Introduction
Depression is associated with the greatest disease burden among all psychological disorders (Whiteford et al., Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari, Erskine, Charlson, Norman, Flaxman, Johns, Burstein, Murray and Vos2013). The severe disease burden is largely attributable to the early onset, frequent recurrence, and substantial impairment associated with depressive disorders (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Koretz, Merikangas, Rush, Walters and Wang2003; Burcusa and Iacono, Reference Burcusa and Iacono2007). Cognitive dysfunction is a clinical feature of depression, observed in both clinical and community samples (Rock et al., Reference Rock, Roiser, Riedel and Blackwell2013; Snyder, Reference Snyder2013; Mac Giollabhui et al., Reference Mac Giollabhui, Olino, Nielsen, Abramson and Alloy2018), that contributes substantially to functional impairment in depression, both in individuals who meet criteria for a depressive episode as well as those with remitted or subclinical depressive symptoms (Gotlib et al., Reference Gotlib, Lewinsohn and Seeley1995; Woo et al., Reference Woo, Rosenblat, Kakar, Bahk and McIntyre2016). Thus, understanding cognitive dysfunction in depression will advance our understanding of the overall course and etiology of depression as well as providing insight on a substantial contributor of functional impairment.
Dysfunction across a broad range of cognitive functions, such as verbal memory and executive functioning, is a reliable correlate of depression in children, adolescents, and adults (Rock et al., Reference Rock, Roiser, Riedel and Blackwell2013; Snyder, Reference Snyder2013; Wagner et al., Reference Wagner, Müller, Helmreich, Huss and Tadić2015). However, it also has been observed prior to first onset, in unaffected first-degree relatives of depressed individuals, and when depression has remitted (Rock et al., Reference Rock, Roiser, Riedel and Blackwell2013; Scult et al., Reference Scult, Paulli, Mazure, Moffitt, Hariri and Strauman2017; MacKenzie et al., Reference MacKenzie, Uher and Pavlova2018). Thus, it remains unclear whether, in addition to being a correlate of depression, cognitive dysfunction is a risk factor for depression, a consequence of depression, or whether both depression and cognitive dysfunction are caused by a common underlying process (Allott et al., Reference Allott, Fisher, Amminger, Goodall and Hetrick2016; Mac Giollabhui et al., Reference Mac Giollabhui, Olino, Nielsen, Abramson and Alloy2018). It is likely that the inconsistent associations between cognitive dysfunction and depression observed in the literature are, in part, driven by the effect of specific clinical characteristics (e.g. persistent depression), comorbid conditions (e.g. substance use disorder), and risk factors (e.g. chronic stress). In particular, anxiety is highly comorbid with depression and substance use disorders (Clark and Watson, Reference Clark and Watson1991; Grant et al., Reference Grant, Goldstein, Saha, Chou, Jung, Zhang, Pickering, Ruan, Smith, Huang and Hasin2015; Grant et al., Reference Grant, Saha, Ruan, Goldstein, Chou, Jung, Zhang, Smith, Pickering, Huang and Hasin2016), is associated with elevated peripheral inflammatory activity (Michopoulos et al., Reference Michopoulos, Powers, Gillespie, Ressler and Jovanovic2017), has been implicated in the up-regulation of inflammatory responses in depression (Slavich and Irwin, Reference Slavich and Irwin2014), and could explain some of the variability in the association between depression and executive functioning (Levin et al., Reference Levin, Heller, Mohanty, Herrington and Miller2007). In order to advance our understanding of who will experience cognitive dysfunction in depression and under which conditions, a more mechanistic understanding is required (Carvalho et al., Reference Carvalho, Miskowiak, Hyphantis, Kohler, Alves, Bortolato, PM, Machado-Vieira, Berk and McIntyre2014).
Two putative mechanisms underpinning the association of depression and cognitive dysfunction are inflammation and body mass index (BMI). Sickness behaviors (e.g. anhedonia/social withdrawal) are common correlates of heightened inflammation as well as core characteristics of depression (Maes et al., Reference Maes, Meltzer, Bosmans, Bergmans, Vandoolaeghe, Ranjan and Desnyder1995); indeed, when an inflammatory response is experimentally induced, depressive symptoms reliably follow (Dantzer, Reference Dantzer2001). Meta-analyses report that depressed patients – or, at least, a subsample of depressed patients – exhibit consistently elevated inflammatory biomarkers, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) (Raison and Miller, Reference Raison and Miller2011; Haapakoski et al., Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimäki2015; Kohler et al., Reference Kohler, Freitas, Maes, de Andrade, Liu, Fernandes, Stubbs, Solmi, Veronese, Herrmann, Raison, Miller, Lanctot and Carvalho2017). However, the direction of the relationship between depression and inflammation also remains unclear, with some studies reporting that elevated inflammation prospectively predicts higher depressive symptoms (Stewart et al., Reference Stewart, Rand, Muldoon and Kamarck2009; Au et al., Reference Au, Smith, Gariepy and Schmitz2015; Moriarity et al., Reference Moriarity, Mac Giollabhui, Ellman, Klugman, Coe, Abramson and AlloyIn Press) and others the reverse (Duivis et al., Reference Duivis, Vogelzangs, Kupper, de Jonge and Penninx2013; Zalli et al., Reference Zalli, Jovanova, Hoogendijk, Tiemeier and Carvalho2016). Higher BMI also is associated with increased risk of depression across the lifespan (de Wit et al., Reference de Wit, Luppino, van Straten, Penninx, Zitman and Cuijpers2010; Quek et al., Reference Quek, Tam, Zhang and Ho2017); however, as is the case with inflammation, the temporal relationship between obesity and depression remains unclear, with some evidence for a bidirectional association (Preiss et al., Reference Preiss, Brennan and Clarke2013).
