Introduction
Small size at birth, following intrauterine growth restriction (IUGR) and/or subsequent neonatal catch-up growth are implicated in the initiation of permanent metabolic and/or physiological adaptations that persist through adult life. This ‘programming’ may lead to increased appetite, adiposity (particularly of visceral depots) and cardiovascular and metabolic diseases including insulin resistance and glucose intolerance in adults.Reference Barker, Hales and Fall 1 , Reference Gluckman and Hanson 2 Therefore, animal models that mimic human IUGR aetiology, develop cardiovascular and metabolic sequelae with ageing after IUGR and/or catch-up growth and are comparable with the human in their relative maturity at birth are required so that underlying mechanisms and intervention strategies can be investigated.
Small animal models have logistical advantages for such studies, including relatively rapid development, and short lifespans facilitating study of the development of progeny with ageing and intergenerational effects. Many small animal models of IUGR have used maternal feedReference Kind, Simonetta, Clifton, Robinson and Owens 3 – Reference Vickers, Breier, Cutfield, Hofman and Gluckman 7 or protein restrictionReference Bertin, Gangnerau, Bailbe and Portha 6 , Reference Fernandez-Twinn, Wayman and Ekizoglou 8 – Reference Desai, Crowther, Lucas and Hales 11 to restrict fetal growth and investigate long-term outcomes. In developed countries, however, placental insufficiency rather than maternal undernutrition accounts for the majority of human IUGR.Reference Bleker, Buimer, van der Post and van der Veen 12 , Reference Pardi, Marconi and Cetin 13 Placental restriction induced by uterine ligation in rats causes IUGR and programmes many components of the metabolic syndrome.Reference Simmons, Templeton and Gertz 14 – Reference Houdijk, Engelbregt, Popp-Snijders and Delemarre-Van der Waal 17 These IUGR rat models often lack catch-up growth in the early neonatal period,Reference Simmons, Templeton and Gertz 14 , Reference Jansson and Lambert 16 , Reference Houdijk, Engelbregt, Popp-Snijders and Delemarre-Van der Waal 17 which is an independent risk factor for the development of adult metabolic disease in humans.Reference Eriksson, Forsen, Tuomilehto, Osmond and Barker 18 – Reference Fagerberg, Bondjers and Nilsson 21
Guinea pigs provide an alternate species to investigate developmental programming of health and disease. The guinea pig has a smaller litter size than the rat,Reference Vickers, Breier, Cutfield, Hofman and Gluckman 7 , Reference Simmons, Templeton and Gertz 14 , Reference Eckstein and McKeown 22 , Reference Eckstein, McKeown and Record 23 is relatively precocial at birth and resembles the human fetus in having a body fat composition of around 10% at term.Reference Engle and Lemons 24 In addition, and unlike the rat, the guinea pig spontaneously develops a phenotype resembling type 2 diabetes and including hyperglycaemia at 4 months of ageReference Arbeeny, Nordin and Edelstein 25 , Reference Vannevel 26 making it a good species to investigate whether IUGR accelerates the development of metabolic disease with ageing. Maternal feed restriction in the guinea pig at 85 or 70% of ad libitum intake reduces fetal and placental weights, increases visceral adiposity in late gestation fetuses and alters placental structure impairing function.Reference Kind, Roberts and Sohlstrom 27 – Reference Roberts, Sohlstrom and Kind 29 Offspring of feed-restricted mothers do not undergo neonatal catch-up growth, but are hyperphagic post-weaning, and as adults have impaired glucose tolerance and cholesterol homoeostasis, increased blood pressure and visceral adiposity,Reference Kind, Simonetta, Clifton, Robinson and Owens 3 – Reference Kind, Clifton and Katsman 5 demonstrating that prenatal restriction programmes metabolic dysfunction in this species. These adverse effects are mostly seen in males, suggesting developmental programming in the guinea pig is sex specific as described in other species, including humans.Reference Gilbert and Nijland 30 Similarly, uterine artery ablation in the mid-gestation guinea pig produces offspring with disproportionate IUGR (also known as asymmetrical IUGR), and male offspring are hyperphagic post-weaning and have increased epididymal adiposity (postnatal outcomes were not assessed in females).Reference Sarr, Thompson, Zhao, Lee and Regnault 31 , Reference Palliser, Kelleher, Welsh, Zakar and Hirst 32 Furthermore, these surgical models impose sudden restriction on normal fetal growth at ~0.5 of term, and induce fetal death of between ~50 and 70% of pups.Reference Turner and Trudinger 33 Interestingly, at least in the case of the maternal undernutrition studies where correlation analyses were reported, size at birth was a stronger predictor of adult outcomes than was nutritional group.Reference Kind, Simonetta, Clifton, Robinson and Owens 3 – Reference Kind, Clifton and Katsman 5 In the present study, we therefore chose to investigate the effects of spontaneous fetal growth restriction that occurs with variation in litter size in the guinea pig.
Spontaneous variation in litter size in the guinea pig also restricts fetal and placental growth in larger litters.Reference Eckstein, McKeown and Record 23 , Reference Ibsen 34 Importantly, IUGR progeny from large litters undergo neonatal catch-up growth.Reference McKeown and MacMahon 35 Consequences of this spontaneous IUGR due to litter size for subsequent postnatal growth, appetite and adult body composition have not been assessed. We therefore characterized the effects of variation in litter size and hence size at birth on these outcomes in the guinea pig. Because developmental programming is sex specific in other species and in the guinea pig following maternal feed restriction, we investigated outcomes in both male and female progeny.
