Introduction
Peanut allergy is the most common food allergy in children, causing substantial morbidity, mortality, and impaired quality of life.Reference Soller, Ben-Shoshan and Harrington1–Reference Bock, Muoz-Furlong and Sampson4 As there is no cure for peanut allergy, it is critically important to develop effective primary prevention strategies.
Recently, the landmark Learning Early About Peanut (LEAP) trialReference Du, Sayre and Roberts5 showed that early peanut introduction (commencing from 4 to 11 months) helped to prevent peanut allergy in high-risk children, prompting worldwide changes in infant feeding guidelines.Reference Togias, Cooper and Acebal6,Reference Fleischer, Sicherer and Greenhawt7 Consistent with LEAP, we observed that peanut introduction before 12 months was associated with a lower incidence of peanut sensitization at 12 months in the general population CHILD cohort.Reference Tran, Lefebvre and Dai8 However, neither report accounted for maternal peanut consumption nor breastfeeding at the time of peanut introduction. Current guidelines recommend no restriction of maternal peanut ingestion but stop short of recommending consumption due to insufficient evidence.Reference Togias, Cooper and Acebal6,Reference Fleischer, Sicherer and Greenhawt7,Reference Chan, Cummings and Atkinson9–Reference Fleischer, Spergel, Assa’ad and Pongracic11
The impact of breastfeeding on allergy development has been widely studied, with inconsistent resultsReference Lodge, Tan and Lau12,Reference Järvinen, Martin and Oyoshi13 – perhaps due to undocumented differences in maternal diet, breast milk composition, or complementary feeding practices. These are relevant to consider because maternal peanut ingestion could provide oral peanut exposure through lactation.Reference Bernard, Ah-Leung and Drumare14–Reference Schocker, Baumert and Kull16 Also, given the immunomodulatory activity of breast milk cytokines, immunoglobulins, and other bioactives,Reference Munblit, Peroni and Boix-Amoros17,Reference Dawod and Marshall18 the timing of peanut introduction relative to breastfeeding cessation may be important. Indeed, a protective effect of breastfeeding while introducing cereals has been found for celiac disease,Reference Persson, Ivarsson and Hernell19,Reference Ivarsson, Hernell, Stenlund and Persson20 and conflicting reports have suggested that breastfeeding while introducing cow’s milk has a protective effectReference Grimshaw, Maskell and Oliver21 or no effectReference Venter, Maslin, Dean and Arshad22 against cow’s milk allergy. However, to our knowledge, no studies have examined this potential interaction in the context of peanut allergy or sensitization.
Our secondary analysis of the 1995 Canadian Asthma Primary Prevention Study (CAPPS) (N = 342)Reference Pitt, Becker and Chan-Yeung23 suggests that, in high-risk children with immediate family history of asthma or two first-degree relatives with IgE-mediated allergic disease, early peanut introduction combined with breastfeeding and maternal peanut consumption is most effective for preventing sensitization. However, that study had insufficient power to examine the timing of peanut introduction relative to breastfeeding cessation, and findings could not necessarily be extrapolated to the general population. In the current study, using data from the general population CHILD birth cohort (N = 2759), we extend our previous workReference Tran, Lefebvre and Dai8 to examine the role of breastfeeding and maternal peanut consumption in the development of peanut sensitization through 5 years of age.
Methods
Study population
Pregnant women were enrolled in the observational CHILD Cohort Study (www.childstudy.ca) between 2008 and 2012Reference Subbarao, Anand and Becker24. Notably, this period precedes the new recommendations for early peanut introduction.Reference Togias, Cooper and Acebal6,Reference Fleischer, Sicherer and Greenhawt7 This general population cohort was recruited from the general population in Vancouver, Edmonton, Manitoba (Winnipeg and Morden/Winkler), and Toronto; 3455 singleton infants born >35 weeks gestation were eligible at birth and commenced the study. For the current analyses, children in the vanguard cohort (n = 191) or missing data on breastfeeding, peanut introduction, or peanut sensitization (n = 505) were excluded, leaving 2759 for analysis (Fig. S1). This study was approved by the Human Research Ethics Board at McMaster University and ethics committees at the Hospital for Sick Children, and the Universities of Manitoba, Alberta, and British Columbia.
Breastfeeding and timing of peanut introduction
Breastfeeding and peanut introduction were determined from questionnaires at 3, 6, 12, 18, and 24 months.Reference Tran, Lefebvre and Dai8,Reference Azad, Vehling and Chan25 Mothers reported when they stopped breastfeeding (infant’s age in weeks or months) and first introduced peanut (not given, <3, 3–4, 5–6, >6–9, >9–12, or >12 months). To address the potential interaction between timing of peanut introduction and breastfeeding, children were classified into three mutually exclusive groups: “Introduced early with breastfeeding” (introduced peanut to the infant before 12 months and was breastfeeding at the time of introduction), “Introduced early without breastfeeding” (introduced peanut to the infant before 12 months but was not breastfeeding at the time of introduction), or “Avoided >12 months” (infant did not receive peanut before 12 months).