There is considerable evidence linking both inflammation and obesity with cognitive dysfunction. Inflammatory biomarkers, particularly IL-6, IL-1β, and TNF-α, disrupt neuronal processes (e.g. long-term potentiation, synaptic plasticity, and neurogenesis) and negatively impact learning, memory, and executive functioning (Reichenberg et al., Reference Reichenberg, Yirmiya, Schuld, Kraus, Haack, Morag and Pollmacher2001; McAfoose and Baune, Reference McAfoose and Baune2009; Carvalho et al., Reference Carvalho, Miskowiak, Hyphantis, Kohler, Alves, Bortolato, PM, Machado-Vieira, Berk and McIntyre2014; Shields et al., Reference Shields, Moons and Slavich2017). Similarly, obesity is reliably associated with poor cognitive performance in children, adolescents, and adults (Liang et al., Reference Liang, Matheson, Kaye and Boutelle2014; Prickett et al., Reference Prickett, Brennan and Stolwyk2015). Given that adipose tissue also can secrete cytokines and contributes to levels of inflammatory cytokines in systemic circulation, the relationship between obesity and cognition is thought to be mediated, at least in part, by inflammatory processes (Spyridaki et al., Reference Spyridaki, Avgoustinaki and Margioris2016). Although few studies have examined this longitudinally, prior research has reported that higher adiposity predicts impaired cognitive performance via elevated peripheral inflammation (Spyridaki et al., Reference Spyridaki, Simos, Avgoustinaki, Dermitzaki, Venihaki, Bardos and Margioris2014).
Executive functioning refers to cognitive abilities needed to effortfully guide behavior toward a goal, especially in non-routine situations (Banich, Reference Banich2009). Although consensus is lacking on a specific model of executive functioning (Friedman et al., Reference Friedman, Miyake, Young, DeFries, Corley and Hewitt2008; Miyake and Friedman, Reference Miyake and Friedman2012; Diamond, Reference Diamond2013), there is general consensus that it involves multiple cognitive processes, including inhibition, working memory, set-shifting, and planning (Packwood et al., Reference Packwood, Hodgetts and Tremblay2011). Executive dysfunction is reliably observed during depressive episodes and when depression is in remission (Rock et al., Reference Rock, Roiser, Riedel and Blackwell2013; Snyder, Reference Snyder2013); it is also associated with increased peripheral inflammation (Chang et al., Reference Chang, Lee, Gean, Lee, Chi, Yang, Lu and Chen2012; Heringa et al., Reference Heringa, Van den Berg, Reijmer, Nijpels, Stehouwer, Schalkwijk, Teerlink, Scheffer, van den Hurk and Kappelle2014; Huang et al., Reference Huang, Guilleminault, Hwang, Cheng, Lin, Li and Lee2016) and BMI (Smith et al., Reference Smith, Hay, Campbell and Trollor2011; Yang et al., Reference Yang, Shields, Guo and Liu2018). However, no study to date has tested whether peripheral inflammatory cytokines or BMI are predictors of worse executive function in depression, particularly in a diverse community sample of largely unmedicated adolescents, who are less likely to be afflicted by some of the confounding/moderating factors in older adults (e.g. repeated depressive episodes, pre-clinical dementia-related cognitive decline) (Misiak et al., Reference Misiak, Beszlej, Kotowicz, Szewczuk-Boguslawska, Samochowiec, Kucharska-Mazur and Frydecka2018).
