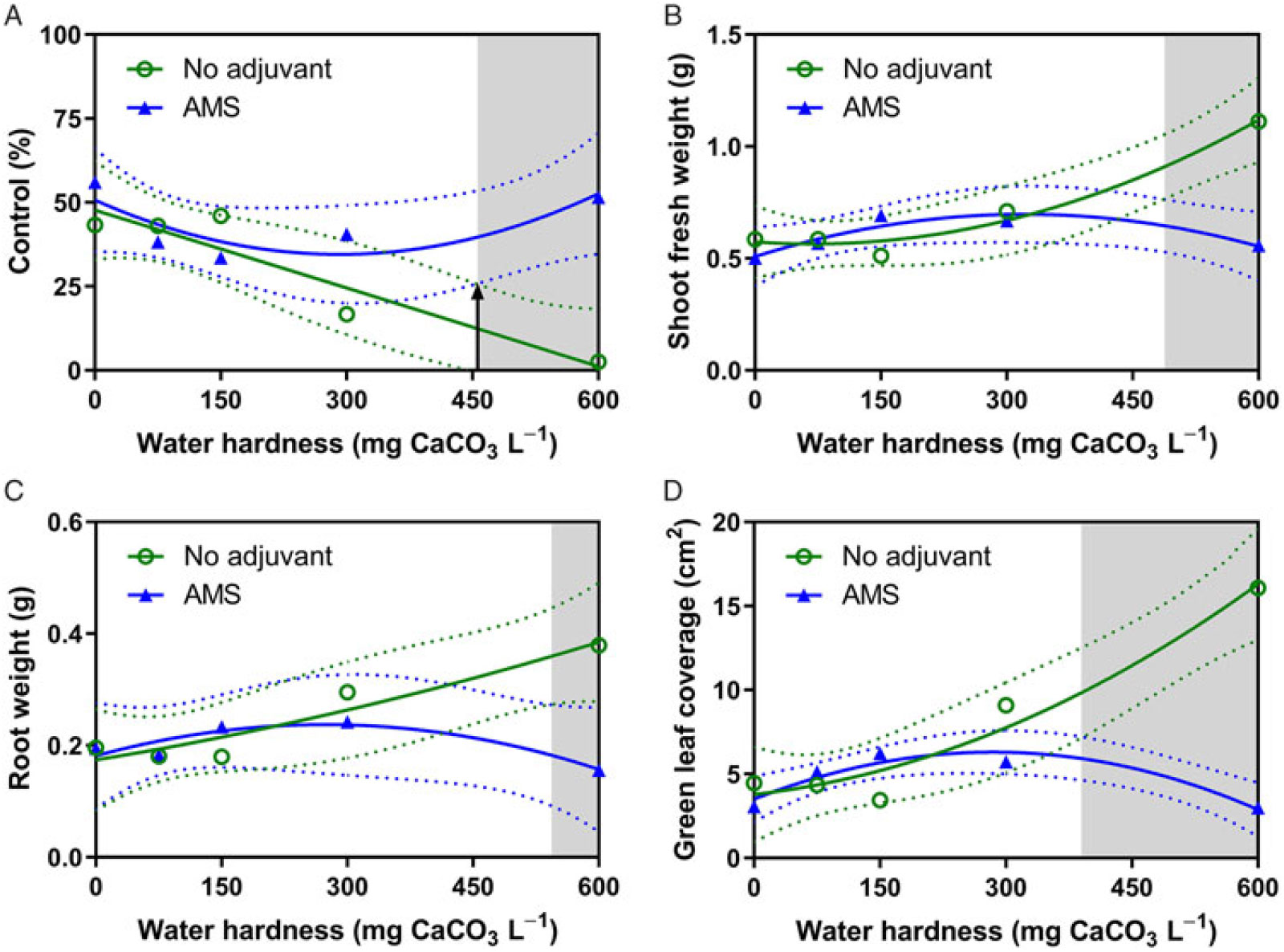
Figure 1. Influence of water-hardness level on 2,4-D dimethylamine efficacy on (A) visual control 4 weeks after application (WAA), (B) shoot fresh weight 4 WAA, (C) root dry weight 4 WAA, and (D) green leaf coverage 4 WAA of horseweed with no adjuvant (○) and with ammonium sulfate (AMS) at 20 mg L−1 (▴). Data were combined across experimental runs. The 95% confidence intervals (CIs) for the treatments are represented by dotted lines. Means with overlapping CIs do not differ. This was confirmed also by Tukey honest significant difference test (α = 0.05). Where the CIs of the two adjuvant treatments no longer overlap (see arrow in A), antagonism was deemed to have occurred (shaded area).