Because breastfeeding duration was reported with more precision (in continuous weeks or months) than peanut introduction (in time windows), it was not possible to determine whether peanut introduction occurred with or without breastfeeding if weaning occurred during the time window of peanut introduction (n = 155). We conservatively classified children as “Introduced early with breastfeeding” only if their breastfeeding duration matched or exceeded the upper range of the peanut introduction window. This definition ensures that children in this group were still breastfeeding when peanut was introduced, with possible misclassification in the “Introduced early without breastfeeding” group. In a sensitivity analysis, we took the opposite approach and required that breastfeeding duration matched or exceeded the lower range of the peanut introduction window.
Maternal peanut consumption
Maternal diet was reported by food frequency questionnaire (FFQ) during mid-pregnancy.Reference Subbarao, Anand and Becker24 We assumed similar peanut intake during pregnancy and lactation, as reported by Frazier et al. Reference Frazier, Camargo, Malspeis, Willett and Young26 and observed in our own data (although the FFQ was not repeated, women were asked if they reduced or increased their intake of any specific foods during pregnancy, and only 2.5% reported modifying their peanut intake). Peanut consumption was determined from two questions asking about dietary intake of “peanuts and other nuts and seeds” and “peanut butter.” Options for intake ranged from “never or <1 per month” to “2+ per day.” For this analysis, we dichotomized consumption as “Regular” (at least once per week) versus “Never or infrequent” (all other groups). In a sensitivity analysis, we excluded mothers (n = 330) with unclear peanut intake (i.e., those who regularly consumed “peanuts and other nuts” but never consumed peanut butter).
Peanut sensitization and allergy
Sensitization was determined by forearm skin prick testing at ages 1, 3, and 5 years,Reference Tran, Lefebvre and Dai8 using a panel of inhalant and food antigens, including peanut (ALK Abello, Mississauga, ON, Canada) and Duotip-Test II devices (Lincoln Diagnostics Inc., Decatur, IL, USA). A positive skin test was defined as a mean wheal diameter ≥2 mm larger than the negative control (or ≥3 mm in a sensitivity analysis). In a sensitivity analysis, we evaluated probable clinical IgE-mediated peanut allergy at 3 years, defined as: sensitization to peanut, not consuming peanut at least once per month, and having a convincing history of signs or symptoms of an allergic reaction to peanut.Reference Simons, Balshaw and Lefebvre27 Sensitization trajectories were ascertained among children tested at all three ages (N = 2185), defined as: never sensitized, transient sensitization (sensitized at 1 and/or 3, but not 5 years), persistent sensitization (sensitized at 1 and/or 3, and 5 years), or late-onset sensitization (only at 5 years). Maternal atopy was defined as a positive skin test to peanut or any of 13 inhalant allergens.
Statistical analysis
Associations were evaluated using penalized logistic Firth regression because of the small number of sensitized children in some groups. Peanut sensitization was investigated cross-sectionally at each age and longitudinally in the form of sensitization trajectories. Crude and adjusted ORs are presented, with adjusted ratios controlling for maternal atopy and study center. These covariates were chosen as potential confounders because they were associated with infant feeding practices and peanut sensitization. We formally tested the interaction between breastfeeding and maternal peanut consumption and explored this interaction in stratified analyses. To address potential reverse causality, we performed a sensitivity analysis excluding infants with a parent-reported diagnosis of atopic dermatitis before 6 months. Analyses were performed using R software (v.3.5.3) and SAS software (v.9.4).
Results
Overall, 74% of mothers were White, 77% had a postsecondary education, and 58% were atopic (Table S1); 4.3% of mothers were sensitized to peanut and 2.3% reported a peanut allergy. The prevalence of peanut sensitization among children was 4.7% (126/2708) at 1 year, 4.1% (100/2460) at 3 years, and 4.1% (96/2328) at 5 years (Table 1).
Table 1. Exposure and outcome frequencies: timing of peanut introduction, breastfeeding, and peanut sensitization in the CHILD cohort (N = 2759)

Timing of peanut introduction was reported according to the time windows listed here. Breastfeeding cessation was reported more precisely, in weeks (before 3 months) or months (after 3 months).
* For 155 children, where breastfeeding cessation occurred within the time window of peanut introduction, we cannot be certain which occurred first. To be conservative, in the main analysis, we assumed these children were NOT breastfed at the time of peanut introduction. In a sensitivity analysis (Table S7), we assumed the opposite.
