INTRODUCTION
Solid ionic conductors, also called solid electrolytes, transport electric current by means of ions. Ionic conduction in solid electrolytes typically peaks if a partial lattice of a solid compound undergoes a transition, usually at elevated temperature, to a quasimolten state (Rickert, Reference Rickert1978). A large number of silver ion conductors have been studied based on silver iodide, AgI (Takahashi, Reference Takahashi1973). At room temperature AgI can be found in both a hexagonal (β-AgI) and a face-centered cubic (γ-AgI) polymorphic form. Upon heating to 147°C, AgI transforms to a body-centered cubic (α-AgI) polymorph that is an ionic conductor (Takahashi, Reference Takahashi1978). Although the structure of α-AgI is presented as having two AgI entities in a bcc unit cell, the Ag ions have been found to be distributed statistically over 42 available sites (Strock, Reference Strock1936; Hoshino, Reference Hoshino1957). Neutron diffraction studies of α-AgI indicate that silver ions are preferentially found in ellipsoidal regions of space centered at tetrahedral sites, and there is a significant anharmonic contribution to the thermal vibrations in the direction of the octahedral sites. This model suggests silver ions move along the ⟨100⟩ direction in channel-like diffusion paths (Eckold et al., Reference Eckold, Funke, Klaus and Lechner1976).
Silver chalcogenides (silver sulfide Ag2S, silver selenide Ag2Se, and silver telluride, Ag2Te) are also known to be solid-state ionic conductors with mobile silver ions along with electrons and/or holes (Okazaki, Reference Okazaki1967). In the case of Ag2S, the ionic conductivity, σ i, particularly at elevated temperature, can be large (>5 Ω−1cm−1), putting this material into the category of superionic conductors (Miyatani, Reference Miyatani1981). At room temperature, Ag2S has a monoclinic structure (space group P21/n, Z=4) and is referred to as β-Ag2S, also known by its mineral name acanthite. Above 176°C, Ag2S undergoes a structural phase transition (Djurle, Reference Djurle1958), becoming body-centered cubic (space group I m 3m, Z=2), referred to as α-Ag2S, with the mineral name argentite. Upon further heating, >586°C, a second phase transition occurs for Ag2S (Frueh, Reference Frueh1961). This third polymorph has a face-centered cubic structure (space group F m 3m, Z=4). There is no mineral name for this FCC Ag2S polymorph, and it is suggested here to refer to this phase as γ-Ag2S. It is the bcc and fcc polymorphs of Ag2S that are of interest as ionic conductors.
The original research project that initiated this study was to investigate possible methods of incorporating an ionic conducting species of Ag2S in a host polymer to generate a conductive extruded film. Evaluation of reference data in the powder diffraction file (PDF) (ICDD, 2010), revealed many calculated diffraction patterns for the high-temperature Ag2S phases. However, high-quality experimental patterns and subsequent refined crystal structure data were not available for the bcc and fcc polymorphs. In an effort to enhance the reference data in the PDF, in situ high-temperature XRD data were collected for Ag2S, with Rietveld refinement results for the β-Ag2S, α-Ag2S, and γ-Ag2S phases reported as part of this study.

Figure 1. (Color online) Film strip plot of in situ high-temperature XRD data for Ag2S.
EXPERIMENTAL
Sample preparation
Into a glass vessel containing 100 ml de-ionized water was charged 12.845 g AgNO3 (Eastman Kodak Co.). Into a second glass vessel containing 100 ml de-ionized water was charged 9.067g Na2S⋅9H2O (Eastman Kodak Co.). Into a glass vessel containing 50 ml de-ionized water, the AgNO3 and Na2S⋅9H2O solutions were added simultaneously, resulting in the immediate formation of a black precipitate, Ag2S. After stirring for 10 min, this dispersion was poured onto a vacuum filtration apparatus, and the precipitate was washed with 1000 ml de-ionized water. The precipitate was collected and placed in a ceramic bowl, followed by drying at 50°C in a vacuum oven for 3 h. The dried powder was analyzed by XRD and identified as β-Ag2S, acanthite PDF 00-014-0072 (ICDD, 2010).
X-ray diffraction
A Rigaku D2000 Bragg–Brentano diffractometer equipped with a copper rotating anode, diffracted beam graphite monochromator tuned to Cu K α radiation, and scintillation detector was used to confirm the phase identification of the black precipitate powder. In situ high-temperature XRD (HTXRD) experiments were performed using a Siemens D5000 diffractometer equipped with a custom XRD furnace (Misture, Reference Misture2003), with measurements performed under pure N2. Rietveld refinements were performed using the software program TOPAS (Bruker-AXS, 2009).

