The most common invasive procedures among hospitalized patients are performed to obtain vascular access.Reference Bodenham, Babu and Bennett 1 Most hospitalized patients have at least a peripheral venous catheter.Reference Davis, Owens and Thompson 2 Millions of intravascular catheters are placed every year; they incorporate an ever-increasing variety of vascular technologies combined with multiple published recommendations and guidelines.Reference Davis, Owens and Thompson 2 – Reference Simonov, Pittiruti, Rickard and Chopra 5 Common vascular access devices (VADs) include peripheral intravenous catheters (PIVs), midline catheters, peripherally inserted central venous catheters (PICCs), centrally inserted central venous catheters (CICCs), tunneled central venous catheters, and ports.Reference O’grady, Alexander and Burns 3 , Reference Chopra, Flanders and Saint 4 , Reference Dariushnia, Wallace and Siddiqi 6 PICCs, CICCs, tunneled catheters, and ports comprise the VAD category of central venous catheters (CVCs).
For healthcare providers, the variety of intravascular catheters can be confusing, which can create unintentional difficulty in choosing the correct VAD for the patient.Reference Simonov, Pittiruti, Rickard and Chopra 5 This level of variety also results in an increased need for vascular access skills, resulting in the development of vascular access as a multidisciplinary medical specialty.Reference Davis, Owens and Thompson 2 Ultimately, the goal is to provide the right vascular access option for the patient’s clinical needs in a way that maximizes the potential benefit while minimizing the inherent risks of vascular access.Reference Chopra, Flanders and Saint 4 , Reference Simonov, Pittiruti, Rickard and Chopra 5
Why is optimization of VAD choice critical?
With the varying options of VADs and anatomical locations available, providers must consider the clinical indications for intravascular access as part of the decision-making process: certain medications, hemodynamic measurements, or monitoring; anticipated duration of use; individual patient characteristics and comorbidities; and potential target vessels.Reference Bodenham, Babu and Bennett 1 , Reference Dariushnia, Wallace and Siddiqi 6 , Reference Moureau, Sigl and Hill 7 CVC indications and medications for infusion should adhere