** Mutually exclusive, among children with skin test data at 1, 3, and 5 years; early = age 1 and/or 3 years, late = age 5 years.
Peanut introduction and breastfeeding
Nearly, all dyads (98%) initiated breastfeeding, and the median duration of any breastfeeding was 11 months. Introduction of peanut was rare before 6 months (n = 48/2759, 1.7%), and still relatively uncommon by 12 months (n = 992/2759, 36.0%) (Table 1). Of those who consumed peanut in the first year, about half (545/992, 54.9%) were breastfeeding at the time of peanut introduction. For subsequent analyses, infant peanut introduction was classified as: introduced early with breastfeeding (n = 545, 19.8%), introduced early without breastfeeding (n = 447, 16.2%), or avoided beyond 12 months, regardless of breastfeeding status (n = 1767, 64.0%).
Association of peanut introduction, breastfeeding, and peanut sensitization
As we have previously reported,Reference Tran, Lefebvre and Dai8 peanut introduction before 12 months was associated with a lower odds of peanut sensitization at 1 year. Here, we found that this protective association persisted at 3 and 5 years, especially among infants who were breastfed at the time of peanut introduction (Fig. 1 and Table S2). At 1 year, the prevalence of sensitization was 101/1734 (5.8%) among infants who avoided peanut beyond 12 months. Regardless of breastfeeding, sensitization was significantly less likely among infants consuming peanut, with 10/437 (2.3%) sensitized among those introduced to peanut early without breastfeeding (aOR 0.39; 95% CI 0.19–0.72), and 15/537 (2.8%) sensitized among those introduced to peanut early while breastfeeding (aOR 0.47; 95% CI 0.26–0.79). Over time, the prevalence of sensitization remained relatively constant in the avoidance group (5.8% at 1 year, 5.2% at 3 years, and 5.8% at 5 years) and was consistently lower among those who received peanut before 12 months without breastfeeding (2.3%, 2.5%, and 1.9%). Notably, beginning at 3 years and especially at 5 years, the prevalence of sensitization was further reduced among children who had been introduced to peanut while breastfeeding. In this group, there was a progressive decline in sensitization prevalence over time (2.8%, 1.6%, and 0.6%) such that by 5 years of age, the odds of peanut sensitization were 88% lower than in the avoidance group (aOR 0.12, 95% CI 0.03–0.31). These associations were essentially unchanged with adjustment for duration of breastfeeding or age at peanut introduction (Table S3). Peanut sensitization was not directly associated with various other measures of breastfeeding (Table S4). Together, these results indicate that the combination of early peanut introduction while breastfeeding is important in this context.
