CHDs are the most common major birth defects worldwide, affecting 6 to 8 children per 1000 live births with 3 per 1000 requiring surgical or catheter-based intervention early in life.Reference Hoffman and Kaplan1,Reference Dolk, Loane and Garne2 Earlier diagnostics, improved treatment, and perioperative care have resulted in higher survival ratesReference Gilboa, Salemi, Nembhard, Fixler and Correa3–Reference Oster, Lee, Honein, Riehle-Colarusso, Shin and Correa5 with more adults than children living with CHD today.Reference Bhatt, Foster and Kuehl6,Reference Dearani, Connolly, Martinez, Fontanet and Webb7 The focus on clinical outcome has therefore shifted from early mortality to long-term morbidity. In recent years, studies have shown that children born with CHD have increased risk of neurodevelopmental deficiencies (such as deficits in language development, social skills, executive functioning, and motor skills)Reference Cassidy, White, DeMaso, Newburger and Bellinger8–Reference Wernovsky14 compared to the background population, and that these neurodevelopmental deficiencies persist in adolescence and adulthood with an increase in psychiatric diagnoses, lower educational achievements, and reduced work participation.Reference Udholm, Nyboe, Dantoft, Jørgensen, Rask and Hjortdal15–Reference Nyboe, Fonager, Larsen, Andreasen, Lundbye-Christensen and Hjortdal17 It is estimated that 33–43% of children born with a major CHD will suffer from impaired neurocognitive development,Reference Bellinger, Wypij and Rivkin9,Reference Khalil, Suff, Thilaganathan, Hurrell, Cooper and Carvalho18 thus making neurodevelopmental deficiencies the most common long-term morbidity in these patients.Reference Gaynor, Stopp and Wypij19 While the association between CHD and educational achievement in Danish adolescents has been studied by Olsen et al, 2011Reference Olsen, Hjortdal, Mortensen, Christensen, Sørensen and Pedersen16, a systematic early neurocognitive blinded follow-up study in Danish children with and without isolated CHD has, to our knowledge, yet to be performed. Early screenings and monitoring for neurodevelopmental deficiencies in this patient group will help facilitate proper and early intervention and support for both child and family and hereby give the child the best possible chances for optimal neurodevelopment.Reference Asschenfeldt, Evald and Heiberg20,Reference Peyvandi, Chau and Guo21
According to the American Heart AssociationReference Marino, Lipkin and Newburger22 and recently the Cardiac Neurodevelopmental Outcome Collaborative,Reference Ware, Butcher and Latal23 early detection of neurodevelopmental deficiencies is of utter importance to the child’s further development and educational achievements. To our knowledge, a neurocognitive screening programme for this patient group is yet to be established in Denmark.
The aim of this cohort study was to investigate the early neurocognitive development in 15 Danish children born with isolated CHD compared with 27 Danish contemporary and age-equivalent children without CHD at 18 and 36 months using the Bayley Scales of Infant and Toddler Development (3rd edition)Reference Bayley24 according to age and the Ages and Stages Questionnaire (3rd edition)Reference Squires25 according to age and 6 months above age. The latter was used in order to discover if above-age challenges would be more sensitive to differences between the groups.Reference Plomgaard, Hansen and Greisen26
Materials and methods
Study design and recruitment
This study is an ongoing prospective cohort study including fetal and neonatal MRIReference Lauridsen, Uldbjerg and Henriksen27,Reference Skotting, Eskildsen and Ovesen28 as well as neurodevelopmental follow-up. The study is taking place at Aarhus University Hospital, Denmark. We here present the neurodevelopmental follow-up using the Bayley Scales of Infant and Toddler Development (3rd edition) and the Ages and Stages Questionnaire (3rd edition) on the same cohort of children with and without CHD.