to guidelines and institutional policies.Reference Marschall, Mermel and Fakih 8 Medications that can be given via a PIV can be given through a midline catheter, including antimicrobials, to avoid PICC placement for courses of therapy that do not exceed 4 weeks. (Duration of therapy is discussed below.) Figures 1 and 2 provide institutional examples of indications for CVC placement and appropriate medications for infusion requirements.

Fig. 1. Nebraska Medicine Indications for Central Venous Catheter Placement (submitted as figure).
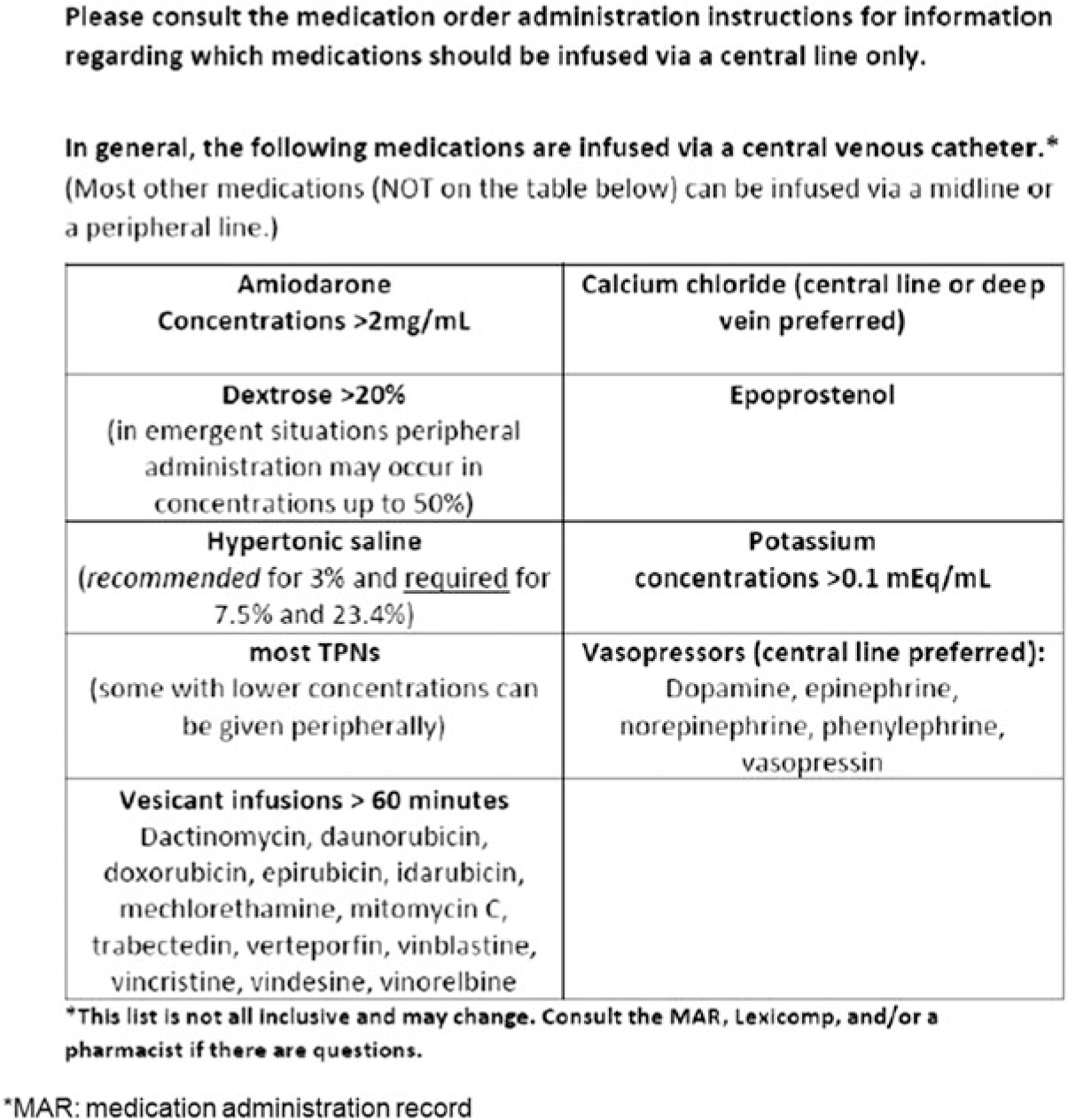
Fig. 2. Nebraska Medicine Infusate Indications for Central Venous Catheters (submitted as figure).
When feasible, current guidelines recommend that catheters in adults be placed in the upper extremity or upper torso to decrease risk of infection.Reference O’grady, Alexander and Burns 3 Consideration of each patient’s unique clinical scenario, anatomy, and the viability of vascular targets are of paramount importance in choosing the optimal location for any catheter because insertion risks vary for each situation. Examples of possible complications include an increased risk of pneumothorax with placement of a subclavian or an internal jugular CVC compared to a femoral vein insertion site. Conversely, an increased risk of infection accompanies catheters inserted in the femoral vein compared to the upper body.Reference Bodenham, Babu and Bennett 1 , Reference O’grady, Alexander and Burns 3 Patients with difficult venous access may be candidates for early placement of a non-PIV catheter, such as a midline catheter.Reference Moureau, Sigl and Hill 7 , Reference Adams, Little, Vinsant and Khandelwal 9 More detail on potential complications is provided in the following section.
Today, these risk–benefit considerations must extend to the choice among a PIV, a midline catheter, a PICC, a tunneled catheter, or a port.Reference Chopra, Flanders and Saint 4 , Reference Dariushnia, Wallace and Siddiqi 6 All VADs carry potential risks of infection, thrombosis, thrombophlebitis, and vascular injury, among others.Reference Dariushnia, Wallace and Siddiqi 6 , Reference Adams, Little, Vinsant and Khandelwal 9 The duration of anticipated need for access, patient characteristics, and the inherent risks of each catheter type should be considered.