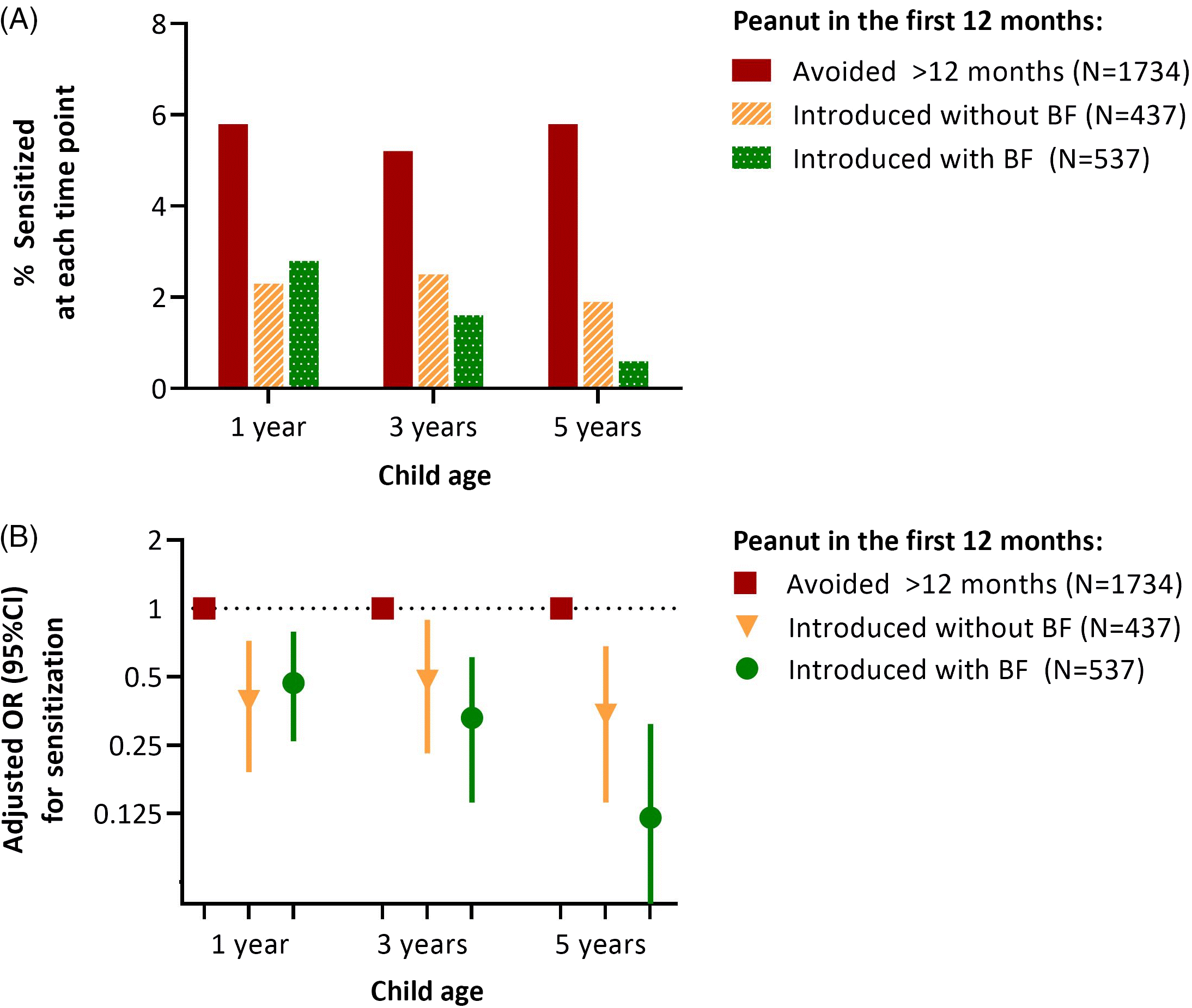
Fig. 1. (A) Frequency and (B) adjusted odds of peanut sensitization at 1, 3, and 5 years in the CHILD cohort, according to breastfeeding status and timing of peanut introduction. BF, breastfeeding (at the time of peanut introduction). Associations are adjusted for maternal atopy and study site. Sensitization was determined by skin prick testing; a mean wheal diameter ≥2 mm was considered positive. The numbers of children assessed at 1, 3, and 5 years were 2708, 2460, and 2328, respectively.
Trajectories of sensitization
Because the apparent effect of introducing peanut while breastfeeding seemed to strengthen over time, we explored different trajectories of peanut sensitization among children who were skin-tested at all three ages (1, 3, and 5 years; Table 1). Overall, 48 children (2.1%) were persistently sensitized at 1 and/or 3 years (“early”) and 5 years (“late”). A similar proportion experienced late-onset sensitization (n = 33, 1.5%) or were transiently sensitized only at the early time point(s) (n = 57, 2.6%). Thus, among the 105 children who were sensitized early, about half (57/105, 54%) “outgrew” their sensitization and tested negative at 5 years of age. Although we had limited power to study these trajectories of peanut sensitization, we observed two interesting trends (Table 2). First, among infants who were sensitized early, those who avoided peanut in the first year were less likely to outgrow their sensitization by 5 years (36/83; 43% resolution) compared to those who received peanut before 12 months (21/22; 96% resolution; regardless of breastfeeding). Second, among infants who were not sensitized early, late-onset sensitization was most common among infants who avoided peanut for the first year (26/1299, 2.0%), less common in those fed peanut early without breastfeeding (5/345, 1.4%), and least common in those fed peanut early while breastfeeding (2/436, 0.5%).
Table 2. Peanut sensitization trajectories from age 1 to 5 years according to timing of peanut introduction and breastfeeding (N = 2185)

Values are n (%) or n/N (%).
* Early = age 1 and/or 3 years; late = age 5 years.
N = 2185 children with skin test data for peanut at 1, 3, and 5 years.