Materials and methods
Animals
All guinea pigs had ad libitum access to a commercially prepared guinea pig and rabbit ration (diet L102; Milling Industries Stockfeeds, Blair Athol, SA, Australia) containing 2640 kcal/kg digestible energy, 19.0% crude protein and 2.5% crude fat, and supplemented with an increased content of vitamin E (165 mg/kg). All guinea pigs had ad libitum access to tap water with added ascorbic acid (400 mg/l; Ace Chemical Company, Camden Park, SA, Australia). Nulliparous female guinea pigs were obtained at 3–4 months of age (Institute of Medical and Veterinary Science Tri-coloured, Gilles Plains Resource Centre, Gilles Plains, SA, Australia) and housed in a 12:12 h day:night cycle throughout the experiment. Females were individually housed in wire cages and after 2–4 weeks acclimatization were weighed three times per week, and checked for oestrus daily as indicated by a rupture of the vaginal membrane.Reference Sisk 36 A single male was placed with the female during her oestrus (2–3 days of ~15 days cycle) and pregnancy was detected by the presence of a copulatory plug on the following morning. Pregnancy was confirmed (n=68) if the animal failed to return to oestrus in the subsequent cycle. At day 60 of gestation, dams were transferred to individual housing in plastic tubs with paper bedding.
After spontaneous delivery at term (range 65–74 days, mean±s.e.m. 69.7±0.1 days), numbers, sex, weights, abdominal circumference and nose to rump lengths of all liveborn offspring were measured and recorded on the day of birth or following morning if delivered overnight (n=158 offspring, males: n=78, females: n=80). Head widths (n=148, males: n=71, females: n=77) and lengths (n=144, males: n=69, females: n=75) were also measured in a subset of the progeny. Each dam was housed with her offspring and provided with ad libitum lucerne chaff in addition to the standard diet. Litters were weighed at least five times per week, until weaned at days 28–30 of age. Absolute growth rates (AGRs) for weight were calculated from the slope of the growth curve from day 10 until weaning (AGR10–28), with data from birth to day 10 excluded due to nonlinear growth over this period (Fig. 2). After weaning all guinea pigs were housed individually and were weighed at least three times per week from day 30 until postmortem at day 115±1. Puberty in the guinea pig occurs between day 56 and day 60 in males, and between day 30 and day 134 in females, with a mean age at puberty for females of 68±22 days (mean±s.d.).Reference Sisk 36 AGRs were, therefore, also calculated for juvenile (weaning – day 60, AGR30–60) and adolescent (days 60–90, AGR60–90) periods. Current fractional growth rate (FGR) for weight for each stage was calculated as AGR divided by the animal’s weight at the beginning of that stage. Feed intakes were recorded daily from day 40 to day 100 (61 males, 58 females) by weighing the filled feed hopper at 9 am, and then again before refilling at 9 am the following day. Relative feed intakes were calculated by dividing daily feed intake by body weight. Average feed intakes for each animal were then calculated for juvenile (days 40–60) and adolescent (days 60–90) periods, and the feed efficiency was calculated as average daily weight gain divided by average daily absolute food intake over each period.
Adult body composition
At day 115±1, a subset of animals (n=41, 22 males, 19 females) selected randomly within each sex were humanely killed between 2 pm and 4 pm by lethal injection of sodium phenobarbitone. Fat depots (interscapular, omental and right side of the neck as well as bilateral axillary, retroperitoneal, perirenal and groin depots) and bilateral skeletal muscles (hindlimb: M. biceps femoris, M. semitendinosus, M. gastrocnemius, M. plantaris and M. tibialis; forelimb: M. biceps brachii) were dissected and weighed. Visceral adipose weight was calculated as the sum of weights of the left and right perirenal and retroperitoneal fat depots. The omental fat associated with the gastrointestinal tract is highly insulin resistant, drains directly into the portal vein and is strongly associated with hepatic insulin resistanceReference Mittelman, Van Citters, Kirkman and Bergman 37 and was, therefore, weighed and analysed separately. Subcutaneous adipose weight was calculated as the sum of weights of left and right axillary and groin fat, right side of the neck fat and interscapular fat depots. Visceral and subcutaneous fats were summed to give a measure of combined adiposity. The weights of dissected skeletal muscles were summed to obtain combined skeletal muscle mass. A high adiposity to lean tissue ratio is a risk factor for many cardiovascular and metabolic diseases.Reference Lim, Yang and Kim 38 A ratio of the combined adiposity to the combined muscle mass was, therefore, calculated as an index of adiposity relative to lean tissue.
Statistical analysis
Data were analysed using SPSS 23.0 for Windows (IBM, Armonk, NY, USA). The effects of litter size on weight before mating, weight gain during gestation and change in weight during lactation were analysed by repeated-measures ANOVA. Effects of litter size on proportions of liveborn and stillborn progeny were analysed by χ2 test. The effects of litter size and sex on birth phenotype and postnatal outcomes were analysed by mixed models ANOVA, including the dam as a random variable to account for effects of a common maternal environment. Bonferroni’s post-hoc comparisons were used to compare differences in maternal and offspring outcomes between litter sizes. Where effects of litter size differed between sexes, outcomes were analysed separately in each sex. Relationships between size at birth and growth rates were examined by Pearson’s correlation analysis, separately in each sex. Because birth weight ranges overlapped between litter sizes, and in order to assess the effect of catch-up growth on postnatal phenotypes, the independent effects of birth weight and neonatal growth rate for weight on post-weaning outcomes were examined by multiple linear regression separately in each sex. Gestation length was not correlated with outcomes when included in initial models and was, therefore, excluded from final multiple linear regression models. A P-value of ⩽0.05 was accepted as statistically significant. All results are expressed as mean±s.e.m.