Duration of vascular access can be classified into short term, medium term, and long term, with some potential overlap.Reference Bodenham, Babu and Bennett 1 , Reference Chopra, Flanders and Saint 4 Generally speaking, short-term catheters include PIVs and nontunneled CICCs, medium-term catheters include midline catheters and PICCs, and long-term catheters may include PICCs, tunneled catheters, and ports.Reference Bodenham, Babu and Bennett 1 Short-term devices are those needed for <6–10 days; thus, a midline catheter or PICC should be utilized instead of a PIV for patients with an expected duration of intravenous access requirement extending beyond 6 days.Reference Bodenham, Babu and Bennett 1 , Reference O’grady, Alexander and Burns 3 , Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Moureau, Sigl and Hill 7 , Reference Adams, Little, Vinsant and Khandelwal 9 , Reference Caparas and Hu 10 Additionally, PICC placement for phlebotomy access or intravenous administration of <6 days is considered inappropriate according to the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).Reference Chopra, Flanders and Saint 4
Midline catheters have become an increasingly viable option for administration of intravenous medications (including antimicrobials) to outpatients, with a maximum duration of midline indwell time of ~4 weeks (based on individual manufacturer recommendations), although some guidelines suggest limiting use after 14 days.Reference Chopra, Flanders and Saint 4 , Reference Adams, Little, Vinsant and Khandelwal 9 , Reference Caparas and Hu 10 For central venous access requiring >6–10 days, PICC lines are often considered first as a medium-term option if the intended duration is weeks to months.Reference Bodenham, Babu and Bennett 1 , Reference Chopra, Flanders and Saint 4 , Reference Caparas and Hu 10 Tunneled lines and ports are considered long term, with durations of months to years, and ports are considered less intrusive on a patient’s lifestyle than tunneled lines.Reference Bodenham, Babu and Bennett 1 , Reference Chopra, Flanders and Saint 4
With any venous access device, the catheter diameter and number of catheter lumens necessary for medical care must be considered. Both diameter and lumens must be minimized to decrease the risk of complications, including infection and thrombosis.Reference Bodenham, Babu and Bennett 1 , Reference O’grady, Alexander and Burns 3 , Reference Chopra, Flanders and Saint 4
What are the complications of device choice?
Venous access is a cornerstone of medical therapy, and similar to other therapy, it can have unintended consequences and complications. Each type of device for venous access has different risks and benefits. Complications include bloodstream infections, thrombosis, thrombophlebitis, venous stenosis, as well as various types of mechanical injury.
Related literature has shown that CVCs have a rate of all complications of ~1%–32%, with lower rates reported among experienced inserters and with image guidance.Reference Bodenham, Babu and Bennett 1 , Reference Dariushnia, Wallace and Siddiqi 6 , Reference Adams, Little, Vinsant and Khandelwal 9 – Reference McGee and Gould 11 Mechanical complications are more frequent when a subclavian approach is used for a CICC than when femoral or internal jugular venous catheters are used, and the subclavian CICC approach is particularly associated with the risk of pneuomothorax.Reference Bodenham, Babu and Bennett 1 If it is necessary to perform multiple percutaneous punctures, the rates of mechanical complications increase significantly.Reference Eisen, Narasimhan, Berger, Mayo, Rosen and Schneider 12 , Reference Lefrant, Muller and De La Coussaye 13 When placing a CVC, it is also important to consider the depth to which the guidewire is being placed; longer catheter and guidewire insertions have resulted in cardiac arrhythmias or direct cardiac trauma.Reference Bodenham, Babu and Bennett 1 All CVCs carry additional potential risks of arterial or venous injury and air embolism, thus demanding expertise and best-practice adherence during insertion, maintenance and removal.Reference Bodenham, Babu and Bennett 1 PICC lines however, given the peripheral venous puncture, carry a lower risk of mechanical complication during insertion compared to CICCs.Reference Simonov, Pittiruti, Rickard and Chopra 5 Inherently, midline catheters and PIVs do not enter the torso, central veins, or the heart; thus, these devices avoid many of these risks.