Initial cohort
Recruitment took place before birth. Women expecting fetuses with and without isolated heart defects were recruited at Aarhus University Hospital between October 2014 and June 2016. The women expecting children with CHD were approached by the obstetric physician at the time of prenatal diagnosis. The women expecting fetuses without heart defects were approached randomly at the first or second routine, free of charge, prenatal ultrasound scan offered all pregnant women in Denmark at approximately gestational age week 12 and 20. The initial cohort consisted of 16 singleton fetuses with isolated CHDs believed to cause disturbances in the flow of oxygenated blood to the fetal brain and 40 singleton fetuses without CHDs. Exclusion criteria in both groups were multiple gestation, chromosomal, genetic, or multiple abnormalities, language other than Danish, social matters, claustrophobia, and birth prior to intrauterine MRI. The women underwent fetal MRI twice during pregnancy.Reference Lauridsen, Uldbjerg and Henriksen27
Study size and final cohort
After birth, both parents were asked to give written informed consent to continue participation and include their newborn in the present postnatal follow-up study. One child with CHD died before follow-up (see Fig 1) and 13 of the 40 children without CHD declined to participate. Thus, loss to follow-up was 6% among those with CHD and 33% among those without. For the demographic data see Table 1, for CHD types and age of surgery see Table 2.

Figure 1. Flowchart illustrating the recruitment of fetuses with heart defects. n indicates the number of children.
Table 1. Maternal and newborn characteristics in 15 pregnancies with isolated fetal heart defects and 27 pregnancies without fetal heart defects
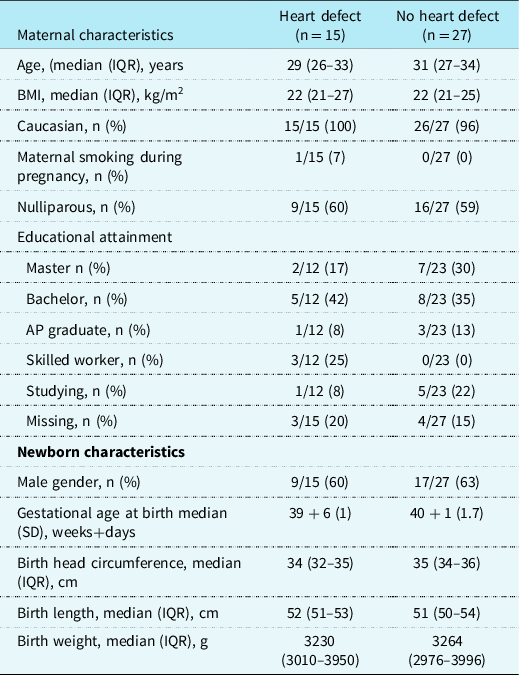
Maternal and newborn characteristics in 15 children with heart defects and 27 children without heart defects, Aarhus Denmark 2014–2016. Data are presented as median (interquartile range) or n (%) or mean (SD). n indicates number. Despite extensive search, we did not have information on educational attainment from all participants
Table 2. CHD and surgery characteristics in 15 children with CHD

CHD and surgery characteristics in 15 children with heart defects, Aarhus Denmark 2014–2016. Data are presented as n (%) or median (interquartile range). n = number; TGA = transposition of the great arteries; VSD = ventricular septal defect
Twelve of the 15 children with CHD underwent prenatal genetic testing; karyotype (n = 2) and array-based comparative genomic hybridization (n = 11 – including one, who had already had a karyotype and one including a single-nucleotide polymorphism analyses). Only one minor abnormality of no clinical significance was detected. The remaining three children with CHD and the 27 children without CHD did not undergo genetic evaluation; however, none of them showed any clinical signs of genetic, chromosomal, or syndromic disorders.
One mother expecting a child without CHD was treated for pregnancy hypertension.
Quantitative variables and the tests
After birth, information on birth weight, birth length, head circumference, placenta weight, and gestational age were collected (see Table 1).
