INTRODUCTION
The Syllidae Grube, 1850 is a geographically widespread and diverse family of polychaetes with more than 700 described species in 74 genera occurring in all marine habitats (Aguado et al., Reference Aguado, Nygren and Siddall2007, Reference Aguado, San Martín and Siddall2012; San Martín & Aguado, Reference San Martín, Aguado and Viacheslav2012). It is therefore not surprising that Day (Reference Day1967) recorded 25 genera in southern Africa. Within them Syllis Savigny, 1818 is the most speciose genus, with nine species recorded in South Africa, of which only Syllis benguellana Day, 1963 was described as endemic. In southern Africa, these species show variation in distribution and abundance. Syllis vittata Grube, 1840 was fairly common on the west coast, while a single specimen was found on the KwaZulu–Natal coast. Syllis amica Quatrefages, 1865 was found in low numbers only on the KwaZulu–Natal coast in the east, while S. benguellana was found only at Lambert's Bay on the west coast where it was locally abundant. The remaining five species, S. gracilis Grube, 1840, S. cornuta Rathke, 1843, S. prolifera Krohn, 1852, S. variegata Grube, 1860 and S. armillaris (Müller, 1771) are common to very common along the entire South African coast. Syllis hyalina Grube, 1863 was also found along the entire South African coast, but Day (Reference Day1967) suggested that it might be a young stage of S. variegata. The remaining ten species listed in Day (Reference Day1967) have since then either been referred to other genera, synonymized with other species or are now considered nomina dubia (Licher, Reference Licher1999). Although syllids have been found in studies conducted locally since 1967, none have increased our understanding of the known distributions of these species, nor have new species been described.
Syllids are known for their diverse reproductive strategies; mainly epigamy (where the whole animal is involved) and stolonization (by stolons derived from the posterior part of the body) (Garwood, Reference Garwood1991; Franke, Reference Franke1999; Aguado et al., 2007). Epigamy and stolonization (i.e. epitoky) are typical for Eusyllinae and Exogoninae, and for Syllinae and Autolytinae, respectively (Franke, Reference Franke1999). These species are mostly free spawners, with early larval development occurring in the water column before the larvae become benthic and continue development (Franke, Reference Franke1999). Exceptions are some species of the Autolytinae, where the eggs are brooded in a ventral pouch of the female stolon where early development occurs before the larvae are released to continue development externally. However, all species of Exogoninae brood eggs, dorsally or ventrally (San Martín, Reference San Martín and Ramos2003, Reference San Martín2005) and in some genera juveniles develop attached externally to the mothers' bodies while vivipary may be found in some species of Syllinae and Exogoninae. This parting from the ‘norm’ in the form of internal and external brooding is considered as an adaptation to small body size or, in some cases, a specialized life style (Franke, Reference Franke1999). Although vivipary in syllids has been known since the description of Syllis vivipara Krohn, Reference Krohn1869, information is generally limited to the number of embryos or larvae present within the mother's body cavity (Goodrich, Reference Goodrich1900; Ben-Eliahu, Reference Ben-Eliahu1977; San Martín, Reference San Martín1984; Russell, Reference Russell1995; Ding et al., 1998; Aguado & San Martín, Reference Aguado and San Martín2006). Even though drawings and photographs of the juveniles do exist (e.g. Goodrich, Reference Goodrich1900; Pocklington & Hutcheson, Reference Pocklington and Hutcheson1983; Russell, Reference Russell1995; Ding et al., Reference Ding, Licher and Westheide1998), almost nothing is known about the development of the larvae or juveniles before birth for any species. Goodrich (Reference Goodrich1900) suggested that larval development in S. vivipara (and by extension, other viviparous species), followed the same path as that of larvae that are not brooded, but this has not been shown conclusively. Similarly, Heacox (Reference Heacox1980) suggested that larval development among the syllids is similar, but that they differ with respect to the timing and duration of larval stages and the development of cephalic structures and parapodia formation. Such differences are suggestive in a family that includes the full range of larval developmental modes from pelagic, planktotrophic development, through degrees of brooding to the extreme of vivipary, and might reflect differences in the immediacy of the need for these organs.