Figure 2. (Color online) XRD patterns for Ag2S collected at (a) 20°C β phase, (b) 250°C α phase, and (c) 650°C γ phase.
RESULTS
An aliquot of β-Ag2S powder was thermally processed using in situ high-temperature XRD. In Figure 1, HTXRD results plotted in film strip format show changes in the respective XRD patterns as Ag2S is heated and then cooled. Corresponding one-dimensional diffraction patterns for data collected at 20, 250, and 650°C (collected during heating) are presented in Figure 2. The nonambient XRD patterns have few diffraction peaks that help to explain the limited availability of good experimental reference patterns. At 250°C, the observed diffraction peaks indicate that α-Ag2S is present, consistent with the β>α transition at 176°C. The diffraction peaks at 650°C are due to γ-Ag2S, as expected since the data were collected above the 586°C β>γ transition. The Ag2S monoclinic >bcc>fcc phase transition order with increasing temperature is in contrast to other ionic conductors. For example, Ag2Te transforms from monoclinic >fcc>bcc and AgI, CuBr, CuI, and CuCl all
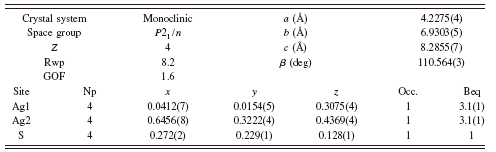
TABLE I. Rietveld refinement structure of β-Ag2S at 20°C.
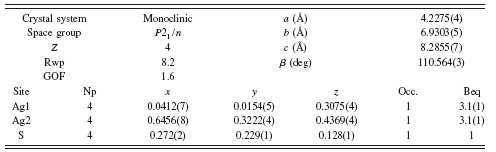

Figure 3. (Color online) Rietveld refinement (blue dots) vs raw data (red line) diffraction patterns for β-Ag2S at 20°C. Inset shows the refined β-Ag2S crystal structure.
show a high-temperature phase transition from fcc or hexagonal close packed (hcp) to bcc (Hull et al., Reference Hull, Keen, Sivia, Madden and Wilson2002).
Examination of the 250°C [Figure 2b] and 650°C [Figure 2c] XRD data show the presence of broad diffuse peaks at ∼35 and 65°2θ in both diffraction patterns. This observation was reported by Tsuchiya et al. (Reference Tsuchiya, Tamaki, Waseda and Toguri1978) in an early attempt to determine the crystal structure of α-Ag2S. The diffuse scattering seen in α and γ-Ag2S is attributed to Ag+ ions in a highly disordered state resembling a liquidlike distribution. Powder neutron diffraction and molecular dynamics simulations (Hull et al., Reference Hull, Keen, Sivia, Madden and Wilson2002) found that Ag+ ions predominantly reside in tetrahedral cavities of a S2− lattice in α-Ag2S and in both tetrahedral cavities and octahedral holes of a S2− lattice in γ-Ag2S. Using the partial structural results known for Ag2S and the data collected in this study, structure refinement of the three Ag2S phases was performed. Refinement was based on the sharp diffraction peaks, whereas the broad diffuse scattering was not modeled.