Influence of Spray Solution Storage Time
A second experiment was conducted in the Purdue University horticulture greenhouses to determine the influence of spray solution storage time, water hardness level, and adjuvant inclusion on the efficacy of 2,4-D dimethylamine and to assess if hard-water antagonism would increase, decrease, or stay the same the longer the spray solution was stored. Dandelions were used to bioassay the effects of treatments on 2,4-D dimethylamine efficacy. Propagation, transplanting, and plant culture were similar to the first experiment. 2,4-D dimethylamine was applied at 1.60 kg ae ha−1 in water with four levels of hardness (0, 150, 300, and 600 mg CaCO3 L−1 equivalents), with or without AMS, in the same manner as in experiment 1. Measurements were taken at each step of mixing to determine solution pH.
The experiment was initiated in the greenhouse in October 2015 and repeated in December 2015 with each run arranged as a randomized complete block design with five blocks. Temperatures for experimental run 1 averaged 24.6 C, with an average daily light integral of 18.0 m−2 d−1. Temperatures for experimental run 2 averaged 23.9 C, with an average daily light integral of 9.6 mol m−2 d−1.
Treatments were applied to dandelion rosettes of 5- to 12-cm diameter. Applications were made on October 24, 2015, in experimental run 1 and on December 10, 2015, in experimental run 2. The same mixing order and procedure were used for experiment 2 as in experiment 1. Treatments were agitated for 5 s, set aside, and stored at 22 C to achieve the five predetermined storage times of 0, 1, 4, 24, or 72 h before spraying. After waiting the set storage times, bottles were agitated again, and treatments were applied using compressed air in a track spray chamber, as described for experiment 1. All storage-time treatments were sprayed on the same date.
Weed epinasty, control, digital images, fresh weights, and dry weights were assessed in the same manner as for the first experiment. A nontreated control for all spray solution storage times was included in experiment 2. ANOVA was conducted with PROC GLIMMIX in SAS, version 9.4. Main effects were storage time (five periods), hardness (four levels), and adjuvant (with or without). For data sets in which main effects or interactions were significant at P ≤ 0.05, the Tukey honest significant difference test (α = 0.05) was used for mean separation. No significant treatment by experimental run interactions were present, so data were analyzed across experimental runs. Regression analysis and determination of antagonism were performed in the same manner as described in experiment 1.
Results and Discussion
Effects of Water Hardness and Adjuvant Inclusion on Weed Control
Water pH was measured at each step of mixing to determine whether significant changes occurred with the addition of AMS or 2,4-D dimethylamine (Table 1). The addition of AMS reduced the pH levels by approximately 0.5 (from pH 5.7 to 5.2). The addition of 2,4-D increased the solution pH 0.46 to 1.63 units. However, the final solution pH of all mixtures was greater than 2.5 pH units above the pKa (2.64) of 2,4-D dimethylamine and the pH of 4.64, which is when 99% of the 2,4-D molecules become dissociated (Tan and Crabtree Reference Tan and Crabtree1994). Thus, we can infer that greater than 99% of the 2,4-D dimethylamine was dissociated, based on the Henderson-Hasselbalch equation (Tan and Crabtree Reference Tan and Crabtree1994).
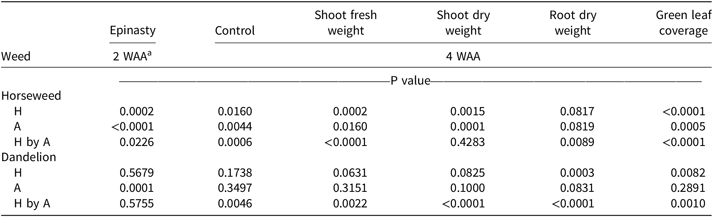
ANOVA for 2,4-D treated horseweed revealed a hardness solution-by-adjuvant interaction for epinasty, control, shoot fresh weight, root weight, and green leaf coverage (Table 2). By 2 WAA, epinasty was 17% higher when spraying 2,4-D dimethylamine at 600 mg CaCO3 L−1 with AMS than without (Table 3). Treatments applied with water at 600 mg CaCO3 L−1 provided less horseweed control 4 WAA than treatments applied with water at 600 mg CaCO3 L−1 plus AMS (Figure 1A). Shoot fresh weight and root dry weight displayed a higher weight when water hardness was 600 mg CaCO3 L−1 without AMS (Figure 1B and 1C). Green leaf coverage (area) was also greater when 2,4-D was applied in 600 mg CaCO3 L−1 hard water without AMS (Figure 1D). Overall, the analysis indicated the ability of 2,4-D dimethylamine to control horseweed was diminished at 600 mg CaCO3 L−1 when AMS was not included in the mixture. When AMS was included in the mixture, horseweed control was similar for all water hardness levels.