Results
Maternal outcomes
Maternal weight at the oestrus before mating did not differ between the litters, however, weight over the 10 days before mating differed over time (P<0.001) and differed between the litter sizes over time (P=0.048, Fig. 1). Dam weight on the day of mating (G0) correlated positively with subsequent litter size (r=0.280, P=0.025, data not shown).
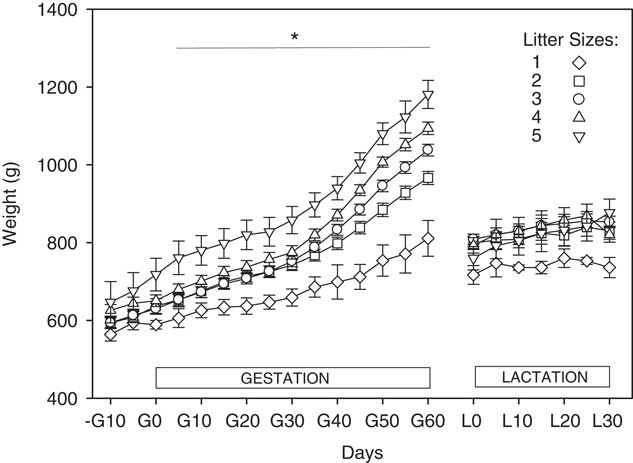
Fig. 1 Maternal weight during the first 60 days of gestation (G) and throughout lactation (L). Dams carrying litters of five are shown in downwards triangles, four in upward triangles, three in circles, two in squares and singletons in diamonds. *P<0.05 for litter size effects at these time points.
Weight increased with time over gestation, and differed between litter size groups, and the change in weight with time also differed between litter sizes (P<0.001 for all, Fig. 1). The change in maternal weight in absolute terms from mating to day 60 of gestation (G60) increased with litter size (P<0.001, Table 1). Dams with litter size of three, four and five gained more weight over the first 60 days of gestation than those with litter sizes of one or two (Table 1). In contrast, change in maternal weight from mating to the day after delivery, reflecting weight of the dam herself, was lower in dams that delivered five pups than in all other groups (Table 1). Maternal feed intakes in mid-gestation were greater in dams carrying five fetuses than in those with smaller litters (Table 1). In late gestation, dams carrying four or five fetuses ate more than dams carrying one or two fetuses (Table 1).
Table 1 Effect of litter size on maternal weights, absolute growth rates and feed intake during pregnancy

Data are expressed as mean±s.e.m. for dams carrying each of the litter sizes (LS).
a,b,c,dMeans with different superscripts differ, P<0.05.
Weight during lactation also differed between dams that delivered different litter sizes (P=0.036), changed with day (P<0.001) and the change in weight over time differed between the litter sizes (P=0.023, Fig. 1). Dams that gave birth to litters of five gained more weight during lactation than all other litter sizes (Table 1).
Litter outcomes
Gestation length differed between litter sizes, and was shorter in dams that delivered five pups compared with those with one or two (Table 1). Gestation length correlated negatively with the total pup weight in the litter (r=−0.268, P=0.035, n=67). The proportion of pups born alive decreased with increasing litter size (P<0.001), falling from 100% in litter sizes of one and two, to 94% in litters of three pups, 82% in litters of four pups and 50% in litters of five pups. Stillbirths were unevenly distributed between litters and were not consistently smaller than liveborn litter mates (data not shown). Total litter weight increased with increasing litter size (P<0.001), and was greater than three-fold higher in litters of four or five than in singleton litters (Table 1).
Birth phenotype
Size at birth of liveborn pups included in later studies in terms of weight, length, abdominal circumference and weight:length ratio decreased as litter size increased (Table 2). Birth weight averaged 97±1 g and ranged from 57 to 134 g across the cohort. Pups from singleton litters were all heavier than pups from litters of five, whereas some overlap in the range of individual birth weights was seen between pups from other litter sizes (Fig. 2). Average birth weight of pups from a litter size of five was 38% lower than those from litter size of one, whereas other birth size measures were reduced to a lesser degree (length, 18%; abdominal circumference, 17%; weight:length, 26%, Table 2). Head lengths and widths were relatively conserved in large litters (Table 2). Weight:length ratio and body mass index (BMI), measures of disproportionate growth, decreased with increasing litter size (Table 2).
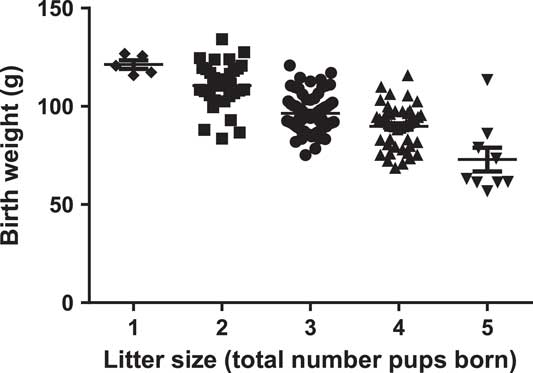
Fig. 2 Litter size and birth weights of individual guinea pig pups included in postnatal studies. Each symbol indicates an individual pup that survived to young adulthood, lines and whiskers indicate means and s.e.m. for each litter size group.