The present study
We used a structural equation model (SEM) framework to simultaneously examine the concurrent and prospective associations between peripheral inflammatory biomarkers, BMI, depressive symptoms, and executive functioning in a diverse sample of adolescents. These measures were obtained across three timepoints, thereby assessing if inflammation and BMI are two processes that can explain the association between depression and impaired executive functioning over time. Two measures of executive functioning (selective attention, switching attention) and one self-report questionnaire assessing future orientation, a construct reliant on executive functioning (D'Argembeau et al., Reference D'Argembeau, Ortoleva, Jumentier and Van der Linden2010), were administered. It was hypothesized that a higher BMI would predict more severe depressive symptoms and worse cognitive functioning via elevated peripheral inflammatory activity. Although five inflammatory biomarkers were assayed (IL-6, IL-8, IL-10, TNF-α, and CRP), the main analyses primarily focus on IL-6 because it is the inflammatory cytokine most reliably associated with BMI (Amaral et al., Reference Amaral, Krueger, Ryff and Coe2015), depression, and executive dysfunction in humans (Kohler et al., Reference Kohler, Freitas, Maes, de Andrade, Liu, Fernandes, Stubbs, Solmi, Veronese, Herrmann, Raison, Miller, Lanctot and Carvalho2017). Supplemental analyses investigated whether (i) associations observed for depressive symptoms also were present for anxiety and substance use, (ii) results differed for TNF-α, CRP, IL-8, and IL-10, (iii) results were affected by sex and race, and (iv) whether the reported results held after controlling for additional confounds.
Methods
Participants
Participants were drawn from a prospective, longitudinal study of adolescent-onset depression. A diverse community sample of 642 adolescents aged 12–13 years and their primary female caregivers were recruited from the Philadelphia area for the Adolescent Cognition and Emotion (ACE) Study. Inclusion criteria for the original study were: adolescents aged 12–13 years and their mothers were willing to participate, and that adolescents self-identified as Caucasian, African-American, or biracial (examining racial differences in depression was a goal of ACE). Exclusion criteria included if the adolescent/mother had insufficient English reading/speaking skills to complete assessments or a psychotic, developmental, or learning disorder.
From the 642 adolescents initially enrolled in the study, 307 agreed to participate in a supplementary section of the study, introduced 4 years after initial enrollment, to assess peripheral inflammation. From the 307 participants with blood assayed to date, IL-6 data were available for: 91 individuals at one assessment, 73 at two assessments, 73 at three assessments, 54 at four assessments, and 16 at five or six assessments. Following enrollment in this supplementary section of the study, the 307 participants took part in an average of 4.03 assessments (s.d. = 2.73) per person, with blood draws at 3.01 of these assessments (s.d. = 1.20), totaling 753 blood samples. From the 753 blood samples, 151 observations were removed either because CRP values were > 10 (indicative of a possible acute infection), participants reported a pertinent medical condition (autoimmune disease, diabetes, blood-clotting disorder), were pregnant at time of blood draw, or the observation at which the blood was drawn represented an individual's fourth or fifth blood draw, leaving a total of 288 participants with 602 observations; time to first follow-up was 1.39 years (s.d. = 0.61) and to second follow-up was 1.01 years (s.d. = 0.51). Observations occurring at the fourth or subsequent blood draw were not included in analyses because of the small number of participants who had completed this number of blood collections.
Missing data analyses tested whether the analytic sample differed significantly from the complete ACE sample. The analytic sample did not differ significantly from the complete sample in the proportion of females, χ2 (1, 642) = 0.57 , p = 0.45. However, the racial composition of the analytic sample differed significantly from what was expected based on the complete ACE sample, χ2 (1, 642) = 9.16, p = 0.002. There were fewer Caucasians present in the analytic sample (standardized residual = −1.6) and more African-Americans (standardized residual = 1.5) than expected. Similarly, a trend was observed for socioeconomic status (SES), χ2 (1, 642) = 3.46, p = 0.06, with fewer participants of low SES present in the analytic sample than anticipated (standardized residual = −1) and more participants not of low SES (standardized residual = 1).
Measures
Depressive symptoms
The Children's Depression Inventory (CDI) is a valid, reliable self-report measure of current depressive symptoms in youth (Kovacs, Reference Kovacs1992). The CDI consists of 27 items scored on a three-point scale ranging from zero to two. Items were summed, with higher scores indicating more severe depressive symptoms. The CDI was administered at every timepoint in the study and demonstrated consistent reliability; Cronbach's α over time was consistently > 0.80.