The follow-up presented in the present study includes the Bayley Scales of Infant and Toddler Development 3rd edition assessments (designed to asses developmental functioning of children aged 1 to 42 months through structured play)Reference Bayley24 at 18 and 36 months of age. The children were tested in the three domains: cognitive, language, and motor. As far as it was possible, the therapists were blinded to whether the child had CHD or not. The administration of the tests followed manual guidelines with optimal test conditions in place. At 18 and 36 months, the parents also completed the Ages and Stages Questionnaire 3rd edition.Reference Squires25
The Ages and Stages Questionnaire is a self-administered (parents) 30-item screening questionnaire related to child development from 1 month to 5 years of age. The items cover five domains of development: communication, gross and fine motor skills, problem-solving, and personal-social issues.Reference Squires25
When Ages and Stages Questionnaire is used as a screening tool, children who perform better-than-average will score near maximum. This results in an underestimation of the mean. To get a normal distribution of the scores, the children were therefore given questionnaires meant for a 6-month-older child.Reference Plomgaard, Hansen and Greisen26
Reference values of both the Ages and Stages Questionnaire and the Bayley Scales of Infant and Toddler Development assessment are based on the performance of American children.Reference Bayley24,Reference Squires25 In the Bayley Scales 3rd edition, 9.8% of the normative sample were clinical cases.Reference Bayley24 Results were included if the child participated in at least one of the planned examinations.
Statistics
From each of the three domains in the Bayley Scales of Infant and Toddler Development test, a composite raw score was achieved, and this raw score was converted to a scale score according to normative data disclosed in the Bayley Scales manual.Reference Bayley24 The mean scale score is 100, and one standard deviation corresponds to 15 scale points.Reference Bayley24 For statistical analysis purposes, Van Chau and colleagues (2013)Reference Chau, Synnes, Grunau, Poskitt, Brant and Miller29 suggested a novel classification of the scale score into four scale score categories given previous findings that the Bayley-III seems to overestimate neurodevelopmentReference Acton, Biggs and Creighton30: normal (>100), low normal (85–100), mild impairment (70–84), and significant impairment (<70).
From each of the five domains in the Ages and Stages Questionnaire, a score was achieved and the corresponding z-scores were calculated according to normative data disclosed in the manual.Reference Squires25
We used Student’s t-test and z-test to calculate the mean difference between the results of the Bayley Scales assessments and the Ages and Stages Questionnaire presented with 95% confidence intervals (CIs).
Ethics
The study was approved by the Danish Data Protection Agency (chart: 1–16–02–86–14) and by the National Committee on Health Research, Denmark (chart: 1–10–72–61–14). The protocol of the project conforms to the ethical standards of the Helsinki Declaration revised in 2008.
Results
The final cohort consisted of 42 children, 15 with isolated CHD and 27 without. Table 1 shows maternal and newborn characteristics and Table 2 shows CHD and surgery details.
At the mean age of 18 months and 8 days (standard deviation: 21 days) and at the mean age of 36 months and 28 days (standard deviation: 46 days), respectively, a physiotherapist and an occupational therapist performed the Bayley Scales of Infant and Toddler Development (3rd edition) assessments at Aarhus University Hospital. The neurodevelopmental test results are presented in Tables 3 and 4.
