INTRODUCTION
Scuticociliatosis caused by histophagous opportunistic scuticociliates is an emerging problem in farmed flatfish including olive flounder Paralichthys olivaceus in Korea (Kim et al. 2004a,b; Jee, Kim and Park, 2001) and turbot Scophthalmus maximus in Europe (Dyková and Figueras, 1994; Sterud, Hansen and Mow, 2000; Iglesias et al. 2001). The scuticociliate species responsible for outbreaks in turbot farms in NW Spain has been identified on the basis of morphological and biometric characteristics as Philasterides dicentrarchi (Iglesias et al. 2001). Other authors working in other areas of Spain, Portugal and France have reported outbreaks due to scuticociliates that have similar morphology to species of the genera Philasterides and Miamiensis, but that show some differences from previously described species of these genera in both morphology and virulence, suggesting the existence of new species or strains (Álvarez-Pellitero et al. 2004). In several studies, light microscopical examination of silver-stained sections has raised questions about the taxonomy of the well-known scuticociliates (see Song and Wilbert, 2000).
There have been no comprehensive ultrastructural studies of P. dicentrarchi, though some ultrastructural findings have been reported for P. dicentrarchi in sea bass, Dicentrarchi labrax in the Mediterranean (Dragesco et al. 1995). Here, we report a detailed ultrastructural study of P. dicentrarchi from farmed turbot from NW Spain, with the aim of identifying morphological features useful for diagnosis. In addition, and in view of the existing uncertainty about the phylogenetic relations of the parasites responsible for scuticociliatosis in farmed flatfish, we have investigated relationships with other Philasterides species and with species of related genera (among others, Miamiensis, Entodiscus and Uronema) on the basis of comparison of the small-subunit rRNA gene sequence.
MATERIALS AND METHODS
Fish and experimental infections
Juvenile turbot Scophthalmus maximus L. (50–100 g) were obtained from a local farm in the north of Galicia (north-western Spain). Prior to experiments, the fish were acclimatized for at least 15 days in 10 litre tanks with a constant flow of water (18±1 °C, pH 6·5±0·5) and aeration. The fish received a standard semi-dried pelleted food daily. The ciliate Philasterides dicentrarchi was obtained from ascitic fluid of naturally infected turbot, as previously described (Paramá et al. 2003).
Ciliate isolation and culture
Ciliates were isolated by abdominocentesis under aseptic conditions from cultured turbot, and maintained in the laboratory in modified L-15 medium (Iglesias et al. 2003), with subsequent axenic culture at 18 °C in ‘complete’ L-15 medium (Leibovitz, 10‰ salinity, pH 7·2; Iglesias et al. 2003) containing 90 mg/l each of adenosine, cytidine and uridine, 150 mg/l of guanosine, 5 g/l of glucose, 400 mg/l of L-α-phosphatidylcholine, 200 mg/l of Tween 80, 10% heat-inactivated foetal bovine serum (FBS) and 10 ml/l of 100× antibiotic antimycotic solution (=100 units/ml of penicillin G, 0·1 mg/ml of streptomycin sulfate and 0·25 mg/ml of amphotericin B) (all from Sigma-Aldrich, USA).
Transmission electron microscopy
Ciliates in culture were collected by centrifugation at 1000 g for 5 min. Cells were fixed in 2·5% (v/v) glutaraldehyde in 0·1 M cacodylate buffer at pH 7·2. They were then washed several times with 0·1 M cacodylate buffer and post-fixed in 1% (w/v) OsO4, pre-stained in saturated aqueous uranyl acetate, dehydrated through a graded acetone series and embedded in Spurr's resin. Semi-thin sections were then cut with an ultratome (Reichart-Jung, Ultracut E, Austria) and stained with 1% toluidine blue for examination with a light microscope. Ultrathin sections were stained in alcoholic uranyl acetate and lead citrate and viewed in a Philips CM12 transmission electron microscope (Philips, Eindhoven, Netherlands) at an accelerating voltage of 80 kV.
Extraction of DNA
Cultured ciliates (106 cells/ml) were harvested by centrifugation at 1000 g for 5 min. The ciliates were washed twice with PBS and total DNA was extracted with DNeasy Tissue Kit (Qiagen, USA) following the protocols supplied by the supplier. Extracted DNA was stored at −20 °C until required for PCR.