Impact of maternal peanut consumption
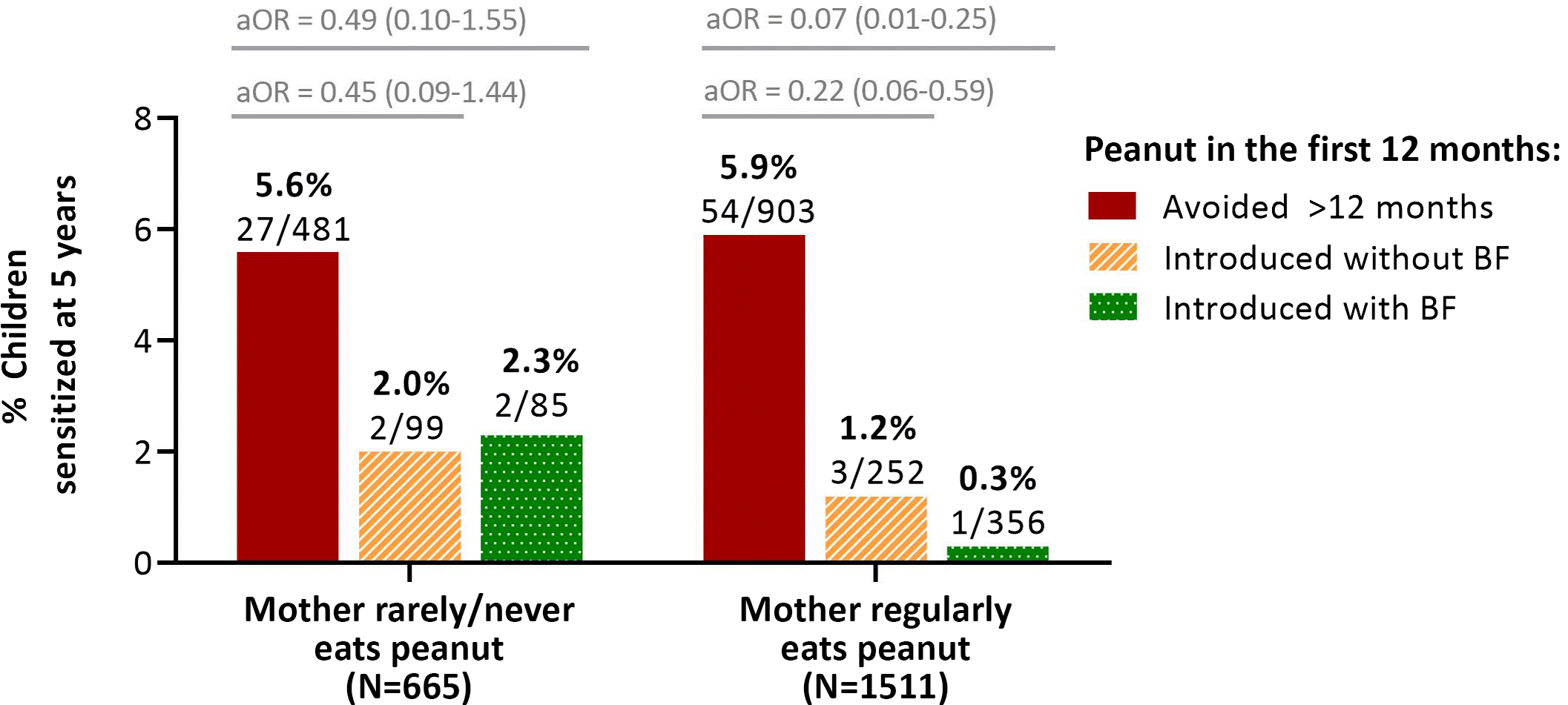
Finally, we assessed maternal peanut consumption. About two-thirds (69%) of mothers reported regularly consuming peanut butter or “peanuts, other nuts or seeds” (Table 1). A possible interaction was detected between maternal peanut consumption and breastfeeding at the time of peanut introduction (p for interaction = 0.059), and stratified analyses showed that these exposures were most beneficial in combination (Fig. 2, Table S5). Among infants who did not receive peanut in the first year, the prevalence of sensitization at 5 years of age was similar whether or not their mothers consumed peanuts (5.9% and 5.6% sensitization with and without regular maternal peanut consumption, respectively). Maternal peanut consumption made only a modest difference among infants who received peanut early without breastfeeding (1.2% and 2.0% sensitization with and without maternal peanut consumption, respectively). However, among infants fed peanut early while breastfeeding, the prevalence of sensitization was substantially reduced with maternal peanut consumption (0.3% and 2.3% sensitization with and without maternal peanut consumption, respectively). Similarly, the enhanced protection from breastfeeding at the time of peanut introduction only occurred among mothers who regularly consumed peanuts (0.3% and 1.2% sensitization following early peanut introduction with and without breastfeeding); this enhanced benefit was not observed among mothers who never or rarely ate peanut (2.3% and 2.0% sensitization following early peanut introduction with and without breastfeeding) (Fig. 2).

Fig. 2. Frequency of peanut sensitization at 5 years in the CHILD cohort, according to breastfeeding status and timing of introduction, stratified on frequency of maternal peanut consumption. BF, breastfeeding (at the time of peanut introduction). Adjusted for maternal atopy and study site. “Regularly” = at least once per week.