Table 3. The Bayley Scales of Infant and Toddler Development and the Ages and Stages Questionnaire test results at, respectively, 18 months and 36 months in 15 children with isolated CHD and 27 without CHD
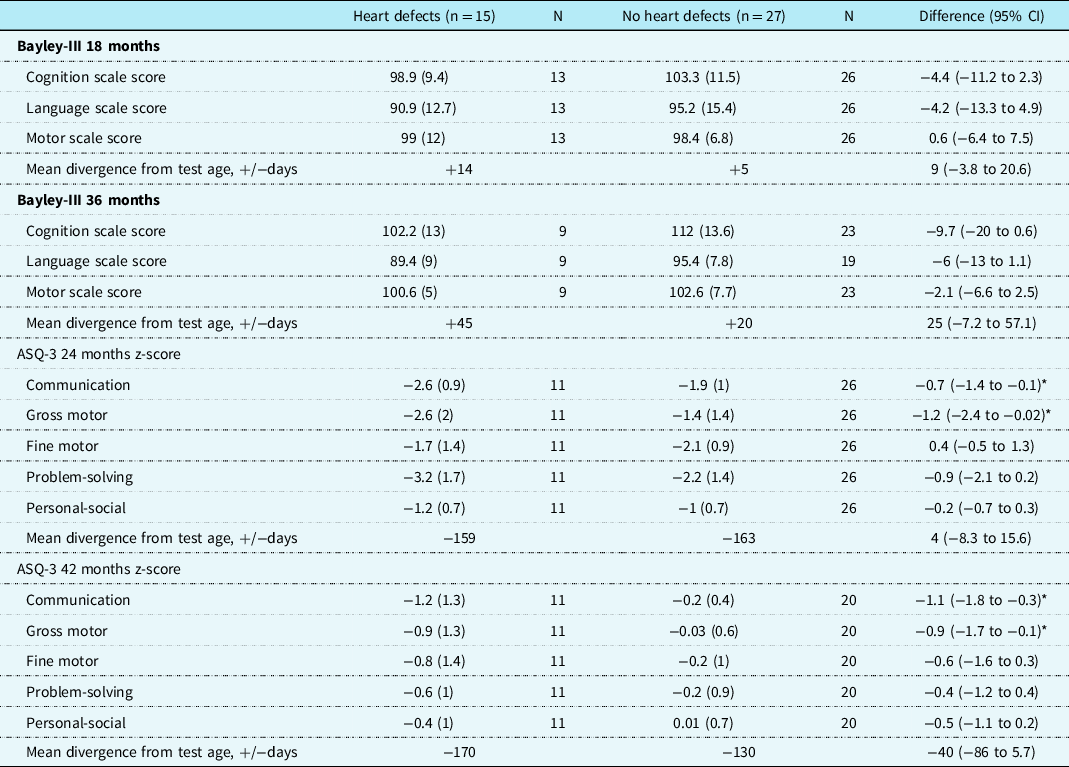
Data are presented as mean (SD). Measures were compared by Student’s t-test and z-test. CI indicates 95% confidence interval; Bayley-III, the Bayley Scales of Infant and Toddler Development-Third Edition; ASQ-3, the Ages and Stages Questionnaires Third Edition; n = the number of children in each group; N = the number of measurements in each group.
* Statistical significance at p < 0.05
Table 4. The Bayley Scales of Infant and Toddler Development composite scale scores descriptive classification relative risk at 18 and 36 months in 15 children with CHD
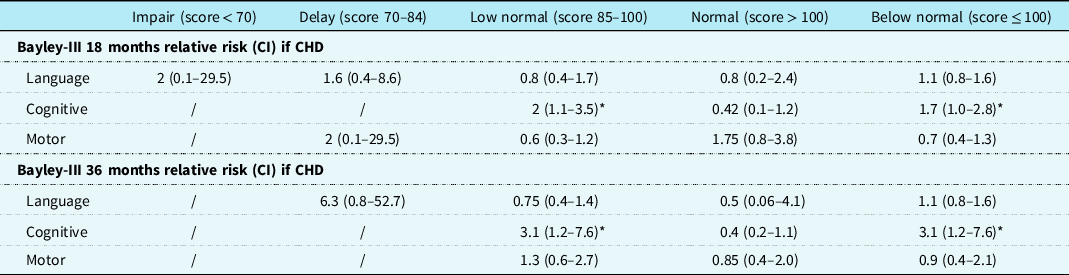
Data are presented as relative risk (CI). Bayley-III indicates the Bayley Scales of Infant and Toddler Development-Third Edition; CI = confidence interval.
* Statistical significance at p < 0.05
In the Bayley Scales of Infant and Toddler Development assessments at 18 and 36 months, we were not able to detect performance difference between the children with and without CHD when comparing mean scale scores in the different categories.
Table 4 shows the relative risk (CI) of scoring normal (>100), low normal (85–100), mild impairment (70–84), or significant impairment (<70).Reference Chau, Synnes, Grunau, Poskitt, Brant and Miller29 Additionally, we calculated the risk of scoring below normal (≤100) compared to normal. Children with CHD had, compared with the children without CHD, an increased risk of scoring below normal in the Bayley Scales cognition category at 18 and 36 months; relative risk 1.7 (95% CI: 1.0–2.8) and 3.1 (CI: 1.2–7.5), respectively. See Fig 2 for a histogram of the distribution of the children.