Executive attention
Four subtests of the Test of Everyday Attention for Children (TEAch) and the Test of Everyday Attention (TEA) were administered assessing switching, sustained, divided, and selective attention (Robertson et al., Reference Robertson, Ward, Ridgeway and Nimmo-Smith1994; Manly et al., Reference Manly, Anderson, Nimmo-Smith, Turner, Watson and Robertson2001). However, given the inadequate reliability of sustained and divided attention when transitioning from TEAch to the TEA and the focus of this study on executive functioning, only behavioral measures of selective and switching attention were used (Mac Giollabhui et al., Reference Mac Giollabhui, Olino, Nielsen, Abramson and Alloy2018). Scaled scores, where a score of 10 is indicative of performance in the 50th percentile (s.d. = 3), are reported for each domain of attention, with higher scores indicating superior performance. The ‘Sky Search’ subtest is a non-linguistic measure of selective attention that controls for motor speed (in the TEAch), in which participants were asked to identify cases in which identical stimuli are paired together on a page. Different stimuli were used for the TEA and TEAch to improve the ecological validity for their respective age groups. Both speed and accuracy are encouraged and the outcome is based both on the participant's ability to correctly identify targets and the speed with which they complete the task. Attentional switching is assessed in the TEAch using the ‘Creature Counting’ subtest and in the TEA using the ‘Elevator Counting’ subtest. Both tests measure the temporary slowing that is associated with switching from one task or mental set to another. Separate scores are computed for the speed and accuracy with which participants complete all test items. Both the TEA and TEAch are ecologically valid measures of attentional functioning that are extensively used in both research and clinical settings with demonstrable validity and reliability (Robertson et al., Reference Robertson, Ward, Ridgeway and Nimmo-Smith1994, Reference Robertson, Ward, Ridgeway and Nimmo-Smith1996; Manly et al., Reference Manly, Anderson, Nimmo-Smith, Turner, Watson and Robertson2001).
Future orientation
The Future Orientation Scale (FOS) is a reliable and valid measure of the degree to which adolescents tend to perceive, anticipate, and plan for the future (Steinberg et al., Reference Steinberg, Graham, O'Brien, Woolard, Cauffman and Banich2009). Participants are presented with a series of contrasting statements with the word ‘BUT’ between them (i.e. ‘Some people like to think about all the possible good and bad things that can happen before making a decision’ BUT ‘Other people don't think it's necessary to think about every little possibility before making a decision’) and are asked to select the statement that best describes them. They then are asked to indicate whether the selected descriptor was really true or sort of true. Responses for each pair of statements then were coded on a four-point Likert scale, ranging from really true for one descriptor to really true for the contrasting descriptor. The internal consistency of this measure in the current sample was adequate with Cronbach's α ranging from 0.71 to 0.82 across assessments.
Inflammation
Blood (10 mL) was obtained primarily in the late afternoon to control for diurnal variation and collected via antecubital venipuncture by a certified phlebotomist. The blood was centrifuged to separate the plasma fraction (BD Hemogard with K2 EDTA) and stored at −80 °C until the day of assay. Medication use, medical disorder status, time of last meal, time of day, and participant's BMI were recorded at each blood draw. Cytokines were quantified by multi-cytokine array (IL-6, IL-8, IL-10, and TNF-α) and high-sensitivity CRP (hsCRP) determined via singleplex assay using an electrochemiluminescence platform and a QuickPlex SQ 120 imager for analyte detection (Meso Scale Discovery, Gaithersburg, MD, USA). The analytes were run in duplicate, with intra-assay coefficients varying from 1.94% to 4.38%. Values were referenced to a standard curve generated from seven calibrators with known concentrations. The lower limit of detection for cytokines was 0.1 pg/mL, with a dynamic range up to 2000 pg/mL. However, hsCRP is present in sera at higher concentrations, and thus, plasma was diluted to correspond to the standard curve. Values were converted to mg/L units and were quantified down to 0.1 mg/L (Breen et al., Reference Breen, Reynolds, Cox, Jacobson, Magpantay, Mulder, Dibben, Margolick, Bream, Sambrano, Martinez-Maza, Sinclair, Borrow, Landay, Rinaldo and Norris2011).
Body mass index
BMI was quantified as weight (kg), determined with an electronic scale, divided by height.
Demographics
Age, sex, race, and SES were assessed via self-report. Mothers indicated whether their child received federally-subsidized school lunch, with receipt of free lunch assumed to indicate low SES.
Procedure
Participants completed two types of assessments. The first type was a comprehensive assessment scheduled to occur annually. During comprehensive assessments, participants completed the CDI, FOS, and interviewers conducted a behavioral assessment of attentional functioning (TEAch/TEA). Shorter 6-month assessments also were scheduled in which participants completed measures of negative life events and the CDI assessing depressive symptoms. Blood draws could occur at either annual or 6-month assessments.