Together, our findings suggest that early peanut introduction is beneficial regardless of maternal peanut consumption, and that breastfeeding provides additional benefit if the mother is regularly consuming peanut. Neither breastfeeding nor maternal peanut consumption were associated with peanut sensitization on their own (i.e., without accounting for each other and the timing of peanut introduction) (Table S4), emphasizing the importance of these three exposures in combination.
Sensitivity analyses
Results were similar in several sensitivity analyses using a less conservative definition to classify breastfeeding status at the time of peanut introduction, using an alternative cutoff (≥3 mm wheal) for sensitization, and excluding mothers with peanut allergy or infants with atopic dermatitis diagnosed before 6 months (Table S6). Results were also similar using probable peanut allergy as an alternative outcome to sensitization at 3 years (Table S2). Results for maternal peanut consumption were consistent after excluding mothers with unclear peanut consumption due to the questionnaire wording of “peanuts and other nuts” (Table S7).
Discussion
In the general population CHILD cohort, we found that peanut introduction before 12 months was associated with a 50%–70% lower risk of peanut sensitization throughout early childhood. At 1 year, this association appeared to be independent of breastfeeding. However, by 5 years, the risk was further reduced if the infant was breastfed at the time of early peanut introduction and the mother regularly consumed peanuts. Longitudinal analyses revealed that these associations were driven by higher odds of outgrowing early sensitization and lower odds of late-onset sensitization. Together, these findings highlight the value of longitudinal studies in evaluating trajectories of allergy development, and identify breastfeeding as an important focus for allergy prevention efforts.
Taken together and building on previous evidence, our results support a “triple exposure” hypothesis (Fig. 3): first, breastfeeding facilitates transmission of immunomodulatory factors in mother’s milk (Exposure 1). If the mother is regularly consuming peanut, breastfeeding also provides oral exposure to peanut antigens or peanut immune complexes in breast milk, beginning at birth and throughout lactation (Exposure 2), which could “prime” the developing immune system for direct peanut oral exposure later in infancy. Peanut exposure may also occur in utero and/or through postnatal environmental exposures when the mother is regularly consuming peanut; these pathways were not studied in our analysis but warrant further investigation. Finally, peanut introduction during infancy (Exposure 3) promotes peanut tolerance regardless of breastfeeding; however, this effect is enhanced through breastfeeding when the mother regularly consumes peanut. We found no direct effect of breastfeeding in the absence of maternal peanut consumption and early peanut introduction, suggesting that all three exposures are required for optimal development of tolerance and prevention of peanut sensitization.
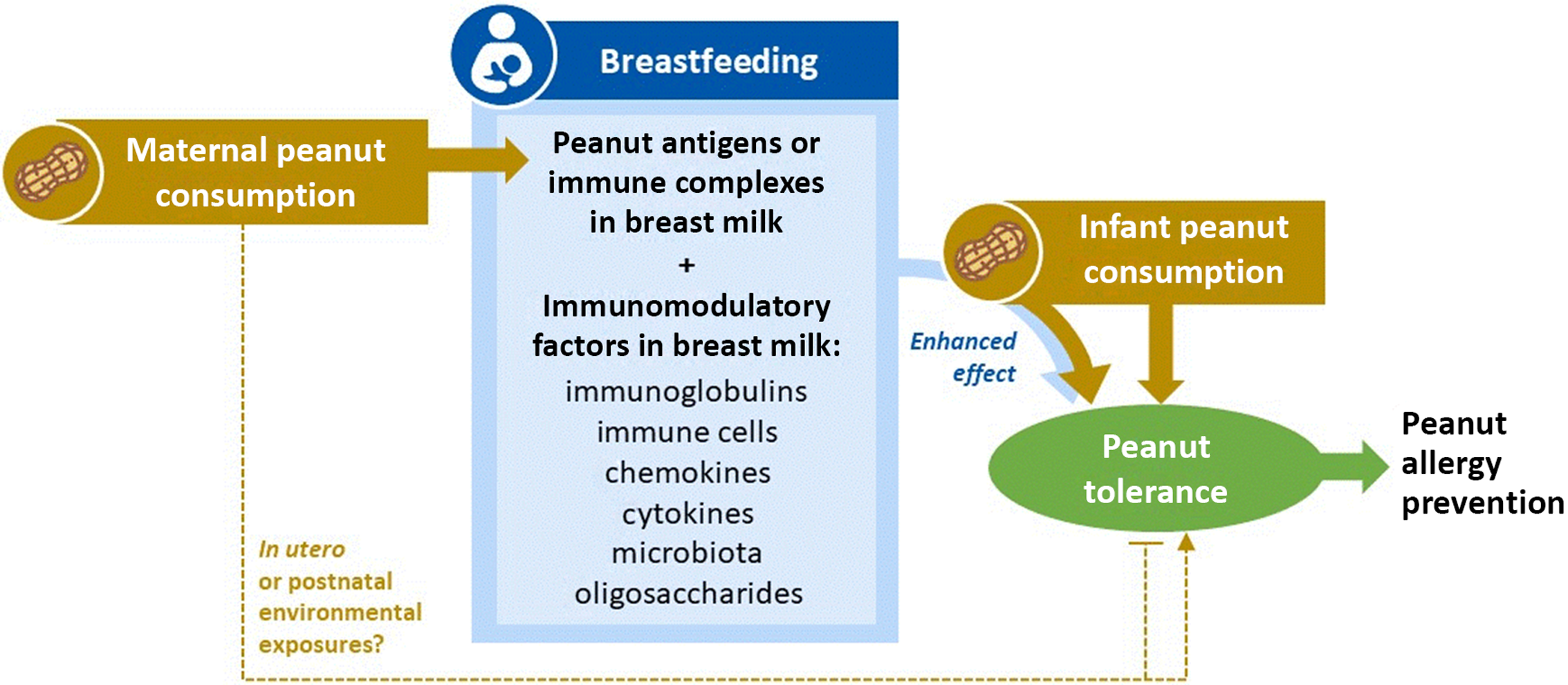