Table 2 Effect of litter size on birth phenotype of live-born pups

LS×sex, interaction between litter size and sex; NSD, not significantly different, P>0.1.
Data are expressed as actual means±s.e.m. of offspring in each of the litter sizes (LS) for pups that survived to adulthood and were included in growth measures. Statistical models included dam to correct for the common intrauterine environment in multiple birth litters.
a,b,c,dMeans with different superscripts differ, P<0.05.
e Head lengths were not measured in four offspring from litter size of three and 10 offspring from litter size of four.
f Head widths were not measured in four offspring from litter size of three and six from litter size of four.
Postnatal growth
Neonates
Growth rates were nonlinear in the first 10 days of life in both sexes (Fig. 3a and 3b). In repeated-measures analysis of weight measures during the neonatal period, weight decreased with increasing litter size in both sexes, and this difference in weight amplified with ageing in males but not females (Fig. 3a and 3b). AGR10–28 did not differ between litter sizes. Effects of litter size on neonatal FGR differed between sexes, such that in males FGR10–28 increased with each increase in litter size from two to four pups (Table 3). In females, FGR10–28 was greater in pups from a litter size of four than those from litter sizes of two or three (Table 3). Neonatal AGR and FGR were higher in males than females (Table 3). AGR10–28 correlated positively with birth weight across the full range of litter sizes (r=0.296, P<0.001) and in males and females separately (r=0.323, P=0.003; r=0.303, P=0.001, respectively, Fig. 4a). FGR10–28 correlated negatively with birth weight overall (r=−0.525, P<0.001) and in males and females separately (r=−0.522, P<0.001; r=−0.701, P<0.001 respectively, Fig. 4b).

Fig. 3 Body weights of male (a, c, e) and female (b, d, f) progeny that survived to adulthood and were included in growth measures, from birth to weaning (a, b), during the juvenile period (c, d) and during adolescence (e, f). Males are shown in closed symbols and females in open symbols with offspring from litters of four in upward triangles, three in circles and two in squares. Bar in panels c and d indicate weaning of offspring from the dam between 28 and 30 days after birth.

Fig. 4 Relationships between absolute (a) and fractional (b) neonatal growth rates from days 10–28 and birth weight for male (n=78 closed symbols, solid regression line) and female (n=80, open symbols, dashed regression line) guinea pigs. (a) Absolute neonatal growth rate correlated positively with birth weight in males (r=0.323, P=0.003) and females (r=0.303, P=0.006). (b) Fractional neonatal growth rate correlated negatively with birth weight in males (r=−0.522, P<0.001) and females (r=−0.701, P<0.001). Offspring from litters of five are shown in downwards triangles, four in upward triangles, three in circles, litters of two in squares and singletons in diamonds.
Table 3 Effect of litter size and sex on postnatal growth rates and food intake

LS×sex, interaction between litter size and sex; AGR, absolute growth rate; NSD, not significantly different, P>0.1; FGR, fractional growth rate.
Feed efficiency was calculated as average daily weight gain over each period divided by average daily feed intake. Data are expressed as means±s.e.m. Statistical models included dam to correct for common intrauterine environment in multiple births.
a,b,cMeans with different superscripts differ, P<0.05.
d In males, neonatal FGR10–28 differed between all litter sizes (P<0.01 for all), while in females, offspring of litters of four grew faster than litters of two and three only (P<0.02 for both).
e Overall, juvenile AGR30–60 was greater in offspring from litters of four compared with litters of two (P=0.004) only.
f Overall, juvenile FGR30–60 differed between all litter sizes (P<0.02 for all).
g Adolescent growth rates did not differ between litter sizes in males or females analysed separately.
h Relative juvenile feed intake was greater in offspring from litters of three and four than in offspring from litters of two (P<0.02 for both).
i Juvenile (40–60 days) feed efficiency did not differ between litter sizes in males or females analysed separately.
j Adolescent feed efficiency was greater in females, and tended to be greater overall, in progeny from litters of three pups than in those from litters of two pups (females: P=0.013, overall: P=0.066), and did not differ between litter sizes in males.
Juveniles
Similar to the neonatal period, body weights during the juvenile period did not differ between litter size groups in females (Fig. 3d). In males, juvenile body weights increased more rapidly in those from larger litters during this period (Fig. 3c). AGR and FGR of juvenile guinea pigs were higher in those from litter sizes of four compared with those in litter sizes of two, and higher in males than in females (Table 2). AGR30–60 was not correlated with birth weight overall, or in males or females separately. FGR30–60 correlated negatively with birth weight overall (r=−0.371, P<0.001), and in males (r=−0.398, P<0.001) and females (r=−0.452, P<0.001) separately. In males, AGR30–60 was independently and positively correlated with neonatal FGR, but not with birth weight (Table 4). AGR30–60 was not independently correlated with birth weight or neonatal FGR in females (Table 4). In both males and females, FGR30–60 was negatively correlated with birth weight but not neonatal FGR (Table 4).
Table 4 Relationships between birth weight and neonatal FGR and subsequent postnatal growth rates and food intake

n, Total number of offspring from all litters; FGR10–28, fractional growth rate for weight in neonates from 10–28 days of age; AGR, absolute growth rate.
*Significant correlations (P<0.05) are shown in bold. Feed efficiency was calculated as average daily weight gain over each period divided by average daily feed intake.