Another mechanical complication critical to consider in VADs is the potential development of venous stenosis. A study assessing stenosis due to placement of both PICCs and CICCs found the incidence of stenosis to be about 7% of all insertions.Reference Gonsalves, Eschelman, Sullivan, DuBois and Bonn 14 Central venous stenosis has been observed as early as 4 days following insertion, although the duration of catheterization increases the likelihood of the stenosis as well.Reference Gonsalves, Eschelman, Sullivan, DuBois and Bonn 14 This complication limits future potential arterio-venous fistulae creation among patients with acute or chronic kidney disease who may require future dialysis.Reference Bodenham, Babu and Bennett 1 , Reference Simonov, Pittiruti, Rickard and Chopra 5 Notably, although midline catheters can potentially result in vascular damage, they do not result in central venous stenosis; therefore, they may not result in full loss of potential for future arterio-venous fistulae.Reference Chopra, Flanders and Saint 4
The most common complication seen with PIVs is thrombophlebitis. Several studies have indicated that the process of thrombophlebitis could be mechanical, infectious, or possibly chemical from the infusate through the PIV.Reference Mermel 15 , Reference Zingg and Pittet 16 The rates of thrombophlebitis from PIV also vary, with estimations ranging from 2% for catheterizations up to 80%.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Zingg and Pittet 16 Some of this variance may be due to the broad array of anatomic sites use for peripheral venous access, including hands, antecubital fossae, limbs, feet, and other locations.Reference Rupp, Tandon, Danielson, Cavalieri and Sayles 17 PIVs cause thrombophlebitis at a higher rate than that of CVCs.Reference Pikwer, Åkeson and Lindgren 18 One study found the rate to be ~10 times higher than the rate in CICCs (78 vs 7.5 per 10,000 indwelling days).Reference Pikwer, Åkeson and Lindgren 18 Rates of phlebitis among midline catheters are similar, and possibly higher, than those of PIVs.Reference O’grady, Alexander and Burns 3 , Reference Moureau, Sigl and Hill 7 , Reference Adams, Little, Vinsant and Khandelwal 9 , Reference Caparas and Hu 10 , Reference Xu, Kingsley and DiNucci 19 , Reference Sharp, Esterman, McCutcheon, Hearse and Cummings 20
Two specific concerns frequently raised regarding the utilization of midline catheters are (1) whether there is an increased risk of complications compared to other vascular catheters and (2) whether vancomycin specifically can be given through a midline. Midlines are noted to have decreased rates of phlebitis and bloodstream infection compared to PIVs and PICCs/CICCS, respectively.Reference O’grady, Alexander and Burns 3 , Reference Caparas and Hu 10 However, among complications considered minor or mechanical (eg, pain, leaking, edema and nonpatency, among others) midlines had a higher rate of complications (2.6%–11.5%) than that of PICCs (1.5%) and CVCs (0.3%), but the overall event rate remains low.Reference Moureau, Sigl and Hill 7 , Reference Xu, Kingsley and DiNucci 19 – Reference Mushtaq, Navalkele and Kaur 21
Historically, certain antibiotics have been considered as indications for CVC placement due to pH or vesicant properties. Vancomycin infusions with a pH < 5 have frequently been called into question, including a 2011 Infusion Nursing Standards of Practice stating that infusions with a pH < 5 or >9, should be given via a CVC.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Caparas and Hu 10 , 22 However, more studies are confirming that infusion of vancomycin via midline catheters is safe for most patients, and in some instances, may even be protective against phlebitis.Reference Caparas and Hu 10 , Reference Xu, Kingsley and DiNucci 19 , Reference Caparas and Hu 23 A prospective, nonrandomized study of 153 surgical patients receiving vancomycin versus other antibiotics via peripheral intravenous catheters revealed no statistically significant difference in phlebitis.Reference Roszell and Jones 24 Caparas et alReference Caparas and Hu 10 demonstrated in a single-center, prospective, randomized controlled trial that short-term vancomycin (<6 days) infusions were not associated with a statistically significant increase in complications, including phlebitis and thrombosis, among novel midlines compared to PICCs. In a follow-up of these results, the institution removed pH as an absolute indication for central venous access and subsequently published an observational study demonstrating no thrombosis or phlebitis among 24 patients receiving vancomycin infusions via a midline catheter for >6 days (range, 6–23).Reference Caparas and Hu 23 A 5-year retrospective study of vancomycin through a midline also demonstrated no (DVTs), rare phlebitis (0.6%), and no extravasation injuries among 1,086 patients who received vancomycin infusions via midline catheters.Reference Caparas and Hung 25
Additional benefits include findings that infusion of vancomycin via midlines has been associated with decreased overall cost compared to PICCs.Reference Adams, Little, Vinsant and Khandelwal 9 , Reference Caparas and Hu 10 In a 2015 article in the Journal of Infusion Nursing, Gorski et alReference Gorski, Hagle and Bierman 26 performed a literature review regarding the pH criteria for CVC placement and concluded that “pH alone is not an evidence-based indication for central line placement.”