Figure 4. (Color online) Rietveld refinement (blue dots) vs. raw data (red line) diffraction patterns for α-Ag2S at 250°C. Inset shows the refined α-Ag2S crystal structure.
Refinement of the monoclinic β-Ag2S phase yielded a structure solution similar to the structure reported by Sadanaga and Sueno (Reference Sadanaga and Sueno1967), although the refined structure from this study shows some differences in the x y z coordinates and required a large microstrain term. Refinement parameters are shown in Table I, and the Rietveld refinement diffraction pattern and β-Ag2S structure are shown in Figure 3.
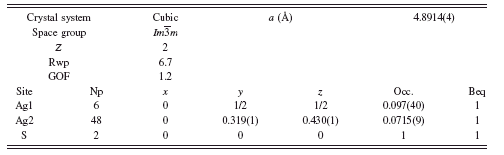
For α-Ag2S at 250°C, it was found that refining a previously reported structure model (Cava et al., Reference Cava, Reidinger and Wuensch1980) yielded unrealistic displacement parameters for the Ag ions of 17 and 6 Å2 for the Ag atoms on the 12d and 6b Wyckoff sites. An improved refinement was obtained by allowing additional disorder in the system in the form of shifting the 12d Ag atoms to the 48j Wyckoff site and refining the occupancy. The result was a stable refinement with Rwp=6.7, GOF=1.2, and a refined stoichiometry of Ag2.01S without using constraints. The final structure parameters are shown in
TABLE II. Rietveld refinement structure of α-Ag2S at 250°C.
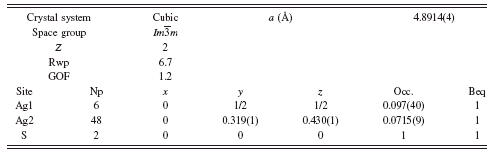
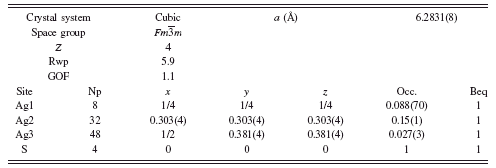
TABLE III. Rietveld refinement structure of γ-Ag2S at 650°C.
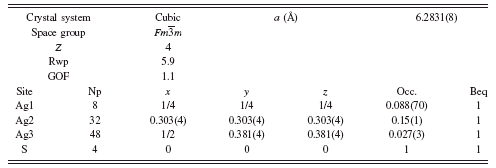

Figure 5. (Color online) Rietveld refinement (blue dots) vs. raw data (red line) diffraction patterns for γ-Ag2S at 650°C. Inset shows the refined γ-Ag2S crystal structure.
Table II and the Rietveld refinement diffraction pattern and α-Ag2S structure are shown in Figure 4.
At 650°C, the fcc γ-Ag2S was also refined, beginning with the model of Frueh (Reference Frueh1961). The result of the refinement was Rwp=6.1, GOF=1.1, but required unreasonable Beq values as high as 20 Å2. Similar to α-Ag2S, the Ag site was split to allow disorder along a logically possible Ag diffusion path, by adding a new Ag atom at the 48f site. The refinement proceeded without constraints to a final result shown in Table III, with Rwp=5.9 and GOF=1.1. The Rietveld refinement diffraction pattern and γ-Ag2S structure are shown in Figure 5. A comparison of the newly adopted crystal structure to that reported by Frueh is shown in Figure 6. The model remains imperfect, as the refined model suggests some Ag deficiency, refining to Ag1.7S. However, we interpret the refinement result as a substantial improvement over earlier models.
A possible explanation for the Ag deficiency in the refined γ-Ag2S structure is the observation of Ag whiskers (confirmed by XRD) growing out of the surface of the Ag2S powder at 650°C (Figure 7). Silver whiskers are also known to form when silver electrical contacts are exposed to hydrogen sulfide (pollutant in air), resulting in short circuits in electrical components (Wikipedia, 2010).
SUMMARY
Structures for acanthite (β), argentite (α), and fcc (γ) Ag2S phases have been successfully refined using the HTXRD data. The resulting powder diffraction patterns are

Figure 6. (Color online) Comparison of γ-Ag2S crystal structure reported by Frueh (left) and the refined γ-Ag2S crystal structure from this study with an additional Ag position (right).

Figure 7. (Color online) Silver whiskers formed during HTXRD thermal processing of Ag2S at 650°C. (a) Ag2S powder on a HTXRD heating strip, postheating; (b) silver whiskers attached to Ag2S removed from the heating strip; (c) SEM micrograph (500×) of a silver whisker growing out of Ag2S powder; (d) SEM micrograph (500×) of a bundle of silver whiskers.
an improvement to current patterns in the powder diffraction file and will be submitted for inclusion in the ICDD PDF database. The α and γ phases diffract very weakly and show diffuse scattering peaks due to mobile Ag atoms. These high-temperature phases are appropriate candidates for further study using maximum entropy modeling to determine the diffusion path(s) of Ag in the α and γ polymorphs. Heating up to 650°C resulted in the formation of Ag whiskers, consistent with a refined silver-deficient structure for γ-Ag2S.