Data analysis
Analyses were conducted in Mplus (Version 7.4) and missing data were handled using Full Information Maximum Likelihood. Path analysis within a SEM framework simultaneously examined concurrent and prospective relationships between IL-6, BMI, depressive symptoms, and four measures of executive/cognitive functioning. All pathways are graphically presented in Fig. 1, which consists of all concurrent, prospective, and auto-regressive pathways among the four variables in each model. Models assumed that the relationship between variables across all timepoints were equivalent (i.e. the relationship between Time 1 depression and Time 2 inflammation was the same as Time 2 depression and Time 3 inflammation) and, therefore, equality constraints were applied to associations between the same variables across different timepoints. Although sex and race were variables of theoretical interest given their association with BMI, inflammation, and depression, they were not included in analyses to limit the number of parameters being simultaneously estimated by the model. Model fit was estimated using: χ2 estimate of goodness of fit, comparative fit index (CFI), and root-mean-square error of approximation (RMSEA). The χ2 test of model fit was reported according to convention, but not interpreted given its limited utility in large samples (Cheung and Rensvold, Reference Cheung and Rensvold2002; Chen, Reference Chen2007). For the CFI, ‘good’ fit is indicated by a value > 0.90 and ‘excellent’ fit by a value > 0.95. A RMSEA statistic between 0.05 and 0.10 is indicative of ‘good’ fit and a value ⩽0.05 is indicative of ‘excellent’ fit (Schermelleh-Engel et al., Reference Schermelleh-Engel, Moosbrugger and Müller2003). Finally, indirect pathways were tested, based on 5000 bootstrapped samples, where significant associations between variables of interest were observed. Supplemental analyses also investigated whether (i) findings from the model predicting depressive symptoms also were evident when predicting anxiety symptoms and substance use, (ii) the model would differ if different biomarkers were considered as predictors (i.e. TNF-α, CRP, IL-8, and IL-10), (iii) the results were different if sex and race were considered, and finally (iv) if the significance of the model was affected by considering several factors known to influence the predictor and outcome variables – complete details are provided as online Supplementary material.
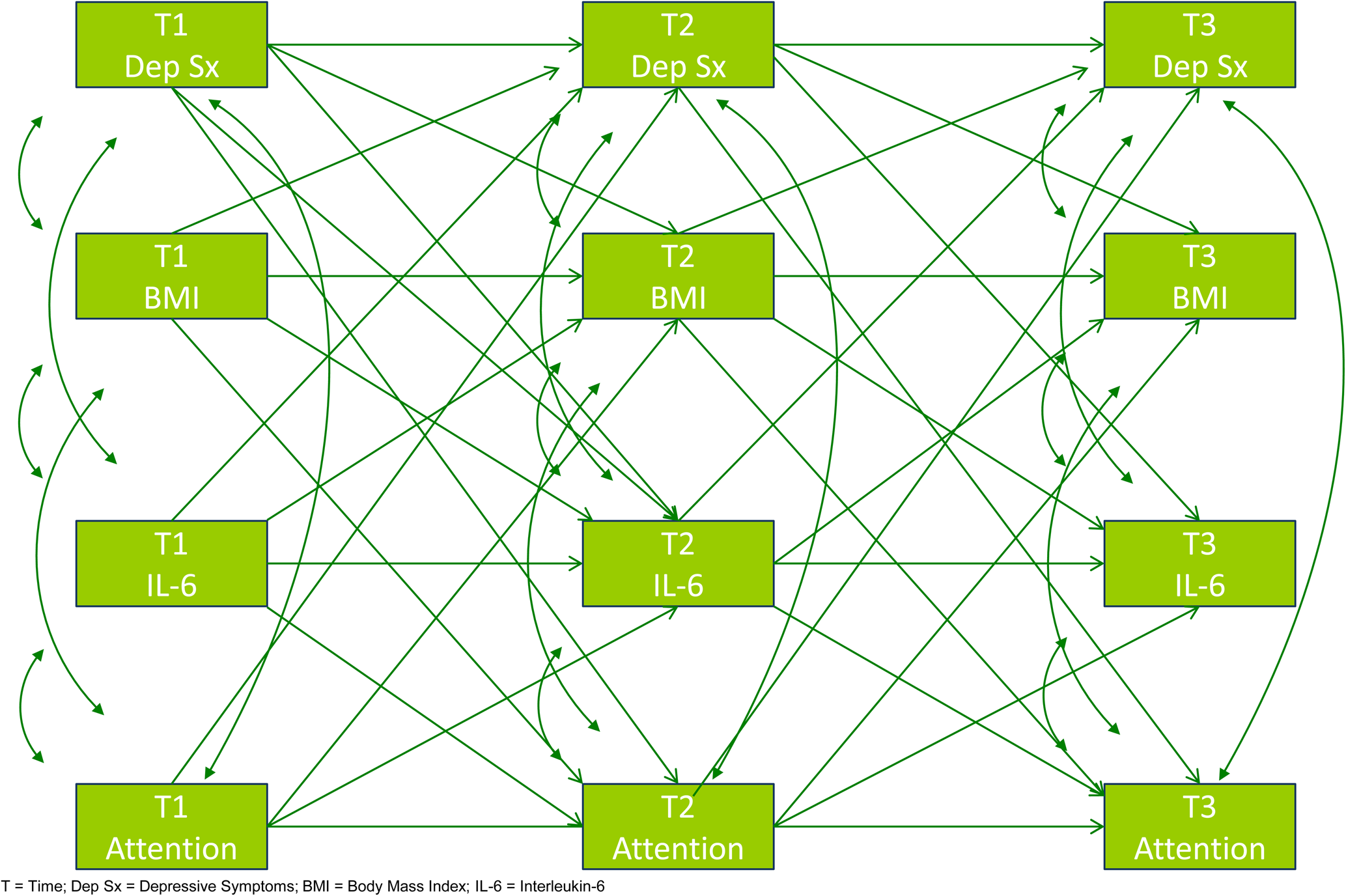
Fig. 1. Path Analysis Model Examining Associations of Depressive Symptoms, Cognitive Functioning, Inflammation and BMI over three years.