In 2016, the updated Infusion Therapy Standards of Practice, removed pH < 5 as a criterion for requirement of central venous access.Reference Gorski, Hadaway, Hagle, McGoldrick, Orr and Doellman 27 In 2017, the Infusion Nurses Society (INS) published the results of a taskforce addressing vesicant medications; the following antimicrobials were removed from the INS vesicant list: amphotericin B, ampicillin, cloxacillin, doxycycline, gentamicin, metronidazole, oxacillin and penicillin.Reference Gorski, Stranz and Cook 28 Retained on the vesicant list as having intermediate risk are acyclovir, nafcillin, and vancomycin. However, the guidelines also note that each facility should develop a consensus on what is considered a vesicant based on organizational formularies and recognizes that a VAD choice should “generally not be based on a single factor, such as the medication or solution category of vesicant or irritant.”Reference Gorski, Stranz and Cook 28
Catheter-related infections are a significant burden on the healthcare system in patient morbidity, increased length of stay, and increased financial burden.Reference O’grady, Alexander and Burns 3 , Reference Maki, Kluger and Crnich 29 , 30 Incidence does appear to be decreasing, although >80,000 catheter-related line infections still occur each year in US ICUs, and as many as 250,000 such infections occur annually among hospitalized patients.Reference O’grady, Alexander and Burns 3 , Reference Simonov, Pittiruti, Rickard and Chopra 5 The incidence rate of catheter-related infection varies due to several different factors, including possible nonmodifiable patient characteristics, such as patients who are immunocompromised and/or have skin or mucosal membrane integrity breakdown due to medical conditions, trauma, and burns. Infusions with lipid formulations may also increase the potential risk of infection. The rate of infection changes based on location of insertion and duration of catheterization indwell time.Reference Bodenham, Babu and Bennett 1 , Reference O’grady, Alexander and Burns 3 , Reference Simonov, Pittiruti, Rickard and Chopra 5 One 2010 study showed the overall incidence density rate of catheter-related bloodstream infections to be 1.3 per 1,000 catheter days. 30 , Reference Fagan, Edwards, Park, Fridkin and Magill 31 The rate of CICCs has been shown to differ over time, with older studies demonstrating differences in catheter-related bloodstream infections based on anatomical site of insertion (comparing internal jugular, subclavian, and femoral veins); however, more recent data suggest no difference.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Marschall, Mermel and Fakih 8 , Reference Merrer, De Jonghe and Golliot 32 , Reference Marik, Flemmer and Harrison 33 Avoiding the femoral vein as a method to prevent infection, particularly among the obese, is still recommended.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Marschall, Mermel and Fakih 8 The effect of the type of CVC on catheter-related infections has been mixed, and multiple studies have shown that PICCs have similar rates of catheter-related infection as CICCs, particularly in hospitalized patients. Thus, PICC placement should not be used as a primary methodology to decrease CLABSI rates.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Marschall, Mermel and Fakih 8 , Reference Caparas and Hu 10 , Reference Nolan, Yadav, Cawcutt and Cartin-Ceba 34 , Reference Safdar and Maki 35 Catheter colonization is a risk for bloodstream infection that is time dependent; the longer the catheter remains in place, the higher the risk of colonization.Reference Raad, Costerton, Sabharwal, Sadlowski, Anaissie and Bodey 36 Catheterization time is thus critical because catheter hub colonization is associated with bacteremia.Reference Holroyd, Vasilopoulos, Rice, Rand and Fahy 37 Due to colonization risks, disinfection of catheter hubs is also recommended before accessing any catheter.Reference O’grady, Alexander and Burns 3 , Reference Marschall, Mermel and Fakih 8
Previous studies have indicated that the rate of infection with PIVs was 0.1%.Reference Maki, Kluger and Crnich 29 Current literature examining PIVs continue to show rates of 0.1%–0.2%.Reference Mermel 15 Midline catheters have a slightly higher incidence of bloodstream infection of ~0.2%–2.5%, although several recent evaluations of midline catheters have found varying results, including lower and similar rates of midline-related bloodstream infections as PIVs.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Adams, Little, Vinsant and Khandelwal 9 , Reference Caparas and Hu 10 , Reference Xu, Kingsley and DiNucci 19 – Reference Mushtaq, Navalkele and Kaur 21 Compared to PICCs and CICCs, however, midline catheter programs have resulted in decreased overall CLABSI rates.Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Moureau, Sigl and Hill 7 , Reference Mushtaq, Navalkele and Kaur 21