Fig. 3. Triple exposure hypothesis for the combined effects of maternal peanut consumption, breastfeeding, and infant peanut consumption in the prevention of peanut allergy. Breastfeeding (Exposure 1) facilitates exposure to immunomodulatory factors in mother’s milk. If the mother is regularly consuming peanut (Exposure 2), breastfeeding further provides oral exposure to peanut antigens, beginning at birth and throughout lactation. Peanut exposure may also occur in utero and/or through postnatal environmental exposures when the mother is regularly consuming peanut, which could promote or prevent tolerance. Peanut introduction during infancy (Exposure 3) promotes peanut tolerance in all children, regardless of breastfeeding; however, this effect can be further enhanced through breastfeeding when the mother regularly consumes peanut. There is no direct effect of breastfeeding (1) in the absence of maternal peanut consumption (2) and early peanut introduction (3). All three exposures are required for optimal development of tolerance and prevention of peanut allergy. Further research is needed to refine and test this hypothesis.
Our findings could help explain why previous studies have failed to demonstrate a clear impact of breastfeeding on food allergy development. In a systematic review, Lodge et al. Reference Lodge, Tan and Lau12 found no consistent evidence of this association; however, none of the included studies assessed the interaction of breastfeeding with maternal diet or timing of allergenic food introduction. Consistent with Lodge et al., we found that breastfeeding was not directly associated with peanut sensitization when these factors were ignored. However, when we accounted for them, significant associations emerged: breastfeeding was associated with less peanut sensitization if the mother regularly consumed peanuts and introduced peanut to her infant before 12 months.
Further, our results may help explain the mixed evidence for maternal peanut consumption and peanut allergy in offspring. A case-control study by DesRoches et al. (N = 403 infants <18 months with or without peanut allergy diagnosis)Reference Desroches, Infante-Rivard, Paradis, Paradis and Haddad28 identified maternal peanut consumption as a risk factor for peanut allergy, and another study by Sicherer et al. (N = 503 high-risk infants aged 3–15 months)Reference Sicherer, Wood and Stablein29 found that maternal peanut consumption during pregnancy (although not during lactation) was associated with higher peanut IgE in offspring. However, these retrospective studies were limited by potential recall bias. By contrast, in the prospective general population GUTS2 cohort (N = 8205 children followed to age 13 years), Frazier et al. found that maternal peri-pregnancy peanut or tree nut consumption was protective against physician-diagnosed peanut or tree nut allergy,Reference Frazier, Camargo, Malspeis, Willett and Young26 although this was not replicated for peanut allergy by Lack et al. in the general population ALSPAC cohort (N = 12,090 children followed to age 38 months).Reference Lack, Fox, Northstone and Golding30 However, both cohorts were established in the early 1990s when peanut avoidance was common, and neither study assessed the potential interaction between maternal peanut consumption, breastfeeding, and the timing of peanut introduction to offspring. To our knowledge, ours is the first study to evaluate these exposures simultaneously, finding that all three are relevant in combination.