PCR amplification, cloning and sequencing of the SSUrRNA gene
PCR amplifications were performed as previously described (Leiro et al. 2000). Briefly, a part of the gene coding the small-subunit ribosomal RNA (SSUrRNA) of P. dicentrarchi was amplified using forward primer 5′-GAGAAACGGCTACCACATCTA-3′ (PSSU1) and reverse primer 5′-CAGGTAAAGAGCCTACTCCA-3′ (PSSU2). PCR reaction mixtures (100 μl) contained reaction buffer (10 mM Tris-HCl, 50 mM KCl, 1·5 mMMgCl2, pH 9·0), 0·2 mM of each deoxynucleoside triphosphate (Roche), 0·4 mM of each primer, 3 units of rTaq polymerase (Roche) and 50 ng of genomic ciliate DNA as template. The reactions were run in an automatic Thermal Cycler GeneAmp PCR System 2400 (Perkin Elmer), initially at 95 °C for 5 min, then 35 cycles at 94 °C for 30 sec, 55 °C for 30 sec and 72 °C for 1 min. After completion of the 35 cycles, a 10-min extension phase at 72 °C was performed. PCR products (25 μl aliquots) were separated on a 1% agarose gel in TBE buffer, stained with ethidium bromide, and visualized under a variable-intensity 312 UV transilluminator (Spectroline, USA).
The PCR product obtained (350 bp) was purified by Microcon-PCR (Millipore, USA) and cloned in the pGEM-T Easy vector (Promega, USA) using the kit supplied by the manufacturer as previously described (Leiro et al. 2002). Briefly, after ligating the PCR fragment, the DH5α cells were transformed by electroporation in a Gene Pulser II (Bio-Rad, USA) using Gene Pulser®/E. coli Pulser™ cuvettes, with a 0·1 cm gap at a voltage of 1·8 kV, capacitance of 25 μF, and resistance of 2000 Ω. Transformed cells were selected on the basis of antibiotic sensitivity and α-complementation by culture on LB agar plates containing 100 μg/ml ampicillin, with 50 μl of a stock solution of 20 mg/ml of 5-bromo-4-chloro-3-indolyl-β-galactoside (X-Gal) and 20 μl of a 0·5 M solution of isopropylthio-β-d-galactoside (IPTG) spread over the surface: white colonies were amplified in LB overnight at 37 °C and plasmid DNA was purified using the Plasmid Mini Kit (Qiagen, Germany) following the manufacturer's instructions. To confirm the presence and size of the cloned fragment, we amplified it by PCR using primers SP6 and T7, which flank the region of insertion of plasmid pGEM-T Easy, under the following thermocycling conditions: 94 °C for 5 min, then 35 cycles each of 30 sec of denaturation at 94 °C, 30 sec of annealing at 42 °C, and 2 min of polymerization at 72 °C, with an additional 7 min at 72 °C at the end of the reaction. The PCR-amplified products were analysed by agarose gel electrophoresis to verify the presence of a single band of the correct size. The SSUrRNA gene fragment cloned in the pGEM-T Easy vector was sequenced in complementary directions using the Dye Terminator Cycle Sequencing kit (CEQ™ DTCS Kit, Beckman Coulter, USA) and loaded into the automatic CEQ™ 2000 DNA Analysis System (Beckman Coulter, USA).
Phylogenetic analyses
The sequences obtained for the SSUrRNA gene fragment of Philasterides dicentrarchi from Galician turbot farms were compared with equivalent sequences from diverse other related parasites (Table 1). The sequences were aligned using CLUSTAL W software (Higgins et al. 1994) and edited with the Jalview Multiple Alignment Editor V1.8. Sites containing gaps were excluded. Phylogenetic trees were constructed using MEGA version 3.1 (Kumar, Tamura and Nei, 2004), using the neighbour-joining (NJ) method applied to Kimura two-parameter distances. Confidence estimates were obtained on the basis of bootstrap generation of 1000 trees.