Table 2. Combined ANOVA by weed species for various characteristics, with P values for the main effect of hardness solution (0, 75, 150, 300, and 600 mg L−1), adjuvant (none or ammonium sulfate), and their interaction shown by weed species for the first experiment.
a Abbreviations: A, adjuvant; H, hardness; WAA, weeks after application.
Table 3. Horseweed epinasty 2 wk WAA of 2,4-D dimethylamine in solutions of various water hardnesses at two AMS levels.
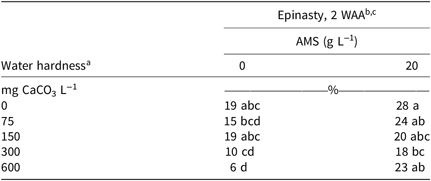
a Solutions of 75, 150, 300, and 600 ppm CaCO3 equivalents were created by dissolving 110.9, 221.8, 443.5, or 887.1 mg CaCl2 2[H2O] L−1, respectively.
b Means followed by the same letter within date type do not differ according to the Tukey honest significant difference test (α = 0.05). Data were combined across experimental run.
c Abbreviations: AMS, ammonium sulfate; WAA, weeks after application.
Previously, Devkota and Johnson (Reference Devkota and Johnson2016) reported no interaction between water hardness and AMS inclusion when controlling horseweed with 2,4-D choline, which is contrary to our research and to the findings of Roskamp et al. (Reference Roskamp, Chahal and Johnson2013) with 2,4-D dimethylamine. Devkota and Johnson (Reference Devkota and Johnson2016) found that horseweed control 3 WAA of 2,4-D choline was decreased 20% as water hardness was increased from 0 to 1,000 mg L−1 in the absence of AMS, but they also reported that adding 10.2 g L−1 AMS did not alleviate the hard-water antagonism. In the present study, horseweed control 4 WAA of 2,4-D dimethylamine was increased 49% when AMS was added to our highest level of water hardness (i.e., 600 mg CaCO3 L−1) (Figure 1A). This is similar to the report of Roskamp et al. (Reference Roskamp, Chahal and Johnson2013), in which they reported AMS increased horseweed control from 2,4-D dimethylamine by 48% in hard water. Each of these experiments used different water hardness levels. For comparison, our experiment tested 0 to 600 mg CaCO3 L−1. Devkota and Johnson (Reference Devkota and Johnson2016) used calcium (CaCl2 2[H2O]) and magnesium (MgSO4 anhydrous) mixed at a 3:1 ratio to generate solution levels from 0 to 1,000 mg L−1 (equivalent to 0 to 718 mg CaCO3 L−1 hardness). Readers are directed to Boyd (Reference Boyd2015) for information on converting from ionic concentration to total hardness expressed as calcium carbonate equivalence.
According to our analysis, horseweed shoot dry weight was the only measurement that did not indicate a hard-water solution-by-adjuvant interaction (Table 2), but analysis did show that adjuvant and hardness treatments, as main effects, were significant. Shoot dry weight was 22% lower when AMS was included in the mixture (data not shown), and shoot dry weight averaged across AMS rate was highest when hardness was 600 mg CaCO3 L−1 (Figure 2).
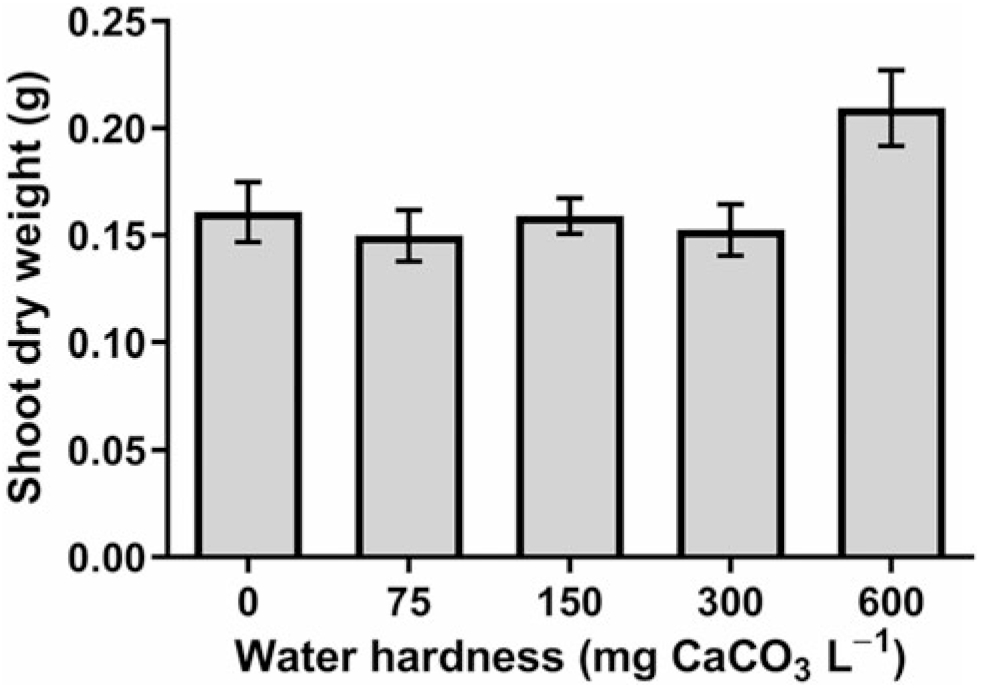
Figure 2. Influence of water hardness on horseweed shoot dry weight 4 weeks after application of 2,4-D dimethylamine. Data were combined across levels of ammonium sulfate and experimental runs. The SE of the mean is shown.