Adolescents
In adolescents, the change in body weight with age differed between litter sizes in males (Fig. 3e), but litter size did not affect weights in females (Fig. 3f). During the adolescent period, neither AGR nor FGRs differed between litter sizes (Table 3). AGR60–90 was higher in males than in females, and FGR60–90 did not differ between sexes (Table 3). AGR60–90 was not correlated with birth weight, in males or in females, and was independently and positively correlated with neonatal FGR in females only (Table 4). Similarly, FGR60–90 was not correlated with birth weight, in males or in females, and was independently and positively correlated with neonatal FGR in females only (Table 4).
Feed intake
Food intake in juvenile progeny did not differ between litter sizes, and was greater in males than in females, in absolute terms (Table 3). In contrast, relative food intake in juveniles differed between litter sizes (Table 3). Overall, and in females, progeny from litters of three or four pups ate more in relative terms than those from litters of two pups (Table 3). Feed efficiency (weight gain per intake) in juvenile guinea pigs did not differ between litter sizes and was higher in males than in females (Table 3). In males, absolute juvenile feed intake was independently and positively predicted by birth weight and neonatal FGRs; these correlations were not significant in females, or for relative feed intake in the juvenile period (Table 4). Feed efficiency was correlated negatively with birth weight in juveniles of both sexes (Table 4).
Feed intake of adolescent progeny did not differ between litter size groups, and was higher in absolute but not relative terms in males than in females (Table 3). Feed efficiency (weight gain per intake) in adolescent guinea pigs varied with litter size and was higher in progeny from litters of three than those from litters of two in females, with a similar trend overall, but not in males (Table 3). In males, adolescent absolute feed intake was predicted independently and positively by neonatal FGR, but not by birth weight (Table 4). In females, adolescent absolute feed intake was predicted by the overall model and independently and positively by both birth weight and neonatal FGR (Table 4). Relative adolescent feed intake was not correlated with birth weight or neonatal growth rate in males. In females, however, relative adolescent feed intake was predicted independently and positively by neonatal FGR, but not by birth weight (Table 4). Feed efficiency in adolescent males was not correlated with either birth weight or neonatal FGR (Table 4). Feed efficiency in adolescent females was correlated independently and negatively with birth weight (Table 4).
Adult phenotype and body composition
Body size
Adult body size did not differ between litter size groups in males or females (Table 5). Males were heavier, longer and had a higher weight:length ratio than females, but BMI did not differ between sexes (Table 5). In males, adult body weight and weight:length ratio correlated independently and positively with neonatal FGR with similar trends for birth weight (Table 6). In females, adult body weight correlated independently and positively with birth weight with a similar trend for neonatal FGR (Table 6). Other measures of adult size were not correlated (P>0.05 for all) with size at birth or neonatal FGR in either sex.
Table 5 Effect of litter size and sex on adult size and body composition

LS×sex, interaction between litter size and sex; NSD, not significantly different, P>0.1.
Data are expressed as mean±s.e.m. Mean age±s.e.m. at postmortem was 115±1 days. Statistical models included dam to correct for common intrauterine environment in multiple births.
a Relative subcutaneous, absolute and relative visceral and absolute total dissected fat weights did not differ (P>0.05) between any two litter sizes.
b Overall, offspring from litters of three had higher absolute and relative omental fat and relative total dissected fat than those from litters of two (P<0.05 for all).
Table 6 Relationships between birth weight, neonatal FGR and adult phenotype in the guinea pig

n, Number of offspring; FGR10–28, fractional growth rate for weight in neonates from 10 to 28 days of age.
Adult body composition is expressed as an absolute weight (g) and as a percentage of the body weight at the time of postmortem (%). Age at postmortem was 115±1 days.
*Significant correlations (P<0.05) are shown in bold.
Body composition
Relative weights of subcutaneous, visceral, omental and total dissected fat depots and absolute weight of visceral and omental fat differed between litter sizes overall, with similar trends for absolute weights of subcutaneous and total dissected fat depots, and effects of litter size were similar in each sex (Table 5). Nevertheless, absolute weight of visceral and total dissected fat and relative weights of subcutaneous and visceral depots did not differ between any two litter sizes. Overall, offspring from litters of three had higher absolute and relative omental fat weights (P=0.049 and 0.013, respectively) and higher relative total dissected fat (P=0.038) than those from litters of two; absolute and relative weights of fat depots did not differ between litter size pairs in sex-specific analyses. Absolute but not relative weights of subcutaneous, visceral and total dissected fat depots were greater in males than in females (Table 5). Skeletal muscle weights did not differ between litter sizes, whilst absolute skeletal muscle weights were higher in males than in females (Table 5). The ratio of dissected fat:skeletal muscle weights did not differ between litter size groups or sexes (Table 5). In males, absolute and relative weights of multiple fat depots, but not absolute or relative skeletal muscle weights, were independently and positively correlated with birth weight and neonatal FGR (Table 6). These correlations were strongest for weights of visceral fats and with the ratio of dissected fat to skeletal muscle weights (Table 6). In females, in contrast, absolute skeletal muscle weight was independently and positively correlated with birth weight but not neonatal FGR, and fat depot weights were not correlated with either birth weight or neonatal FGR (Table 6).