All catheters carry a potential risk of thrombosis, although the risk appears to vary by both catheter, anatomic location, and patient characteristics. Up to one-third of patients with CICCs may develop thrombosis, with potential increased risk with femoral and subclavian placements, although this is not consistent in all studies.Reference Bodenham, Babu and Bennett 1 , Reference Marik, Flemmer and Harrison 33 PICCs, compared to CICCs are associated with increased thrombosis in some studies, potentially at least in part due to the longer possible duration of use.Reference Bodenham, Babu and Bennett 1 A meta-analysis by Chopra et alReference Chopra, Anand and Hickner 38 placed the overall risk of PICC-related DVTs of 2.7%, which was significantly greater than for CICC.Reference Chopra, Anand and Hickner 38 This risk increased in critically ill patients and those with malignancies. Although the risk of catheter-related DVT increased with PICCs, the same study did not find and increased risk of pulmonary emboli with PICCs.Reference Chopra, Anand and Hickner 38 Some studies have indicated a correlation between diameter size of catheters and risk of thrombosis, but this has not been clearly defined by all studies.Reference Gonsalves, Eschelman, Sullivan, DuBois and Bonn 14 , Reference Grove and Pevec 39 Compared to PICCs, midline catheters have been reported to have lower overall rates of associated thrombosis in some, but not all, studies.Reference Adams, Little, Vinsant and Khandelwal 9 , Reference Mushtaq, Navalkele and Kaur 21
Patient dissatisfaction should also be considered a potential complication in today’s healthcare arena. Decreasing the number of needle sticks noted with catheters is a potential area for improved satisfaction.Reference Adams, Little, Vinsant and Khandelwal 9 A decreased rate of attempted PIV cannulations with implementation of a midline catheter program has been reported.Reference Moureau, Sigl and Hill 7 , Reference Adams, Little, Vinsant and Khandelwal 9 Patient satisfaction was directly affected by complications from central venous ports, and not the cosmetic appearance of the port.Reference Ignatov, Hoffman and Smith 40 Patients who receive PICC lines are more satisfied with a PICC if it is placed above the elbow than at the elbow.Reference Polak, Anderson, Hagspiel and Mungovan 41
How, in the face of the complexity of vascular access options and potential complications, can frontline clinicians make the best possible decisions for their patients?
Several opportunities exist for improved decision making; they include, but are not limited to, implementation and utilization of a multidisciplinary vascular access team, continued education and training regarding device choice and use, guidance through algorithms and policies, intelligent decision-support tools and ongoing quality improvement processes.
Vascular access as a specialty continues to evolve, with its own consensus statement on scope of practice from the national society (the Association for Vascular Access) speaking to the complexity of the practice from the time of device choice through removal of the catheter.Reference Davis, Owens and Thompson 2 Vascular access teams exist at many institutions to assist with decision making, to maximize efficiency, and to perform maintenance on many different catheter types and insertion sites. However, such teams are frequently only focused on the placement and maintenance of PIVs, midlines, and PICCs.Reference Chopra, Flanders and Saint 4 , Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Moureau, Sigl and Hill 7 , Reference Marschall, Mermel and Fakih 8
For organizations without a vascular access team, ongoing education regarding VADs and their inherent risks and benefits combined with procedural training on insertion, maintenance, and removal seems intuitive, yet with the changing landscape of device options, education remains of paramount importance.Reference O’grady, Alexander and Burns 3 , Reference Dariushnia, Wallace and Siddiqi 6 , Reference Marschall, Mermel and Fakih 8 Further opportunities include standardization of care through implementation of algorithms to provide guidance on VAD choice based on institutional guidelines and best practices (Figure 3).Reference Simonov, Pittiruti, Rickard and Chopra 5 , Reference Marschall, Mermel and Fakih 8 Implementation of a robust midline program has been associated with both decreased complications, specifically CLABSI, combined with decreased overall cost.Reference Moureau, Sigl and Hill 7 Finally, with the evolution of the electronic medical record and innovative technology in the healthcare sector, implementation of intelligent, integrated decision support for vascular-access decision making may be an appealing future option to help guide clinicians at the time they are placing an order.

Fig. 3. Nebraska Medicine vascular access algorithm.