Results
Bivariate correlations for the main study variables are presented in Table 1 for the 288 participants aged 16.34 (s.d. = 1.44) who were present at first blood draw. Four SEM-based path analyses were conducted for each of the following four cognitive variables sequentially considered as outcomes: selective attention, switching attention (timing), switching attention (accuracy), and future orientation.
Table 1. Descriptive statistics and bivariate correlations of study variables for 288 participants at Time 1

T1 Age, age at baseline; SES, socioeconomic status; Race: 1, African-American; BMI, body mass index; CDI, Children's Depression Inventory; FOS, Future Orientation Scale; T1: Time 1; ATT, TEA or TEAch Selective Attention; CCT, TEA or TEAch Switching Attention Timing; CCA, TEA or TEAch Switching Attention Accuracy
*p < 0.05; **p < 0.01; ***p < 0.001
Model fit
Model fit statistics are provided for each of the four models in Table 2. The χ2 test of model fit was statistically significant, indicating that the observed data differed significantly from the expected for this model. For each of the models, both the CFI (>0.90) and the RMSEA (⩾0.05 and ⩽0.10) indicated adequate model fit.
Table 2. Model fit for each of the four SEM models

*p < 0.05; **p < 0.01; ***p < 0.001
Concurrent and prospective associations between depressive symptoms, cognitive
Functioning, IL-6, and BMI
All of the pathways tested in the four models are presented in Fig. 1. The pathways presented in the models can be divided into three conceptual components: concurrent, prospective, and auto-regressive associations.
All pathways are reported in Table 3; a number of statistically significant patterns were evident in the results. Concurrent associations represent the partial correlation of any two variables constrained to be equal across all timepoints. Across all timepoints, higher levels of IL-6 were associated with worse selective attention, switching attention (accuracy), and future orientation. Higher depressive symptoms and higher BMI both were concurrently associated with worse switching attention (timing and accuracy, respectively). Across all models, higher BMI was concurrently associated with higher IL-6. Similarly, a number of significant findings were evident in the prospective associations of depressive symptoms, cognitive functioning, IL-6, and BMI. First, higher BMI prospectively predicted worse selective attention and switching attention (accuracy). Second, across all models, higher BMI predicted both more depressive symptoms and a higher level of IL-6. Finally, higher depressive symptoms prospectively predicted higher IL-6.
Table 3. Concurrent and prospective associations for each of the four SEM models

IL6, interleukin-6; COG, cognitive variable of interest; BMI, body mass index; CDI, Children's Depression Inventory. Bolded coefficients represent statistically significant results.
Ψp < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001
Indirect effects: BMI on executive functioning via IL-6
Given the prospective associations observed between BMI and cognition, additional analyses were conducted using 5000 bootstrapped samples to test whether higher BMI predicted worse cognition indirectly via increased IL-6 levels in circulation, thereby determining whether baseline BMI predicted levels of circulating IL-6 in the future, which, in turn, predicted concurrent executive functioning. Indirect pathways via IL-6 were found when predicting to future orientation (b = −0.02; 95% CI −0.001 to −0.05) and selective attention (b = −0.21; 95% CI −0.07 to −0.41). No indirect pathway was observed for switching attention timing (b = −0.01; 95% CI −0.15 to 0.15) or accuracy (b = −0.14; 95% CI −0.31 to 0.001).
Given the prospective association of depression and IL-6, an indirect pathway also was tested to determine the influence of depression on cognitive functioning via inflammatory activity. Significant indirect effects were evident, including the effect of depressive symptoms on selective attention (b = −0.06; 95% CI −0.02 to −0.17), switching attention (accuracy) (b = −0.04; 95% CI −0.002 to −0.13), and future orientation (b = −0.01; 95% CI −0.0001 to −0.02) via IL-6, but not for switching attention (timing) (b = −0.01; 95% CI −0.05 to 0.05). No indirect effects of BMI on prospective depressive symptoms via IL-6 were obtained.