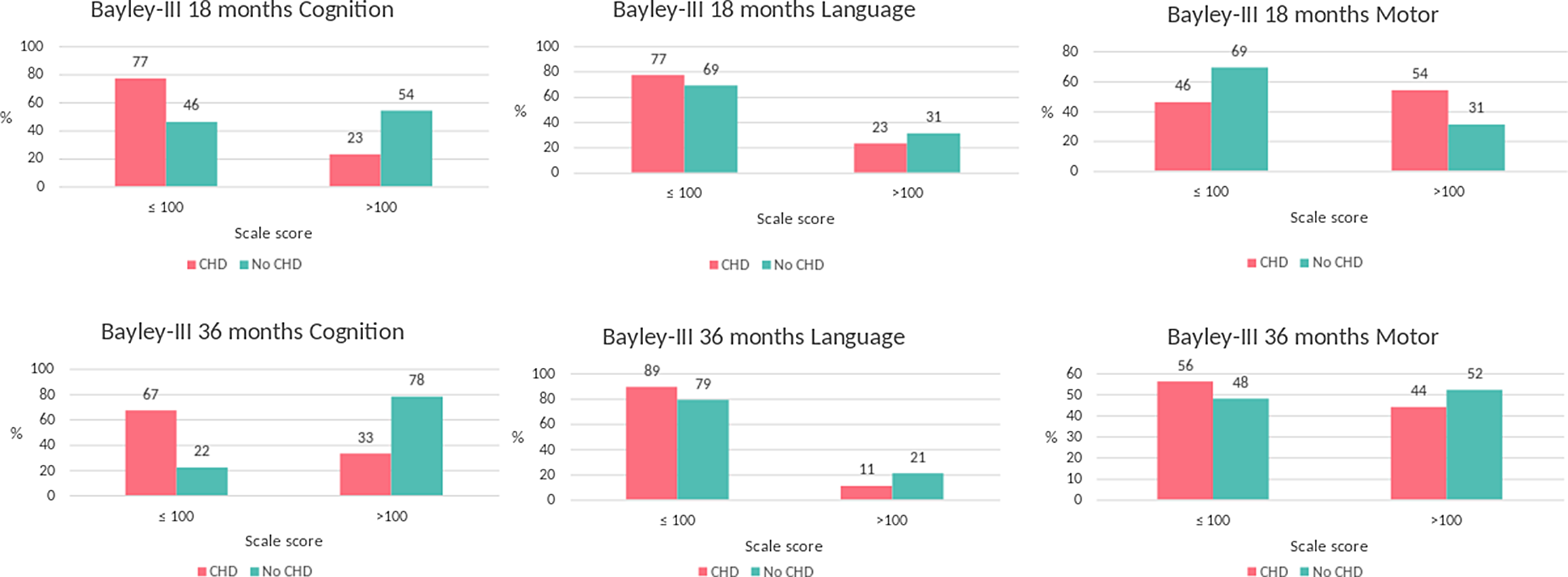
Figure 2. The Bayley Scales of Infant and Toddler Development scale scores descriptive classifications at 18 and 36 months in 15 children with CHD and 27 without CHD. Bayley-III indicates the Bayley Scales of Infant and Toddler Development-Third Edition.
In both the Bayley Scales assessments, the children with CHD were on average a little older than the children without CHD: +9 days at the 18-month and +25 days at the 36-month assessment.
At 18 and 36 months, the parents completed each time two sets of the Ages and Stages Questionnaire (3rd edition). The Ages and Stages Questionnaire according to age (see Supplementary Table S1) showed that the children with CHD achieved lower communication scores at 36 months of age. When challenged with the milestones of a 6-month-older child, these performance differences persisted as those with CHD achieved lower scores than the children without CHD in the communication and gross motor categories at both follow-ups; communication mean z-score difference −0.72 (CI: −1.4; −0.1) and −1.06 (CI: −1.8; −0.3) and gross motor; mean z-score difference: −0.87 (CI: −1.7; −0.1) and −1.22 (CI: −2.4; −0.02), respectively (see Table 3 for details).