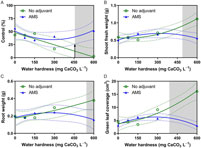
ANOVA for 2,4-D treated dandelion revealed a hardness solution-by-adjuvant interaction for control, shoot fresh and dry weights, root dry weight, and green leaf coverage (Table 2). Treatments of 2,4-D dimethylamine in hard water (600 mg CaCO3 L−1) without AMS were less effective at controlling dandelions (Figure 3A). Like horseweed, dandelions treated with 2,4-D dimethylamine in a water hardness of 600 mg CaCO3 L−1 had higher shoot and root weights compared with those treatments that included AMS (Figure 3B–3D), revealing a loss of control due to hard-water antagonism. Green leaf coverage, likewise, was higher for treatments at 600 mg CaCO3 L−1 without AMS (Figure 3E).
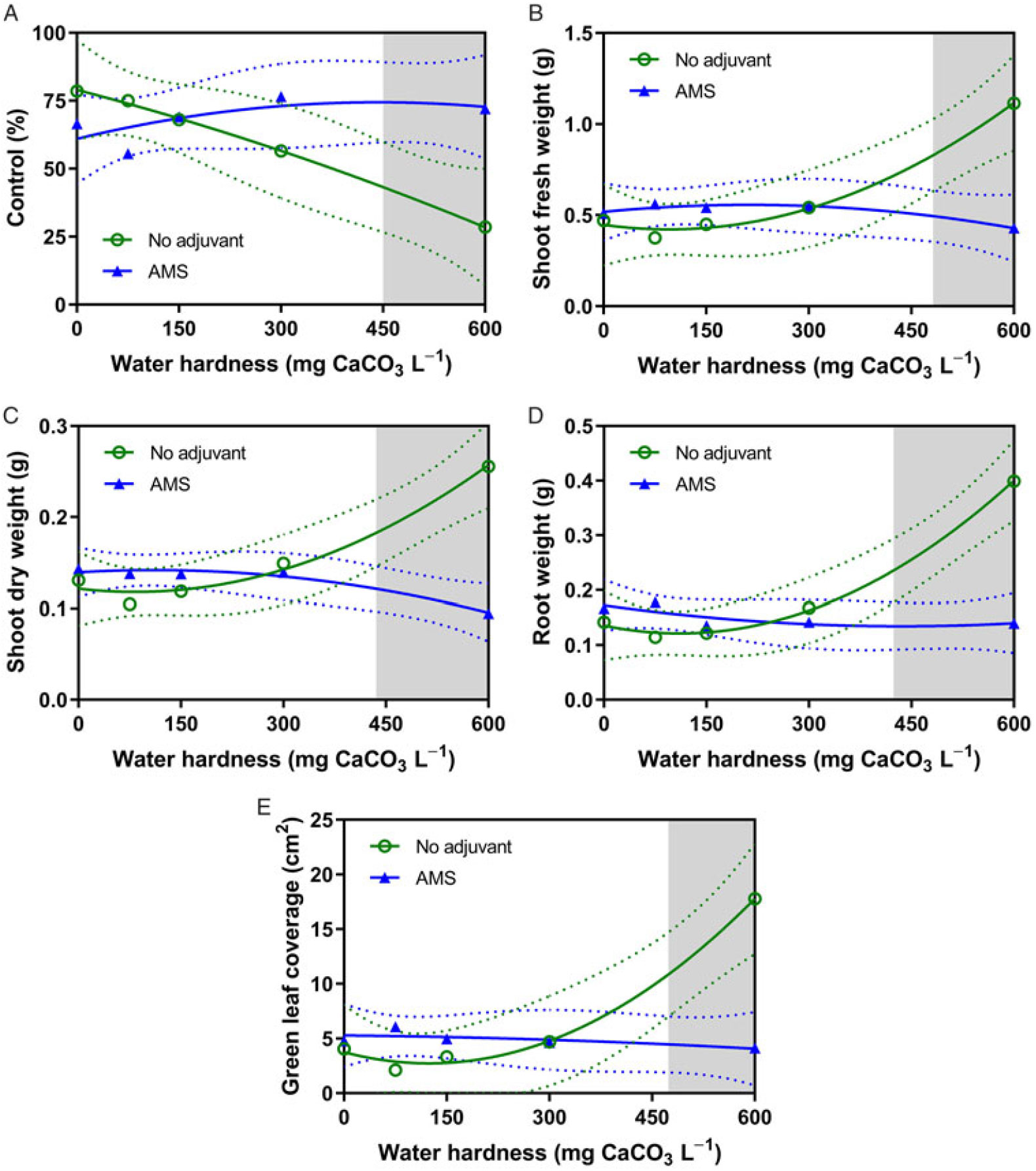
Figure 3. The influence of water hardness level on 2,4-D dimethylamine efficacy on (A) visual control 4 weeks after application (4 WAA), (B) shoot fresh weight 4 WAA, (C) shoot dry weight, (D) root weight, and (E) green leaf coverage 4 WAA of dandelions with no adjuvant (○) and with ammonium sulfate (AMS) at 20 mg L−1 (▴). Data were combined across experimental runs. The 95% confidence intervals (CIs) for the treatments are represented by dotted lines. Means with overlapping CIs do not differ. This was confirmed also by Tukey honest significant difference test (α = 0.05). Where the CIs of the two adjuvant treatments no longer overlap (see arrow in Figure 1A), antagonism was deemed to have occurred (shaded area).
Analysis of dandelion epinasty 2 WAA did not show a solution-by-adjuvant interaction but did reveal differences by treatments (Table 2). Dandelion epinasty was 55% in mixtures with AMS and 36% in mixtures without AMS (data not shown). An increase in foliar absorption of 2,4-D likely occurred from the inclusion of AMS, thereby allowing for 15% to 19% greater epinasty regardless of hard-water levels, which is similar to what has been reported in previous research (Roskamp et al. Reference Roskamp, Chahal and Johnson2013; Thelen et al. Reference Thelen, Jackson and Penner1995; Zollinger et al. Reference Zollinger, Nalewaja, Peterson and Young2010). As the experiment progressed beyond the first 2 WAA, treatment separation among hardness levels became more evident and ANOVA revealed the significant hardness solution-by-adjuvant interactions.