The Syllidae are widely distributed in marine habitats but they are more abundant and diverse in shallow waters where they are often dominant in terms of diversity and abundance (San Martín, Reference San Martín and Ramos2003). Compared to other polychaete groups, Syllidae are relatively commonly associated with other marine invertebrates, representing 9% of the total known symbiotic polychaetes (Martin & Britayev, Reference Martin and Britayev1998; Glasby & Watson, Reference Glasby and Watson2001; López et al., 2001). They are renowned for forming associations with a taxonomically wide range of host species where they may live as ecto- or endosymbionts in commensal or parasitic associations (López et al., Reference López, Britayev, Martín and San Martín2001). At 14%, syllids comprise the third highest percentage of parasitic polychaete species known to date, after the Spionidae (38%) and Oenonidae (24%), and are the second most well-represented family within the ‘non-boring’ parasites (Martin & Britayev, Reference Martin and Britayev1998; López et al., Reference López, Britayev, Martín and San Martín2001). Of the 33 syllid species reported as symbionts, 23 were described as commensals and 11 as parasites (López et al., Reference López, Britayev, Martín and San Martín2001). Sponges and cnidarians were the dominant host groups for both types of association, followed by sipunculids, echinoderms, bryozoans and decapods for commensals and polychaetes, nemerteans and tunicates for parasitic syllids (Martin & Britayev, Reference Martin and Britayev1998; López et al., Reference López, Britayev, Martín and San Martín2001). Echinoderms, including ophiuroids, asteroids and holothuroids, are relatively common hosts of commensal syllids in obligatory or facultative associations, but no symbiotic associations were reported between syllids and molluscs by Martin & Britayev (Reference Martin and Britayev1998). Thus, to the best of our knowledge, these are the first reports of symbiotic associations between a species of syllid with host species from Bivalvia and Gastropoda. In addition, this is the first report of a viviparous species of syllid reported as living in close association with other marine invertebrates.
In this paper, two new species of Syllis from South Africa, Syllis unzima and Syllis amicarmilliaris, are described. These are the first new syllids to be described locally since 1963 (Day, Reference Day1967), and include a new record of viviparous development which is described in detail. We provide a brief review of vivipary in polychaetes in general and syllids in particular and we examine the notion that development in brooded and non-brooded syllid larvae is the same, and briefly discuss the possible benefit to S. unzima sp. nov. of an association with sea cucumbers.
MATERIALS AND METHODS
Both species of worm were collected from coralline algae and sediment in the upper intertidal zone within the effluent outflow path from two abalone (Haliotis midae Linnaeus, 1758) farms in east Walker Bay (34°37′47″S 19°17′56″E and 34°36′6″S 19°20′12″E) on 21 May 2012. Additional specimens of Syllis unzima sp. nov. were collected from the surface of holothurians (Holothuria scabra Jaeger, 1833) in a zero exchange re-circulating aquaculture system in west Walker Bay (34°26′13″S 19°13′5″E) on 17, 22 and 23 August 2012 and on the surface of an oyster (Crassostrea gigas (Thunberg 1793)) shell in an on-shore culture facility in Kleinzee (29°42′42″S 17°4′16″E) on 16 November 2012. Additional specimens of Syllis amicarmillaris sp. nov. were collected from the shells of abalone (H. midae) from an off-shore facility in Saldanha Bay (33°1′17″S 17°58′20″E) on 24 October 2012.
The worms were relaxed with 7% MgCl in tap water before processing or photographing. All photographs were taken with a Leica EZ3 camera attached to either a Leica MZ75 stereomicroscope or a Leica DM1000 compound microscope. After photographing the whole worms, the relaxed worms were fixed in 4% seawater formalin before transferring to 70% ethanol for storage. To prepare the slides of the larvae, gravid chaetigers were cut open with a scalpel blade and the larvae gently pushed out. After photographing the live larvae, they were fixed in formalin as above, before permanent slides were made. Slides were prepared by mounting sections of formalin-fixed specimens or larvae directly in Aquatex® mounting fluid, left to dry at room temperature and sealed with clear nail varnish. All drawings were prepared using a camera lucida.
For scanning electron microscopy (SEM), the specimens were dehydrated through a series of 80%, 90% and 100% ethanol, before being critical point dried over CO2 with an Emitech K850 Critical Point Dryer, gold-coated with a Q150T-S Turbo-Pumper Sputter Coater, and examined with a Hitachi S-3000N electron microscope at Servicio Interdepartamental de Investigación (SIDI), Universidad Autónoma de Madrid (UAM).
Specimens were lodged at Iziko South African Museum, Cape Town (SAM) and the Museum Nacional de Ciencias Naturales, Madrid (MNCN) (Spain).
SYSTEMATICS
Order: PHYLLODOCIDA Dales, 1962
Family: SYLLIDAE Grube, 1850
Subfamily: SYLINNAE Grube, 1850
Genus: Syllis Lamarck, 1818
Syllis unzima sp. nov.
(Figures 1–6)