RESULTS
Morphology
The surface membrane system of P. dicentrarchi comprises 3 components: the outer (limiting) membrane (M), the outer alveolar membrane (OAM), and the inner alveolar membrane (IAM) (Fig. 1A). Within the OAM and IAM an alveolar space (A) of variable size can be clearly seen (Fig. 1A, E). The ciliary axoneme shows 9 doublets of peripheral microtubules and 1 central doublet (Fig. 1A, inset). Figure 1A also shows in longitudinal view a kinetosome forming part of the somatic ciliature, and from which arises a cilium in association with a parasomal sac. In toluidine blue-stained longitudinal sections, and in ultrathin sections, kinetia made up of dikinetids can be seen in the anterior two-thirds of the body (Fig. 1C, E). The microtubular system comprises subpellicular microtubules located directly beneath the cell surface (Fig. 1D) or associated with kinetosomes in the epiplasm and at the ends of the alveolar sacs (Fig. 1F).

Fig. 1. Transmission electron micrographs of Philasterides dicentrarchi. (A) Longitudinal section of cilium accompanied by parasomal sac (ps) and presence at the base of the cilium of a kinetosome (arrow). The surface membrane system can be seen, made up of outer membrane (M), outer alveolar membrane (OAM) and inner alveolar membrane (IAM), with the alveolar space (A) between the OAM and IAM. Inset: cross-section of axonemal 9×2+2-pattern. (B) Transverse section showing the parasomal sac (white arrowhead) between a pair of kinetosomes (black arrows). (C) Semi-thin longitudinal section of a fragment of P. dicentrarchi stained with toluidine blue. One of the kinetia can be seen, formed by 2 dikinetids (white arrowhead) in the anterior two-thirds of the body. In the anterior part oral cilia can be seen (arrow). (D) Longitudinal section of a row of subpellicular microtubules (arrowheads) arranged below the cortex. In the inside of the cell numerous free ribosomes can be seen. (E) Longitudinal section of a dikinetid (arrows). (F) Transverse microtubules located in epiplasm between the mitochondrion (M) and the kinetosome with which they are associated (arrowhead). RER: rough endoplasmic reticulum.
The globular or somatic macronucleus contains chromatin granules dispersed throughout the nucleoplasm, with granular nucleoli located towards the periphery of the macronucleus (Fig. 2A, B). The micronucleus or generative nucleus is closely associated with the macronucleus, and is located close to one of the latter's nuclear pores (Fig. 2C). The micronucleus contains compact chromatin, located a short distance from the double nuclear membrane, which is punctuated by nuclear pores (Fig. 2C).

Fig. 2. (A) Ultrathin section showing the macronucleus (Ma) and micronucleus (Mi) of Philasterides dicentrarchi. Nucleoli (arrowheads) can be seen at the periphery of the macronucleus, while chromatin granules are dispersed throughout this structure. The micronucleus shows the most compact chromatin. In this microphotograph, no connection between the 2 nuclei is observed. (B) Semi-thin transverse section stained with toluidine blue, showing nucleoli (arrowheads) at the periphery of the macronucleus. (C) Nuclear pores in the micronucleus (black arrowhead) and macronucleus (arrow). (D) Rough endoplasmic reticulum (arrowhead) can be seen below the cortical mitochondria (M). (E) Small, stacked Golgi-like saccules (arrow) in the internal part of the ciliate body. (F) The mitochondria (M) are located between the kinetosomes (K). Microtubules in a small protuberance (black arrowhead) can be seen in association with the kinetosomes. In the macronucleus peripheral nucleoli (arrow) and chromatin granules (asterisk) can be seen.
Mitochondria occur beneath the cell cortex, parallel to the cortical surface (Figs. 2D, F), between the kinetosomes (Fig. 2F); only rarely were mitochondria seen in the interior of the cytoplasm. Rough endoplasmic reticulum was generally located in close proximity to mitochondria (Fig. 2D). Structures similar to small dictysomes, possibly forming part of the Golgi apparatus, were also seen close to mitochondria (Fig. 2E).
The extrusomes of P. dicentrarchi show 2 different morphologies: spherical and fusiform (Fig. 3A, B). The spherical extrusomes, located below the cell surface and in direct contact with the plasma membrane, are surrounded by a membrane and have an amorphous content with a diameter of about 1·1 μm (Fig. 3A, C). Fusion of the spherical extrusomes with the plasma membrane and release of their contents to the exterior gives rise to a thin mucilaginous layer over the cell surface (Fig. 3C). The fusiform extrusomes have a pouch-like morphology and length of about 1·6 μm, are arranged perpendicular to the plasma membrane, and have compact content (Fig. 3B).