Treatments with and without AMS were plotted with 95% CIs to determine the level at which hard-water antagonism occurred (arrow in Figure 1A). When the CIs no longer overlapped, it was determined that treatments statistically differed and that hard-water antagonism occurred (shaded areas in Figures 1 and 3). The water hardness level at which antagonism first occurred is listed in Table 4 for all 4 WAA ratings that had a solution-by-adjuvant interaction. Overall, hard-water antagonism reduced control and increased shoot and root weight and green leaf coverage as levels reached approximately 450 mg CaCO3 L−1, with antagonism occurring as low as 390 mg CaCO3 L−1 and as high as 590 mg CaCO3 L−1 (Table 3). This level of hardness validates research by Patton et al. (Reference Patton, Weisenberger and Johnson2016) on dandelion and common plantain (Plantago major L.) and Roskamp et al. (Reference Roskamp, Chahal and Johnson2013) on lambsquarters (Chenopodium album L.), horseweed, and redroot amaranth (Amaranthus retroflexus L.); both groups reported hard-water antagonism of 2,4-D dimethylamine at 404 mg CaCO3 L−1 (594 mg CaCl2 2[H2O] L−1). These and other weak acid herbicides, like aminopyralid, tembotrione, and diflufenzopyr, exhibit reduced control when applied with hard water at levels of 500 mg CaCO3 L−1 or higher (Zollinger et al. Reference Zollinger, Nalewaja, Peterson and Young2010). Research with glyphosate, dicamba, and 2-methyl-4-chlorophenoxyacetic acid (MCPA) also has revealed increased efficacy with the inclusion of AMS in hard-water solutions (Nalewaja and Matysiak Reference Nalewaja and Matysiak1993; Roskamp et al. Reference Roskamp, Chahal and Johnson2013; Thelen et al. Reference Thelen, Jackson and Penner1995; Zollinger et al. Reference Zollinger, Nalewaja, Peterson and Young2010).