Discussion
In the current study, increasing litter size in the guinea pig induced disproportionate IUGR that was followed by catch-up growth commencing in neonatal life and which persisted post-weaning but not into adolescence. Concomitant with this accelerated growth, offspring from large litters had increased relative feed intakes as juveniles. Increased neonatal growth also predicted hyperphagia in juveniles, which persisted into adolescent life in both sexes. Interestingly, perinatal growth was correlated with adult body composition differently in males and females, predicting visceral adiposity in males, but not in females, and consistent with sex-specific relationships between neonatal growth and adiposity in children.Reference Ong, Ahmed, Emmett, Preece and Dunger 39 Spontaneous fetal growth restriction induced by large litter sizes in the guinea pig, therefore, induces sex-specific programming of postnatal phenotype. As the guinea pig also develops diabetes mellitus spontaneously in adulthood,Reference Arbeeny, Nordin and Edelstein 25 , Reference Vannevel 26 this provides a model to investigate developmental programming of susceptibility to metabolic diseases of ageing.
Maternal outcomes
Mothers who were larger at conception tended to have larger litter sizes, consistent with previous reports of a positive relationship between weight and number of corpora lutea at conception,Reference Eckstein and McKeown 22 and suggesting that their greater litter sizes reflect higher ovulation rates. In the present study, the proportions of stillborn pups increased in large litters, particularly in litters of five pups. This may reflect their earlier gestational age at delivery, which is negatively correlated with stillbirth rates in this species.Reference Goy, Hoar and Young 40 Small size at birth relative to gestational age is also a predictor for stillbirth in the guinea pig.Reference Goy, Hoar and Young 40 Restricted nutrient supply in utero due to competition for maternal nutrients and/or restricted delivery due to limited placental growth and/or function may, therefore, also have contributed to poorer neonatal outcomes in large litters in the present study, as these pups were substantially smaller at birth.Reference Roberts, Sohlstrom and Kind 28 , Reference Roberts, Sohlstrom and Kind 29 Not surprisingly, mothers carrying larger litters gained more weight during gestation than those with smaller litters. Nevertheless, the change in maternal weight from conception to the start of lactation, reflecting growth of the mother herself, was lowest in dams that gestated five pups. Guinea pigs give birth to relatively mature pups that account for a significant part of their own weight,Reference Engle and Lemons 24 and pregnancy appears to reduce maternal energy stores in late gestation, reflected in lighter adipose depots.Reference Sohlstrom, Katsman and Kind 41 Together with these previous studies, our results suggest that high fetal demand for nutrients plus inability to further increase feed intake in late gestation limits maternal growth in dams carrying the largest number of pups. Compensatory hyperphagia may, therefore, contribute to the subsequent faster lactation growth rates that we observed in dams that gave birth to large litters. It is also likely that nutrient flow to milk production was lower in these mothers that gestated litters of five pups than in those that delivered three or four pups due to lower perinatal survival of pups and/or earlier weaning. Previous studies have reported that although mothers produce higher yields of milk in larger litters, the milk yield per pup is reduced, suggesting pups in larger litters may be ‘force’ weaned earlier in comparison with those from smaller litters.Reference Davis, Mepham and Lock 42 Interestingly, dams in all litter size groups gained weight in lactation in the present study, in contrast to a previous report.Reference Mepham and Beck 43
Birth phenotype
Although there was some overlap in ranges of individual birth weights between litter size groups, mean birth weight decreased consistently with increasing litter size and singleton pups were 38% heavier than pups from litters of five. This birth weight difference is of a similar magnitude as that reported for weights of pups in late gestation in multiparous females.Reference Ibsen 34 Most measures of size at birth decreased with increasing litter size in the present study, including weight:length ratio (an index of thinness), and head width:birth weight ratio (an index of head sparing). Thus, spontaneous fetal growth restriction in guinea pigs from large litters induces disproportionate IUGR, which in human epidemiological studies is associated with greater increases in risks in cardiovascular and metabolic diseases than those associated with a proportionate reduction in size at birth.Reference Phillips, Barker, Hales, Hirst and Osmond 44 , Reference Law, Gordon, Shiell, Barker and Hales 45 Asymmetrical growth restriction resulting in a thin neonate and characterized by head sparing usually reflects restriction predominantly in late gestation,Reference Rosenberg 46 and is also observed following maternal famine exposure in late gestation in humansReference Ravelli, Vandermeulen and Michels 47 and in experimental models of restricted placental capacity in sheep.Reference De Blasio, Gatford, Robinson and Owens 48
Increased litter size may reduce fetal nutrient availability and hence size at birth via a reduction in placental size and function and/or competition for maternal nutrients together with physical limitations on maternal feed intake.Reference Eckstein and McKeown 22 , Reference Eckstein, McKeown and Record 23 , Reference McKeown and MacMahon 35 Fetal weight in guinea pigs diverges between litter sizes from ~55 days of gestation,Reference Ibsen 34 consistent with progressively increasing limitation of nutrient/oxygen supply in large litters through late gestation. Limited maternal nutrient intake may also contribute to reduced fetal growth in large litters, with similar mean birth weights in litters of four and five in the present study as those induced by feed restriction of guinea pigs throughout gestation to either 85 or 70% of ad libitum feed intake.Reference Kind, Simonetta and Clifton 4 Shorter gestation lengths may also contribute to reduced size at birth. Gestation length is reduced in guinea pig litters with high total fetal weights,Reference McKeown and MacMahon 35 which may have contributed to the 3–4 day reduction in gestation lengths in litters of five compared with those in litters of one or two pups in the present study, and may in turn have contributed at least in part to smaller sizes at birth in this group. Weight of fetal guinea pigs increases by ~10% over the last 3–4 days of gestation, although interestingly weight gain during this period is markedly lower in large litters than in litters with only one or a few pups.Reference Ibsen 34 Differences in gestation length are thus unlikely to explain the majority of difference in size at birth that we observed in large litters. In recent studies of neuroactive steroids in this species, preterm delivery at 62–63 days of gestation (~12% reduction in gestation length) reduced birth weight by only 17%,Reference Kelleher, Hirst and Palliser 49 considerably less than the 38% difference in average birth weight between pups from litters of one and five. In the present study, the negative relationship between size at birth and litter size in most parameters was observed across the full range of litter sizes, further implying that additional factors contribute to small size at birth in pups from large litters.