Discussion
Key results
The children with major isolated CHD had an up to three times higher risk of scoring below normal in the Bayley Scales of Infant and Toddler Development (3rd edition) cognition category.
Additionally, children with CHD achieved lower scores than their age-equivalent peers in the Ages and Stages Questionnaire (3rd edition) communication and gross motor categories, and this performance difference persisted over time.
Interpretation
Our findings indicates that children with CHD have cognitive challenges. In addition, the parents find that the children with CHD are challenged in the communication and gross motor categories compared to their age-equivalent peers. And the neurodevelopmental challenges in children with CHD persist over time. We may only see the tip of the iceberg in our cohort. The significance of the neurodevelopmental deficiencies may increase when the children are growing and experience increasing demands of skills and capabilities. Their skills might be sufficient at 36 months, but these children are at risk of lacking behind when starting school and the complexity of the educational curriculum increases.Reference Olsen, Hjortdal, Mortensen, Christensen, Sørensen and Pedersen16,Reference Asschenfeldt, Evald and Heiberg20,Reference Majnemer, Limperopoulos, Shevell, Rosenblatt, Rohlicek and Tchervenkov31,Reference Bellinger, Wypij and duPlessis32 Brosig et al, 2018Reference Brosig, Bear and Allen33 found that many of their patients who scored in the average range at 2 years of age showed deficits at 4 years of age.
In the present study, we demonstrate that motor and communication deviations are detectable using the Ages and Stages Questionnaire already in early childhood in line with previous research,Reference Gaynor, Stopp and Wypij19,Reference Latal34–Reference Brandlistuen, Stene-Larsen, Holmstrom, Landolt, Eskedal and Vollrath37 and that the children with CHD have a higher risk of scoring below normal in the Bayley Scales cognition category at 18 and 36 months.
The Ages and Stages Questionnaire (3rd edition) has been tested in different high-risk patient groupsReference Skellern, Rogers and O’Callaghan38–Reference Lindsay, Healy, Colditz and Lingwood40 and has been validated in different countries, languages, and settings.Reference Richter and Janson41–Reference Elbers, Macnab, McLeod and Gagnon43 In the CHD patient group, Noeder et al, 2017Reference Noeder, Logan and Struemph44 found the Ages and Stages Questionnaire to be a valid screening tool, especially in the gross motor category where they found excellent sensitivity.
We found that only the Ages and Stages Questionnaire could detect performance differences between the two groups when comparing mean scale scores in the different categories. For some of the children with isolated CHD, where the parent’s response to the Ages and Stages Questionnaire indicated potential neurodevelopmental delay, we found no definite signs of delay in the Bayley Scales assessments. However, these children may still represent a group at-risk for future neurodevelopmental deficiencies,Reference Schonhaut, Armijo, Schonstedt, Alvarez and Cordero45 and this is supported by the fact that the children with CHD had an up to three times increased risk of scoring below normal in the Bayley Scales 3rd edition cognition category. Borderline intellectual functioning has been connected to limitations in social and behavioral capacities in childhood.Reference Blasi, Pirastru and Cabinio46,Reference Baglio, Blasi and Intra47
It is possible that we, with a larger study cohort, would have been able to detect more differences between the children with and without CHD.