Supplemental analyses
Complete details on a series of additional analyses can be found in online Supplementary material. This statistical testing indicated that the effects reported above were specific to depressive symptomatology, and do not generalize to the future experience of anxiety symptoms nor to the initiations of substance use (see online Supplementary Tables S1–S2). The effects observed for IL-6 were not observed consistently in models examining TNF-α, CRP, IL-8, and IL-10, however similar associations were observed inconsistently; for example, higher levels of TNF-α were significantly associated with worse future orientation but not executive functioning (see online Supplementary Tables S3–S6). Finally, the conclusions did not change substantially when participant sex or ethnicity was considered, and the results held when additional control variables were added to the model (see online Supplementary Tables S7–S10).
Discussion
In a diverse community sample of urban adolescents assessed annually over 3 years, we found that a higher BMI predicted increased future depressive symptoms as well as worse executive functioning/future orientation via higher levels of IL-6 in systemic circulation. Depressive symptoms also predicted an increase in peripheral IL-6. Significantly, all observed associations were unique to depressive symptoms and were not observed in models of anxious symptoms or substance use. Thus, these results suggest that higher depressive symptoms and greater BMI lead to worse cognition via a shared pathway of increased IL-6.
More severe depressive symptoms were concurrently and prospectively (trend-level) associated with worse switching attention and, moreover, prospectively predicted worse selective attention, switching attention (accuracy), and future orientation via elevated peripheral IL-6. The direct concurrent and prospective associations between depression and switching attention have been observed in analyses examining the entire ACE sample (Mac Giollabhui et al., Reference Mac Giollabhui, Olino, Nielsen, Abramson and Alloy2018) and are consistent with previous papers reporting that depression is concurrently and prospectively associated with worse executive functioning (Rock et al., Reference Rock, Roiser, Riedel and Blackwell2013; Snyder, Reference Snyder2013). A number of previous studies have shown that inflammation is associated with concurrent and prospective impairment across a number of different cognitive functions (Chang et al., Reference Chang, Lee, Gean, Lee, Chi, Yang, Lu and Chen2012; Goldsmith et al., Reference Goldsmith, Haroon, Woolwine, Jung, Wommack, Harvey, Treadway, Felger and Miller2016). Studies that included control groups found that increased inflammation predicted worse cognition irrespective of diagnostic status (Krogh et al., Reference Krogh, Benros, Jørgensen, Vesterager, Elfving and Nordentoft2014). Thus, depressed individuals experiencing chronic inflammatory activity may be more likely to experience disrupted cognitive functioning (Raison and Miller, Reference Raison and Miller2011). Should inflammation be linked with cognitive dysfunction in a subset of depressed individuals, this may further explain the considerable heterogeneity in cognitive dysfunction observed in depression (Snyder, Reference Snyder2013).
Inflammation is both concurrently and prospectively associated with impaired cognition in medical and community samples (Reichenberg et al., Reference Reichenberg, Yirmiya, Schuld, Kraus, Haack, Morag and Pollmacher2001; Baune et al., Reference Baune, Ponath, Golledge and Varga2008; Jenny et al., Reference Jenny, French, Arnold, Strotmeyer, Cushman, Chaves, Ding, Fried, Kritchevsky, Rifkin, Sarnak and Newman2012; Li et al., Reference Li, Robertson, Yu, Cheypesh, Dinu and Li2014; Singh-Manoux et al., Reference Singh-Manoux, Dugravot, Brunner, Kumari, Shipley, Elbaz and Kivimaki2014; Huang et al., Reference Huang, Guilleminault, Hwang, Cheng, Lin, Li and Lee2016), although there is considerable heterogeneity in the cognitive domains affected as well as the cytokines implicated. This study also found that IL-6 activity was associated with worse executive functioning/future orientation in a healthy adolescent sample. Supplementary analyses suggest that the association between cognitive functioning and inflammation is largely specific to IL-6 (of the cytokines assayed). It is unclear whether this finding will be replicated; however, animal research also has reported that the inflammatory effect on executive functioning is specific to IL-6 (Sparkman et al., Reference Sparkman, Buchanan, Heyen, Chen, Beverly and Johnson2006). It also should be kept in mind that IL-6 is pleiotropic with multiple origins, particularly adipocytes, and thus, peripheral assessment of IL-6 is not solely a measure of immune system activation. There was no evidence of a direct prospective association between inflammation and executive functioning; this may be due to the concurrent modeling of BMI alongside inflammation in all models. Instead, this study suggested that higher BMI prospectively predicts worse cognitive functioning via increased peripheral IL-6. Higher BMI has been consistently associated with a generalized pattern of cognitive dysfunction, including executive dysfunction, across the lifespan (Laitala et al., Reference Laitala, Kaprio, Koskenvuo, Raiha, Rinne and Silventoinen2011; Liang et al., Reference Liang, Matheson, Kaye and Boutelle2014; Prickett et al., Reference Prickett, Brennan and Stolwyk2015; Yang et al., Reference Yang, Shields, Guo and Liu2018) and these findings support previous research hypothesizing that inflammation may be the mechanism underpinning an association between high BMI and cognitive dysfunction (Spyridaki et al., Reference Spyridaki, Simos, Avgoustinaki, Dermitzaki, Venihaki, Bardos and Margioris2014; Spyridaki et al., Reference Spyridaki, Avgoustinaki and Margioris2016). There is considerable interest in understanding how BMI and poor cognitive functioning are related, with physical activity and poor diet quality identified as two putative mechanisms (Pistell et al., Reference Pistell, Morrison, Gupta, Knight, Keller, Ingram and Bruce-Keller2010; Esteban-Cornejo et al., Reference Esteban-Cornejo, Tejero-Gonzalez, Sallis and Veiga2015). Further research is needed to identify the mechanisms by which BMI leads to worse cognitive functioning in humans.