Table 4. Water hardness level resulting in antagonism of 2,4-D dimethylamine for weed control in the first experiment.a
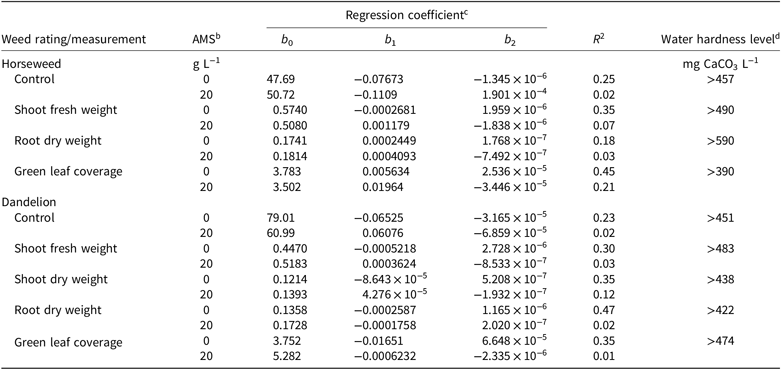
a All ratings and measurements were collected 4 wk after application.
b Abbreviation: AMS, ammonium sulfate.
c Data across experimental run were fit with a second-order polynomial (quadratic) equation: y = b0 + b1x + b2x 2, where y represents the measurement, b0 represents the y intercept, b1 and b2 represent regression coefficients, and x represents the water hardness in CaCO3 equivalents.
Influence of Spray Solution Storage Time
Water pH was measured at each step of mixing in the second experiment, which evaluated the influence of spray solution storage time on 2,4-D dimethylamine efficacy (Table 5). There were changes in pH (<1.5 pH units) with each addition to the mix. The addition of AMS acidified the mixture to a pH near 5.2 for most solutions, and the addition of the herbicide increased the solution pH closer to neutral (pH 7) (Table 5). Storage time had a small and inconsistent effect on solution pH. Solution pH for all mixtures was greater than 2.4 pH units above the pKa of 2,4-D dimethylamine, indicating that greater than 99% of the 2,4-D molecules were dissociated.
Table 5. Solution and herbicide pH during mixture preparation and at each storage time in the second experiment.
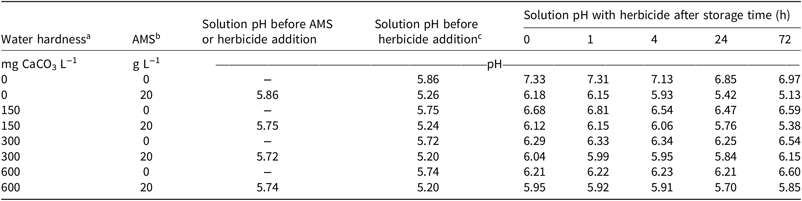
a Solutions of 150, 300, and 600 mg CaCO3 L−1 equivalents were created by dissolving 221.8, 443.5, or 887.1 mg CaCl2 2[H2O] L−1, respectively.
b Abbreviation: AMS, ammonium sulfate.
c 2,4-D dimethylamine was mixed at 4.3 ml L−1 to apply 1.60 kg ae ha−1 at a spray volume of 815 L ha−1.
There was a hardness-by-adjuvant interaction for all measurements (Table 6) similar to what we found for the initial experiment (Table 2). Spray solution storage time as a main effect was not significant for any of the ratings or measurements in this study (Table 6), nor were there hardness-by-time interactions, adjuvant-by-time interactions, or hardness-by-adjuvant-by-time interactions (Table 6). This information provides applicators greater time flexibility, because changing weather conditions may require delaying the application and prolonging storage of mixtures. Although spray water pH between 7 and 8 is common in agriculture (Hem Reference Hem1985) and can lead to insecticide and fungicide alkaline hydrolysis that increases over time (Etō Reference Etō1974; Kuhr and Dorough Reference Kuhr and Dorough1976), spray solution storage time did not affect 2,4-D efficacy in this experiment or the efficacy of other weak acid herbicides in previous experiments (Devkota et al. Reference Devkota, Whitford and Johnson2016; Mahoney et al. Reference Mahoney, Nurse and Sikkema2014; Stewart et al. Reference Stewart, Nurse, Cowbrough and Sikkema2009). In addition, it is important to note that hard-water antagonism occurred immediately when mixing; the 0-h treatment in this experiment was mixed and applied within 1 min. As such, applying quickly after mixing will not reduce the potential for hard-water antagonism.
Table 6. Combined ANOVA for various characteristics, with P values for the main effect of hardness solution (hardness of 0, 150, 300, and 600 mg L−1), adjuvant (none or ammonium sulfate), solution storage time (0, 1, 4, 24, 72 h), and their interactions on dandelion for the second experiment.