Neonatal phenotype
Offspring of larger litters grew faster in fractional terms as neonates, indicating a change in partitioning of nutrient intake towards growth in these spontaneous IUGR guinea pigs. Increased appetite may also contribute to accelerated neonatal growth after IUGR, as it occurs in other species including humans,Reference De Blasio, Gatford, Robinson and Owens 48 , Reference Ounsted and Sleigh 50 , Reference Shin, Dai, Thamotharan, Gibson and Devaskar 51 but neonatal feed intakes have not as yet been reported in IUGR guinea pigs. Accelerated neonatal growth may programme adverse later outcomes in these guinea pigs from large litters, as this is an independent risk factor for the development of cardiovascular and metabolic disease in humans.Reference Eriksson, Forsen, Tuomilehto, Osmond and Barker 18 – Reference Fagerberg, Bondjers and Nilsson 21 Interestingly, despite the more rapid relative neonatal growth in pups from larger litters, these pups exhibited less growth check after weaning at 28 days than those from smaller litters (Fig. 3c and 3d), suggesting that they may have changed their intake from milk to solid feed earlier. This may reflect lower milk production per pup, reported previously in large guinea pig litters,Reference Davis, Mepham and Lock 42 and competition between litter mates for the two available teats in this species which only allows pups to suckle periodically.Reference Sisk 36 Guinea pigs have been successfully weaned at birth or 8 days of age,Reference Davis, Mepham and Lock 42 indicating that there is not an absolute requirement for suckling in this species.
Post-weaning phenotype
Interestingly, the accelerated relative growth in guinea pigs from large litters persisted after the neonatal period, and was also observed in juveniles at 30–60 days of age, but this did not continue subsequently to adolescence. The accelerated juvenile growth does appear to reflect continued catch-up growth following removal of prenatal constraint, as juvenile relative growth rates were negatively correlated with birth weight in both males and females, as were relative growth rates in the neonatal period. The relative duration of catch-up in IUGR guinea pigs thus appears to be somewhat longer than in IUGR humans, where the majority of catch-up growth occurs in the first 6 months of life, and is largely complete in infancy by 2 years of age.Reference Tenovuo, Kero and Piekkala 52 – Reference Hokken-Koelega, De Ridder and Lemmen 55 Juvenile growth rates in guinea pigs from large litters were greater than those from smaller litters, not only in relative, but also in absolute terms. Increased appetite and feed intake probably underlies the accelerated juvenile growth rates of these spontaneously growth-restricted guinea pigs, at least in part, as we also observed increased food intake relative to body size in these animals. This role for increased appetite as a mechanism for early catch-up growth after IUGR is further supported by a lack of difference in absolute or relative feed intakes between litter sizes during adolescence, when the litter sizes also had similar growth rates. Feed efficiency did not differ between litter sizes in the juvenile period in either sex, and was improved in female offspring from litters of three compared with two as adolescents. Although this may suggest decreased relative fat deposition in these adolescent females, as fat has a greater energy content per weight than muscle, this did not reduce fatness in adulthood. Juvenile but not adolescent feed efficiency was negatively correlated with birth weight in both sexes, indicating improved efficiency of conversion of food to weight gain, and thus suggesting greater relative lean tissue deposition in the juvenile period. This pattern of post-weaning accelerated growth may also lead to adverse health consequences, as accelerated growth after IUGR in rats; particularly when allowed increased caloric feed intake or fed western-style diets as adults; is associated with obesity, cardiovascular complications and early death.Reference Vickers, Breier, Cutfield, Hofman and Gluckman 7 , Reference Ozanne and Hales 9 , Reference Holness and Sugden 56 , Reference Benyshek, Johnston and Martin 57 Despite the fact that effects of litter size on growth were similar in both sexes in the present study, relationships between perinatal growth and both post-weaning growth and feed intake were sex-specific. Relative juvenile growth rates correlated negatively with birth weight in both sexes, as discussed above, but absolute juvenile growth rates were not correlated with size at birth in either sex, and correlated positively with neonatal growth in males only. Juvenile feed intake in absolute terms correlated positively with size at birth and neonatal FGR, but in males only, and may reflect effects of perinatal growth on body size, as relative feed intakes did not correlate with perinatal growth in either sex. In contrast to these largely male-specific relationships in juveniles, the majority of relationships between perinatal growth and adolescent growth were observed only in females, where rapid neonatal growth predicted faster growth and higher feed intakes in adolescence. These results suggest that programming of postnatal appetite and nutrient partitioning by prenatal and early life exposures is sex-specific in guinea pig, and furthermore that the sex-specific nature of these effects depend on age. Sex-specific programming of later outcomes has also been reported in humans and in animal models, particularly for adult metabolic and body composition outcomes.Reference Gilbert and Nijland 30 These results further reinforce the need to study progeny of both sexes in future studies investigating developmental programming of metabolic and other outcomes in the guinea pig.