In medicine, the dogmas of ‘do no harm’ and ‘the patient comes first’ echo throughout the halls of many of our institutions. Given that these principles remain of paramount importance when considering the best vascular access choice, discussion of options, benefits and risks, with the patient, when feasible, should always be undertaken.Reference Chopra, Flanders and Saint 4 Formally, this process is that of shared decision making and when combined with evidence-based medicine, provides the optimal opportunity for the best possible outcomes (Figure 4).Reference Hoffmann, Montori and Del Mar 42 Intuitively, we consider shared decision making to be aligned with the informed consent process, but they are not the same. Informed consent is obtained prior to placement of CVCs, but not routinely obtained for PIVs. Midline placement may or may not require consent, potentially varying between institutions. Not all guidelines or recommendations discuss patient preference in vascular access decision making, but overall satisfaction of patients may increase when they are involved in the discussion and adherence to maintenance recommendations may increase as well.Reference Chopra, Flanders and Saint 4 , Reference Moureau, Sigl and Hill 7 , Reference Hoffmann, Montori and Del Mar 42

Fig. 4. Diagram of shared decision making.
Finally, all vascular access programs should have ongoing quality assessment and improvement projects with target rates for complications to provide a benchmark for ongoing performance improvement.Reference Bodenham, Babu and Bennett 1 , Reference O’grady, Alexander and Burns 3 , Reference Dariushnia, Wallace and Siddiqi 6 , Reference Marschall, Mermel and Fakih 8 This may include ongoing educational updates, simulated training, competency assessment, and audits to assess compliance.
Choice isn’t everything: Best practices are needed for line insertion, maintenance, and removal
Optimal management of an intravascular device requires a multidisciplinary effort from the time the decision is made to place a device until after it is removed.Reference O’grady, Alexander and Burns 3 , Reference Marschall, Mermel and Fakih 8 Best practices for catheter insertion, securement, and maintenance of the catheter, dressing, and catheter connectors is crucial to preventing complications.Reference O’grady, Alexander and Burns 3 , Reference Chopra, Flanders and Saint 4 , Reference Marschall, Mermel and Fakih 8 Breaks in aseptic insertion technique raise particular concern for increased risk of infection; therefore, catheter change should be considered once the patient is stabilized. Appropriate maintenance of dressings to prevent infectious and mechanical complications is necessary, yet deficiencies and variation in both securement and dressing of all catheter types (PIVs to CVCs) may occur in ≥30% of hospitalized patients.Reference Rupp, Tandon, Danielson, Cavalieri and Sayles 17 , Reference Rupp, Cassling and Faber 43 Education, situational awareness, and skills competency for catheter maintenance among healthcare workers may improve the quality of care and thus prevent complications. Discontinuation of unnecessary intravascular catheters is the final critical point in providing the best possible vascular access care for patients.Reference Bodenham, Babu and Bennett 1 , Reference Chopra, Flanders and Saint 4 , Reference Marschall, Mermel and Fakih 8 Midline catheters have been used as a way to “de-escalate” from a CVC in some populations.Reference Caparas and Hu 10 Because midline catheters do not “count” as a CVC and infections associated with midlines are not reportable, we must resist the temptation to use midlines inappropriately or exchange one set of reportable complications for another set of unreportable complications. Prompt catheter removal may be complicated by lack of physician and team recognition that a CVC remains in place.Reference Chopra, Flanders and Saint 4 Therefore, catheter surveillance systems and clinician reminders should be in place to better ensure appropriate CVC use and removal.
Vascular access, with its dizzying array of potential device choices compounded with varying risks for complications, requires a systematic approach to optimize device choice for each patient based on clinical indications, anticipated duration of therapy, risks and benefits of each catheter and anatomic option, and finally, patient preference. Institutional development of algorithms or decision-support tools is recommended in conjunction with ongoing education, vascular access expertise, and continual process improvement. Improved vascular access decision making is a critical need, especially with evolving midline utilization, to decrease risks, complications, and costs while improving care and patient satisfaction.
Author ORCIDs
Kelly A. Cawcutt, 0000-0003-3586-0951
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Dr Cawcutt has participated in an advisory council meeting and as a sponsored speaker for Bard (now Becton Dickinson) in the past 36 months.
Dr Rupp is an advisory board member for Citius, has received grant support from XBioTech, and from from Contrafect, has served as a consultant for Bard/Teleflex and t for Ariste. None of the above roles carry direct conflict with content discussed within this manuscript. All other authors report no conflicts of interest relevant to this article.