Fig. 3. (A) Longitudinal section of Philasterides dicentrarchi showing digestive vacuoles (asterisk) and oral microtubules (black arrow) in the anterior part of the body. Extrusomes can be seen at the cell surface, interspersed between mitochondria (black arrowheads). At the bottom of the figure, exocytosis of a spherical extrusome can be seen (double arrow). (B) Detail of fusiform extrusomes (arrows) in the posterior part of the body, showing electron-dense content; 2 of the extrusomes are fused with the cell membrane, and the third is located internally. (C) Exocytosis of a spherical extrusome, showing fusion of the extrusome with the cell membrane. (D) Longitudinal section of the kinetosomes of an oral polykinety, showing the associated vesicles that form part of the cytostome (arrow).
Phylogenetic analysis
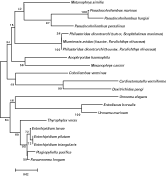
Nested PCR of DNA extracted from the turbot P. dicentrarchi isolate using the primers PSSU1 and PSSU2 amplified a product of 350 bp. Sequence analysis confirmed the P. dicentrarchi product to be 18 S rDNA, corresponding to nucleotides 386–734 of the published P. dicentrarchi small-subunit ribosomal RNA (SSUrRNA) (GenBank Accession number AY642280), and differing from this sequence at positions 649, 660, 678 and 705. The 350-bp fragment of the P. dicentrarchi sequence was aligned with SSUrRNA gene sequences from other Philasterida species in the BLAST database, and a phylogenetic tree was generated with the NJ algorithm. The identity between the SSUrRNA sequence of P. dicentrarchi isolated from turbot and other sequences ranged from 85 to 99% (Table 1). The highest nucleotide identities were seen with species isolated from olive flounder Paralichthys olivaceus, namely Miamiensis avidus (99%) and P. dicentrarchi (98%). The lowest nucleotide identities were seen with Entodiscus borealis (85%) and Uronema marinum (86%) (Table 1). The optimal-topology tree obtained by the neighbour-joining method is shown in Fig. 4. As can be seen, the analysis places the turbot P. dicentrarchi isolate in a branch with the olive flounder M. avidus and P. dicentrarchi isolates, with a bootstrap value of 100%.

Fig. 4. Phylogenetic relationships of diverse species of the order Philasterida, as inferred by analysis of SSUrRNA gene sequences using the neighbour-joining method. Bootstrap values for 1000 replicates are indicated at each node. The scale bar indicates a distance of 0·02.
DISCUSSION
In P. dicentrarchi, the outer membrane (limiting membrane) does not appear to be continuous with the ciliary membrane, as occurs in Ichthyophthirius (Chapman and Kern, 1983). In some ciliates (e.g. Paramecium) transverse connections or septa have been described (Allen, 1971); however, no such structures were observed in our P. dicentrarchi specimens. In the anterior two-thirds of the body dikinetids were present, as reported previously by Iglesias et al. (2001). The presence of dikinetids in the body ciliature is one of the characteristics of the subclass Scuticociliatia, by contrast with the monokinetids generally seen in the subclass Hymenostomatia (Hausmann and Hülsmann, 1996).
Philasterides dicentrarchi has parasomal sacs distributed mainly around the base of the somatic cilia, though sometimes around the oral ciliature. It has been suggested that the parasomal sacs may play a role in endocytosis (Orias and Rasmussen, 1976), pinocytosis (Hausmann and Hülsmann, 1996), or they may simply be small invaginations of the plasma membrane (Allen, 1971). The presence of subpellicular microtubules located beneath the cell surface and associated with kinetosomes is a characteristic shared by various non-scuticociliate ciliates, such as Tetrahymena (Fleury, 1991; Fleury and Laurent, 1994) and the location of the microtubules in the epiplasm are different from those described in Uronema spp. (Kaneshiro and Holz, 1976).
The macronucleus and micronucleus of P. dicentrarchi are morphologically similar to those of Uronema (Kaneshiro and Holz, 1976) and are located in the central part of the body (Iglesias et al. 2001). As in Uronema, the macronucleus shows chromatin granules dispersed throughout the nucleoplasm, while granular nucleoli are seen in the periphery (Kaneshiro and Holz, 1976). The micronucleus, like that of other scuticociliatids and hymenostomatids, is closely associated with the macronucleus (Kaneshiro and Holz, 1976; Gorovski, 1973). In spite of this proximity, we have not detected connections between both nuclei. This finding contrasts with those reported for Uronema and Tetrahymena (Gorovski, 1973; Kaneshiro and Holz, 1976). In addition, we have not observed nucleoli in the micronucleus which are likewise absent in Uronema (Kaneshiro and Holz, 1976).