Adult body size and composition
In the present study, progeny of large litters, despite their smaller size of birth, attained similar adult size as those from small litters who were subject to less restriction in utero. This is consistent with findings of human studies, where ~85–90% of individuals who were born IUGR attain a final height within 2 s.d. of their peers.Reference Albertsson Wikland, Boguszewski and Karlberg 53 – Reference Hokken-Koelega, De Ridder and Lemmen 55 Accelerated neonatal growth was a major determinant of adult size, particularly in male guinea pigs, again consistent with human studies where failure of early catch-up growth is a strong predictor of shorter adult height.Reference Albertsson Wikland, Boguszewski and Karlberg 53 , Reference Karlberg and Albertsson Wikland 54 Few differences were seen in adult body composition between litter size groups, although we were not able to include progeny of litters of one or five pups in these litter size comparisons due to low numbers of adult pups of each sex available as young adults. We did observe greater weights of omental and total dissected fat in offspring from litters of three than in those from either two or four pups. We would have expected greater fatness with increasing litter size, given that increasing litter size was associated with progressive decreases in size at birth and increases in neonatal growth rates. Interestingly, IUGR induced by chronic maternal ethanol consumption throughout guinea pig pregnancy, a model for fetal alcohol syndrome, is followed by neonatal catch-up growth and increased visceral and subcutaneous adiposity in young adults of both sexes.Reference Dobson, Mongillo and Brien 58 Thus, sex-specific effects of fetal growth patterns differ between these causes of IUGR. In male IUGR rats catch-up growth after weaning is followed by development of increased visceral adiposity by 7 weeks and obesity by 26 weeks.Reference Simmons, Templeton and Gertz 14 IUGR induced by uterine artery ablation in guinea pigs is also associated with increased visceral adiposity in male offspring, although in this model progeny do not undergo catch-up growth, and body composition outcomes in females were not reported.Reference Sarr, Thompson, Zhao, Lee and Regnault 31 Small size at birth and catch-up growth in early life are independent risk factors for obesity in human adultsReference Eriksson, Forsen, Tuomilehto, Osmond and Barker 18 and similar effects might explain the greater omental and total adiposity we observed in progeny from litters of three compared with two male pups. The reason why these effects were not even more pronounced in progeny from litters of four pups, which had similar fatness as those from litters of two pups, is not clear, given that these pups also experienced catch-up growth and grew faster than progeny of litters of two or three pups during the neonatal and juvenile periods. It is possible that these progeny of litters of four pups will develop central adiposity with further ageing beyond young adulthood, as these animals achieved similar weights to those of pups from litters of two nearly a month later than the pups from litters of three, particularly in males.
Across the entire range of litter sizes, the relationships between size at birth, neonatal growth and adult body composition were sex-specific, as also seen for predictors of post-weaning growth and feed intake. The increased adiposity, particularly visceral adiposity, observed in adult male guinea pigs who had grown rapidly as neonates is likely to be associated with adverse metabolic outcomes in these animals. Visceral fat is insulin resistant and in humans, visceral fat mass is a stronger predictor of cardiovascular and metabolic dysfunction than subcutaneous fat.Reference Mittelman, Van Citters, Kirkman and Bergman 37 , Reference Lim, Yang and Kim 38 The association between early life catch-up growth and later adiposity seen here in male guinea pigs is consistent with reports in humans and other species.Reference Vickers, Breier, Cutfield, Hofman and Gluckman 7 , Reference Eriksson, Forsen, Tuomilehto, Osmond and Barker 18 , Reference De Blasio, Gatford, Robinson and Owens 48 , Reference Law, Barker, Osmond, Fall and Simmonds 59 – Reference Kensara, Wootton and Phillips 61 Interestingly, our results are consistent with reports from several of these studies where the independent associations of small size at birth and neonatal catch-up growth with adult adiposity were investigated, and neonatal catch-up growth was more predictive of adult adiposity than small size at birth.Reference De Blasio, Gatford, Robinson and Owens 48 , Reference Rogers 60 , Reference Kensara, Wootton and Phillips 61
Conclusion
Spontaneous fetal growth restriction due to litter size in the guinea pig gives rise to offspring with disproportionate IUGR and these offspring undergo catch-up growth which persists from neonatal life post-weaning into the juvenile period. Further programmed adult outcomes described here in progeny of large litters whose growth was restricted in utero, such as increased visceral adiposity and hyperphagia, are similar to effects of small size at birth induced in other models of IUGR in guinea pig,Reference Kind, Simonetta and Clifton 4 ratReference Vickers, Breier, Cutfield, Hofman and Gluckman 7 and in humans.Reference Ravelli, van der Meulen, Osmond, Barker and Bleker 62 The spontaneous IUGR guinea pig may, therefore, be a useful model to investigate the mechanisms underpinning perinatal programming of hyperphagia and obesity. Because many of these relationships are sex-specific, it will be critical to include progeny of both sexes in future studies. Further studies are required to determine if the spontaneously growth-restricted guinea pig develops insulin resistance and other metabolic and cardiovascular pathologies with ageing.
Acknowledgements
We thank Arkadi Katsman for assistance with in vivo studies and Jeffrey Robinson for intellectual input and support.
Financial Support
The study was supported in part by project grants from the National Heart Foundation.
Conflicts of Interest
None.
Ethical Standards
The Animal Ethics Committee of the University of Adelaide approved all studies. The studies on animals were performed in accordance with the Australian code of practice for the care and use of animals for scientific purposes published by the National Health and Medical Research Council of Australia.