a Abbreviations: A, adjuvant; H, water hardness; T, time; WAA, weeks after application.
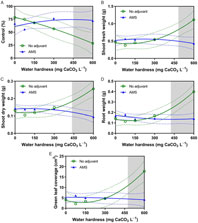
Similar to the first experiment, hard-water antagonism was observed at water hardness as low as 418 mg CaCO3 L−1 for some measurements (Table 7). When 2,4-D dimethylamine was applied in mixtures without AMS at 600 mg CaCO3 L−1, there was a 12% reduction in dandelion epinasty 2 WAA, compared with treatments with AMS (data not shown). Control was reduced in water at 600 mg CaCO3 L−1 hardness when AMS was omitted (Figure 4A). Furthermore, without AMS in water at 600 mg CaCO3 L−1 hardness, shoot fresh weight, shoot dry weight, root dry weight, and green leaf coverage were increased (Figure 4B–4E), which illustrates that dandelion control from 2,4-D dimethylamine was reduced by hard water and that hard water antagonism was overcome by AMS.
Table 7. Water hardness level resulting in antagonism of 2,4-D dimethylamine for the control of dandelion in the spray solution storage time experiment.

a Abbreviations: AMS, ammonium sulfate; WAA, weeks after application.
b Data across experimental run were fit with a second order polynomial (quadratic) equation: y = b0 + b1x + b2x 2, where y represents the measurement, b0 represents the y intercept, b1 and b2 represent regression coefficients, and x represents the water hardness in CaCO3 equivalents.
c Values were determined fitting 95% confidence intervals (CIs) to second-order polynomial (quadratic) curves and determining the point at which the CIs of treatments with and without AMS no longer overlapped by (see Figure 4).
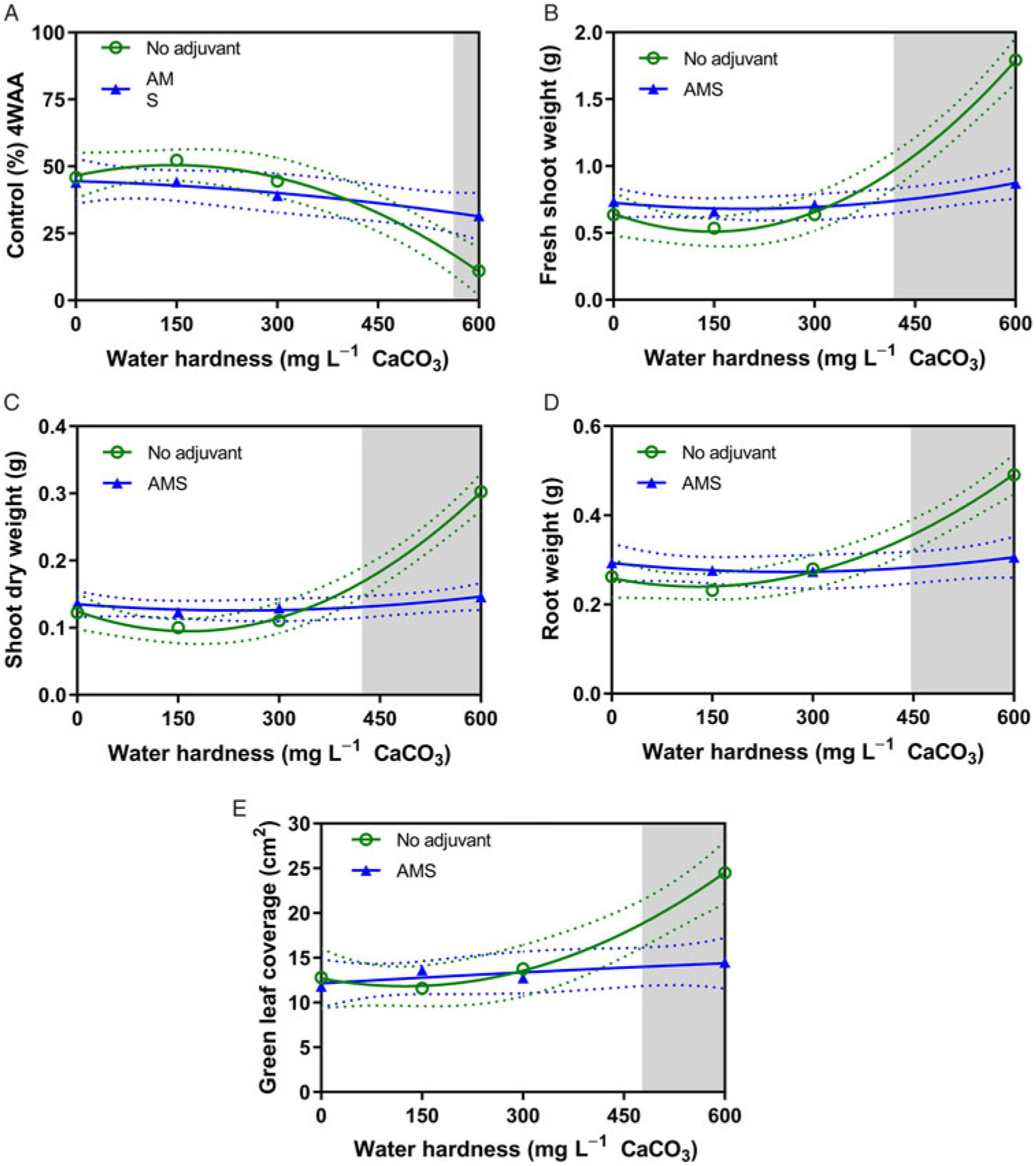
Figure 4. The influence of water hardness level on 2,4-D dimethylamine efficacy on (A) visual control 4 weeks after application (WAA), (B) shoot fresh weight 4 WAA, (C) shoot dry weight 4 WAA, (D) root dry weight 4 WAA, and (E) green leaf coverage 4 WAA of dandelions with no adjuvant (○) and with ammonium sulfate (AMS) at 20 mg L−1 (▴). Data were combined across experimental runs. The 95% confidence intervals (CIs) for the treatments are represented by dotted lines. Means with overlapping CIs do not differ. This was confirmed also by Tukey honest significant difference test (α = 0.05). Where the CIs of the two adjuvant treatments no longer overlap (see arrow in Figure 1A), antagonism was deemed to have occurred (shaded area).