The Bayley Scales of Infant and Toddler Development is by many considered the gold standard of infant developmental assessment and is among the most frequently used standardised instruments for assessing early neurodevelopment in preschool children. However, the Bayley Scales of Infant and Toddler Development 3rd editionReference Bayley24 were published in 2006, and several studies have later argued that the 3rd edition underestimates delays by overestimating the children’s developmentReference Chau, Synnes, Grunau, Poskitt, Brant and Miller29,Reference Acton, Biggs and Creighton30,Reference Long, Galea, Eldridge and Harris48–Reference Johnson, Moore and Marlow53 and when using the Bayley Scales to describe neurodevelopment in early preschool years, caution is warranted.Reference Ware, Butcher and Latal23 This and our use of an Ages and Stages Questionnaire designed for a 6-month-older child might offer an explanation to why we observe more children at risk using Ages and Stages Questionnaire than with the Bayley Scales of Infant and Toddler Development 3rd edition. It is likely that an overestimation of the children’s abilities will affect the poorest performers the most, thus making the children with neurodevelopmental challenges appear to perform better than they actually do.Reference Goldstone, Baiocchi and Wypij50 We have tried to accommodate this overestimation by using the categories suggested by Van Chau and colleagues.Reference Chau, Synnes, Grunau, Poskitt, Brant and Miller29
Limitations
An important limitation is the small group sizes and the heterogenicity of the CHDs included in the study and hence a big difference in the complexity of the surgeries the children with CHD have undergone. The fact that the children without CHD are more than a month older than those with CHD at the time of the late Ages and Stages Questionnaire assessment is also a limitation that probably leads to an overestimation of the difference between the groups.
Strengths
A major strength in our study is that we within the same time period have included and examined age-equivalent peers without CHD. Because of our use of a reference group, confounding variables known to affect neurocognitive development such as, for example, birth weight, gestation age, and maternal education can be minimised. The children with CHD and the reference group are very similar in regard to maternal characteristics and hence socio-economic status. Another big strength worth mentioning is the exclusion of children with both syndromic CHD and genetic abnormalities, since many genetic syndromes are connected with impaired neurocognitive development, and the inclusion of these children may therefore distort the results. We have included children with transposition of the great arteries, but also children with coarctation/hypoplastic arch not so often included in follow-up studies,Reference Cassidy, White, DeMaso, Newburger and Bellinger8–Reference Calderon, Angeard, Pinabiaux, Bonnet and Jambaque11,Reference Peyvandi, Chau and Guo21,Reference Hiraiwa, Ibuki and Tanaka54 and we still find differences between the children with and without CHD indicative of neurodevelopmental deficiencies. This indicates that probably all children with CHD would benefit from systematic follow-up in order to initiate relevant support to the families. The occupational and physiotherapist were blinded as far as possible to whether or not the child had a CHD while assessing the children, and this also strengthen the results of this study. Furthermore, the children in this study have undergone a comprehensive multidimensional neurodevelopmental assessment comprising of both parental reports, direct child testing, and clinical observations as recommended by the Cardiac Neurodevelopmental Outcome Collaborative.Reference Ware, Butcher and Latal23
Generalisability
Both the guidelines from the American Heart AssociationReference Marino, Lipkin and Newburger22 and from the Cardiac Neurodevelopmental Outcome CollaborativeReference Ware, Butcher and Latal23 state the importance of screening and continuously monitoring all children born with CHD for neurodevelopmental impairment. Not just children born with major CHD, but the entire cohort of children and adolescents with CHD. In Denmark, such screening has yet to be established. Based on the results in this present study and in previous studies, we strongly recommend that an extensive screening and monitoring programme is to be established in Denmark, to give these children and families the support they need in order for the children to reach their full potential.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121002195
Acknowledgements
The authors thank the children and families who participated in this study. We would also like to thank Niels Uldbjerg, MD, DMSc1, Olav Bjørn Pedersen, MD2, PhD, and Camilla Linddahl, MD1, for the help with recruitment. 1Department of Gynecology and Obstetrics, Aarhus University Hospital, Aarhus, Denmark, and 2Department of Gynecology and Obstetrics, Copenhagen University Hospital, Denmark.
Financial support
This study received financial support from the Danish Children’s Heart Foundation (Børnehjertefonden) (M.H.L., grant number 100,000 DKK) and Frimodt Heineke Fonden (M.H.L., grant number 50,000 DKK).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (the National Committee on Health Research, Denmark and the Danish Data Protection Agency), and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (the National Committee on Health Research, Denmark).