Philasterides dicentrarchi, like Tetrahymena, has numerous mitochondria located below the cell cortex and parallel to the body surface (Elliot and Bak, 1964). By contrast, Uronema species show a single large mitochondrion occupying most of the subpellicular area (Kaneshiro and Holz, 1976). Mitochondria in P. dicentrarchi are generally associated with rough endoplasmic reticulum, and we also observed structures compatible with small dictyosomes similar to those found in Tetrahymena thermophila (Kurz and Tiedtke, 1993).
Philasterides dicentrarchi has spherical extrusomes, denominated mucocysts by Dragesco et al. (1995), as well as fusiform extrusomes located close to the plasma membrane. It should be noted that the term mucocyst has not been very clearly defined, and these structures are interpreted in different ways by several authors (Hausmann, 1978; Kugrens, Lee and Corliss, 1994). Extrusion vesicles that cannot be readily classified as mucocysts on the basis of shape and/or content are generally considered to be cortical organelles. Thus the term mucocyst (implying the presence of mucosubstances) should be used with caution (Tokuyasu and Scherbaum, 1965). In the present study, spherical extrusomes of P. dicentrarchi were seen to fuse with the plasma membrane and release their content to the exterior. This release, according to some authors, may be induced by a mechanical or chemical stimulus (Rosati and Mondeo, 2003); however, other authors have suggested that the release may be provoked by the glutaraldehyde fixative used (Tokuyasu and Scherbaum, 1965). In addition, in Tetrahymena pyriformis it has been reported that the morphology and content of both types of extrusomes are variable: specifically, the extrusomes may show fusiform morphology before release and spherical morphology during release (Tokuyasu and Scherbaum, 1965). Similar changes may occur in P. dicentrarchi, since we only observed spherical mucocysts (never fusiform mucocysts) releasing their content to the exterior.
On the basis of the morphological characteristics of wet-mounted and stained specimens (cell shape, somatic kinety number, paroral membrane and membranelle morphology, and position of the contractile vacuole pore), Kim et al. (2004a) identified as P. dicentrarchi a ciliate obtained from systemically infected olive flounder Paralichthys olivaceus in Korea. The same authors used PCR to amplify the SSUrRNA gene using two universal oligonucleotide SSUrRNA primers (denominated U 1F and U 1R), obtaining a 1760-bp amplicon that was deposited in the GenBank database with Accession number AY642280. On the basis of this sequence, in the present study we designed 2 nested primers that amplified a 350-bp DNA fragment from our turbot P. dicentrarchi isolates, enabling phylogenetic comparison with sequences previously reported for related species. The partial nucleotide sequence obtained showed high identity (98%) with the corresponding sequence of the Philasterides species isolated from olive flounder (Kim et al. 2004a), and even higher identity (99%) with the sequence of Miamiensis avidus (GenBank Accession number AY550080), likewise isolated from olive flounder showing severe ulceration and haemorrhage in skeletal muscle (Jung et al. 2005). The morphological description of M. avidus given by Jung et al. (2005) basically coincides with published descriptions of P. dicentrarchi from turbot (Iglesias et al. 2001), from sea bass (Dragesco et al. 1995) and from olive flounder (Kim et al. 2004a). The molecular phylogenetic analysis confirms that M. avidus from olive flounder is closely related to P. dicentrarchi. These results agree with previous morphological studies in which both organisms were considered synonyms (Song and Wilbert, 2000). On the other hand, these results also indicate that P. dicentrarchi is not closely related to other scuticociliate genera that have been implicated in scuticociliatosis in fish culture, notably Uronema and Pseudocohnilembus (Cheung, Nigrelli and Ruggieri, 1980; Munday et al. 1997; Sterud et al. 2000; Jee et al. 2001).
This work was supported by grants AGL2003-04644 from the Comisión Interministerial de Ciencia y Tecnología (CICYT), and PGDIT02RMA23701PR from the Xunta de Galicia, Spain.