On the basis of these experiments, it would be beneficial for applicators to include AMS when applying 2,4-D dimethylamine in hard water. Indiana groundwater hardness ranges from 0 to 1,384 mg CaCO3 L−1, with 56% of sources classified as very hard (>300 mg CaCO3 L−1) and 17% at a hardness of greater than 400 mg CaCO3 L−1 (IDNR 1999). Glyphosate labels call for the inclusion of a water-conditioning agent like AMS (Anonymous 2004), but 2,4-D labels do not, even though the benefits of adding AMS to mixtures with hard water are documented in this and other research (Devkota and Johnson Reference Devkota and Johnson2016; Nalewaja et al. Reference Nalewaja, Woznica and Manthey1990; Patton et al. Reference Patton, Weisenberger and Johnson2016; Roskamp et al. Reference Roskamp, Chahal and Johnson2013; Zollinger et al. Reference Zollinger, Nalewaja, Peterson and Young2010). As such, manufacturers of 2,4-D dimethylamine should consider the inclusion of statements to the label similar to that for glyphosate: “The addition of 1 to 2 percent dry ammonium sulfate by weight or 8.5 to 17 pounds per 100 gallons of water may increase the performance of this product on annual and perennial weeds” (Anonymous, 2004). Alternatively, manufacturers may need to consider modifying sequestering agents in the formulation to better protect against hard-water antagonism. Previously, formulations were designed to perform well in 1,000 mg CaCO3 L−1 (Kelly Reference Kelly1953), which is well above the levels of antagonism observed in our research. Future research should assess the influence of hard water on other 2,4-D formulations as well as premixes with 2,4-D to determine hard-water influence on the performance of newer formulations.
This research demonstrates that spray solution storage time does not affect hard-water antagonism or the mitigation of hard-water antagonism with the inclusion of AMS. Considering the low cost and potential uptake benefit of adding AMS, it may be advantageous to add this water-conditioning agent anytime 2,4-D dimethylamine is applied. A caution to applicators is that the inclusion of AMS in a spray tank with a final solution pH greater than 7.5 can lead to ammonia volatilization (Vlek and Craswell Reference Vlek and Craswell1981). Although herbicide mixtures are efficacious after being stored several days, ammonia volatilization can increase over time (Vlek and Craswell Reference Vlek and Craswell1981), causing an unpleasant tank aroma. Therefore, applicators wishing to store herbicide mixtures for later use should consider buffering spray water pH to less than 7.5 to eliminate the ammonium to ammonia transformation.
Acknowledgements
The authors express their gratitude to the Midwest Regional Turf Foundation for funding this project. This work was also supported by Hatch project 1004762 from the US Department of Agriculture National Institute of Food and Agriculture. No conflicts of interest have been declared. The authors also thank Drs. Steve Weller, Fred Whitford, and Bryan Young for their helpful review of this manuscript; and Katherine Wierzbowski, Joe Ikely, Dan Weisenberger, and Julie Young for providing their assistance with this experiment.