A concurrent association between inflammation and depression was not observed; however, higher depressive symptoms prospectively predicted higher IL-6 (supplementary analyses also observed that higher baseline TNF-α predicted increased future depressive symptoms). There is a relatively established body of research linking acute inflammation with depression (Maes et al., Reference Maes, Meltzer, Bosmans, Bergmans, Vandoolaeghe, Ranjan and Desnyder1995; Dantzer, Reference Dantzer2001) as well as evidence of a bi-directional association between inflammation and depression (Messay et al., Reference Messay, Lim and Marsland2012). However, it is likely that elevated inflammation only characterizes a sub-group of depressed individuals, and further, the majority of studies have been conducted in adult clinical samples, rather than an adolescent community sample where significantly lower levels of depression are observed (Kohler et al., Reference Kohler, Freitas, Maes, de Andrade, Liu, Fernandes, Stubbs, Solmi, Veronese, Herrmann, Raison, Miller, Lanctot and Carvalho2017). Additionally, once control variables were introduced, this prospective association disappeared, which may point to a confounding factor (e.g. gender) that is underpinning the increases in both inflammation and depressive symptoms. Thus, it may be that the current sample is not sufficiently powered to observe an effect that is only present in a sub-sample of individuals with depression, or that a concurrent association between depression and inflammation is only present in individuals meeting criteria for MDD (Raison and Miller, Reference Raison and Miller2011; Kohler et al., Reference Kohler, Freitas, Maes, de Andrade, Liu, Fernandes, Stubbs, Solmi, Veronese, Herrmann, Raison, Miller, Lanctot and Carvalho2017).
These results should be interpreted in light of the limitations of this study. Inflammation was based on a single sera assessment and variability in food intake, medication status, and diurnal variation may decrease the precision of our estimates, although significant confounds (e.g. infection) were addressed. Although established measures of attentional functioning, the TEA/TEAch are not normed on large US adolescent samples. Thus, it is possible that the normed scores used in this study do not accurately capture age- and gender-normed performance. Moreover, it is unclear whether these results generalize to a clinical sample of depressed individuals, given that assessment relied on self-report questionnaires. Although the ACE sample is notable for its racial, gender, and socioeconomic diversity, the analytic sample was less likely to include Caucasian participants and participants of low SES compared with the entire ACE sample, which could potentially influence the generalizability of these findings. It should be noted that the SEM models presented in this paper were complex, and required estimation of a large number of parameters, which, even for a sizeable sample, increased the risk that the parameters estimated were biased. These limitations are mitigated by direct and repeated measurement of peripheral inflammatory markers in a large, diverse cohort where cognitive functioning was assessed using reliable instruments across multiple modalities.
Conclusions
This study provides strong evidence that higher BMI leads to both depression and cognitive dysfunction in adolescence. It also highlights that an inflammatory cytokine, IL-6, may play a mechanistic role linking higher BMI with impaired cognitive functioning. Moreover, it may point to a sub-type of depression that is characterized by both metabolic dysregulation and cognitive dysfunction. Importantly, these relationships were found to be largely specific to depression, rather than anxiety, and the significance of the statistical modeling held even when considering several potential confounding factors. Future research should investigate systematically whether BMI and IL-6 are associated with executive functioning impairment alone using established theoretically-based models of executive functioning.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719000564.
Author ORCIDs
Naoise Mac Giollabhui, 0000-0003-4226-5704
Acknowledgements
None.
Financial support
This research was supported by the National Institute of Mental Health Grants MH079369 and MH101168 awarded to Lauren B. Alloy, the National Institute of Mental Health Grant MH096478 awarded to Lauren Ellman, and the National Institute of Mental Health Grant F31MH118808 to Naoise Mac Giollabhui.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