Our findings underscore the value of utilizing prospective, longitudinal cohorts to explore how breastfeeding impacts food sensitization and may contribute to food allergy prevention efforts. The importance of breastfeeding at the time of peanut introduction was not apparent at the 1-year assessment; this interaction emerged at 3 years and strengthened further by 5 years, suggesting a “programming” effect on the immune system that persists long after the period of exposure during infancy. A similar phenomenon was recently reported for egg allergy, where breastfed children had a lower risk of egg allergy at 2.5 years (but not at 1 year), if their mother’s milk contained detectable levels of ovalbumin.Reference Verhasselt, Genuneit and Metcalfe31 Further, while the overall proportion of sensitized children was relatively constant over time, trajectory analyses revealed that it was not uncommon for individual children to outgrow an early sensitization or develop sensitization later in childhood. Continued follow-up will be required to assess the clinical relevance of these early trajectories later in life, and further research is needed to explore underlying biological mechanisms.
It is important to note that while the optimal scenario appears to be “triple exposure” to maternal peanut consumption, breastfeeding, and early peanut introduction, there was no scenario in which early peanut introduction appeared “harmful.” That is, even among mothers who did not consume peanut and infants who were not breastfed at the time of peanut introduction, early peanut introduction was associated with lower risk of sensitization. Thus, early peanut introduction (before 12 months) appears beneficial regardless of maternal diet or breastfeeding, but these benefits may be enhanced by breastfeeding and maternal peanut consumption. This implies that clinical recommendations for early peanut introduction need not depend on breastfeeding status or maternal diet but suggest that advising mothers to breastfeed and eat peanuts during this critical period could provide additional benefits beyond those provided by the current guidelines for early peanut introduction.
Strengths and limitations
The major strength of our study is the prospective design and repeated objective skin testing in a large general population birth cohort. A limitation of conducting this secondary analysis in a general population cohort is that we had relatively few cases of sensitization, limiting our statistical power in multivariable models to assess confounders. Further, 58% of the mothers in this study were atopic, which may have influenced both their breastfeeding practices and decisions around the timing of peanut introduction. Another important limitation is that we evaluated peanut sensitization rather than peanut allergy; however, it is well established that sensitization is a clinically important marker of immune dysregulation.Reference Brockow, Zutavern and Hoffmann32–Reference Illi, von Mutius and Lau34 Since relatively few infants received peanut before 6 months of age, we could not assess the impact of introduction during this time window, and since nearly all dyads initiated breastfeeding, we could not study infants who were never breastfed. Finally, we did not document the frequency of infant peanut consumption, and we estimated maternal peanut consumption during lactation from a FFQ completed during pregnancy. However, we believe this approach was reasonable because only 2.5% of CHILD mothers reported modifying their peanut intake during pregnancy. We may have overestimated maternal peanut consumption because peanuts were not always distinguished from other nuts; however, our results were consistent in a sensitivity analysis excluding mothers where peanut intake was unclear. Finally, our study is observational and should be considered hypothesis-generating. Experimental studies are needed to test these hypotheses and inform clinical recommendations.
Conclusion
Our findings support current guidelines recommending early peanut introduction for allergy prevention and contribute new evidence that maternal peanut consumption and breastfeeding could further enhance this protective effect. Clinical and mechanistic studies are warranted to test this “triple exposure” hypothesis and characterize the underlying mechanisms. The results of such studies will have important implications for clinical practice, maternal and infant feeding recommendations, and the development of supplements or therapeutics for mothers who cannot safely consume peanut or infants who cannot be breastfed.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174420001129
Acknowledgments
We are grateful to all the families who took part in this study, and the whole CHILD team, which includes interviewers, nurses, physicians, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists. We also thank Michelle La and John Schellenberg (University of Manitoba) for assistance with the literature review and formatting.
Financial Support
The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment Network of Centres of Excellence (AllerGen NCE) provided core support for the CHILD Study. This research was specifically funded by a CIHR Project Grant #156,155. This research was supported, in part, by the Canada Research Chairs program. MA holds the Tier 2 Canada Research Chair in Developmental Origins of Chronic Disease and is a CIFAR Fellow in the Humans and the Microbiome Program. SET holds the Tier 1 Canada Research Chair in Pediatric Precision Health.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation in Canada and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the Human Research Ethics Board at McMaster University and ethics committees at the Hospital for Sick Children, and the Universities of Manitoba, Alberta, and British Columbia.
Author contributions
Dr. Azad and Dr. Sears conceptualized and designed the study. Ms. Reyna, Mr. Dharma, and Ms. Dai carried out data analyses. Dr. Lefebvre managed the data. Dr. Becker, Dr. Mandhane, Dr. Turvey, Dr. Subbarao, and Dr. Sears designed the data collection instruments, and coordinated and supervised data collection. Dr. Simons conducted clinical data collection and contributed the peanut allergy outcome. Dr. Marshall and Mr. Tran contributed to the interpretation of results. Dr. Azad drafted the initial manuscript. All authors reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Azad agrees to be accountable for all aspects of the work.