Fig. 1. Syllis unzima, sp. nov: (A) anterior end, dorsal view; (B) compound chaetae, anterior parapodium; (C) aciculae, anterior parapodium; (D) compound chaetae, midbody; (E) aciculae, midbody; (F) compound chaetae, posterior parapodium; (G) acicula, posterior parapodium; (H) dorsal simple chaeta; (I) ventral simple chaeta. Holotype (SAM A608383). Scale bars: A, 375 µm; B–I, 20 µm.

Fig. 2. Syllis unzima, sp. nov. scanning electron microscopy: (A) anterior end, dorsal view; (B) prostomium and most anterior segments, dorsal view; (C) detail of prostomium and nuchal organs; (D) posterior end, and one juvenile emerging; (E) detail of the juvenile (MNCN 16.01/14732).

Fig. 3. Syllis unzima, sp. nov. scanning electron microscopy: (A) midbody parapodia, dorsal view, and compound chaetae; (B) compound chaetae, midbody (MNCN 16.01/14732).

Fig. 4. Syllis unzima, sp. nov: (A) whole, gravid female, with late stage juveniles filling the area behind the black line. Individual juveniles are indicated by the arrows (MNCN 16.01/14712); (B) anterior end; (C) anterior showing distinctive colour patterns, pharynx and tooth; (D) proventricle; (E) juveniles emerging head first from mid-body chaetigers of mother; (F) more juveniles emerging from further back on the same female, one emerging head first, and the other, tail first. Scale bar: F, 200 µm.

Fig. 5. Syllis unzima, sp. nov. Juveniles in different stages of development: (A, B) 2-chaetiger larvae from SAM A60385; (A) clearly shows barrel-shaped structure that develops into the pharynx; (C) 7-chaetiger larvae from SAM A60384; (D) anterior of 7-chaetiger larva showing pre-pharynx and proventricle; (E) posterior of 7-chaetiger larva; (F) anterior of 11-chaetiger larva showing developing pharynx, proventricle and palps; (G) posterior of 11-chaetiger larva; (H) anterior of 16-chaetiger larva from MNCN 16.01/14713, showing fully developed pharynx and proventricle; (I) posterior of 16-chaetiger larvae from MNCN 16.01/14713.

Fig. 6. Ventral view of Holothuria scabra showing Syllis unzima sp. nov. (arrows) within the reduced ambulacral grooves, crevices and entering the oral opening.
TYPE MATERIAL
Holotype: entire specimen (coordinates: 34°26′13″S 19°13′5″E; on-shore closed culture system, on H. scabra) (SAM A60383); coll. G. Robinson, 17 August 2012
Paratypes: 3 specimens (MNCN 16.01/14709), 23 August 2012; 1 female with 2 slides of larvae (MNCN 16.01/14712 and MNCN 16.01/14713, respectively), 22 August 2012; 1 female with emerging offspring on SEM stub (MNCN 16.01/14732); 8 specimens in ethanol (SAM A60386), 17 August 2012; 1 female worm with one slide of larvae (SAM A06385); 1 female worm with two slides of larvae (SAM A60384), 23 August 2012; 1 slide of parapodia (MNCN 16.01/14727), 22 August 2012. All are entire specimens collected by G. Robinson in west Walker Bay (34°26′13″S 19°13′5″E) from the ventral surface of tank cultured H. scabra.
DESCRIPTION
Holotype complete, 8 mm long, 0.51 mm wide, 45 chaetigers. Body of medium size, elongate, cylindrical, with a distinctive colour pattern, two slender transverse dark bands, purple to brown, on each anterior and midbody segment, one just after intersegmental furrow, and other just posterior to the midline of segment (Figures 1A, 4A–C); also, some pigmented areas on prostomium and palps (Figures 1A, 4A–C). Prostomium trapezoidal to oval (Figures 1A, 4B, C). Two pairs of red eyes in trapezoidal arrangement (Figures 1A, 4B, C). One pair of densely ciliated nuchal grooves extending along posterio-lateral margin, between prostomium and peristomium (Figures 1A, 2B, C). Palps triangular, as long as prostomium or slightly longer. Antennae, tentacular and dorsal cirri slender and distinctly articulated (Figures 1A, 2A, B, 4A, B). Median antenna much longer than prostomium and palps together, with about 30 articles, arising between posterior pair of eyes; lateral antennae distinctly shorter than median one, about half the length, with about 16 articles, originating in front of anterior pair of eyes (Figures 1A, 4B, C). Peristomium well differentiated, shorter than subsequent segments, slightly extended anteriorly (Figures 1A, 2B, C); a slender row of cilia on anterior margin of peristomium (Figure 2C); two pairs of tentacular cirri, dorsal pair similar to median antenna, with about 23 articles, ventral pair shorter, with about 15 articles. Dorsal cirri longer than body width, with irregular variations in length anteriorly (the first, second, third, fourth, fifth, sixth and seventh pairs with 35, 18, 26, 30–33, 20, 26 and 15 articles, respectively) and alternating in length in midbody and posteriorly; from proventricle backwards, long dorsal cirri with about 27 articles and shorter ones with 18 articles, diminishing in length posteriorly. Ventral cirri short, digitiform, not extending beyond parapodial lobes. Parapodial lobes distally bilobed (Figures 1A, 3A). Anterior parapodia with 10–12 compound chaetae, 8–9 on midbody parapodia, up to 4–6 chaetae per parapodium in most posterior chaetigers. All falcigers heterogomph, unidentate, distally hooked, with long, thin spines on margin, all similar throughout, but some dorso-ventral and antero-posterior gradation in length (Figures 1B, D, F, 3A, B); blades of anterior falcigers decrease in length from 33–20 µm on anterior (Figure 1B), to 20–17 µm on midbody to 17–16 µm on posterior parapodia, while concomitantly getting broader (Figure 1D, F). Solitary dorsal simple chaeta on posterior chaetigers, slender, unidentate (Figure 1H); ventral simple chaetae thin, sinuose, unidentate (Figure 1I). Up to four aciculae in anterior parapodia, one distally hollow and three straight and broad distally (Figure 1C), two in midbody (Figure 1E) and solitary in mid-posterior and posterior parapodia, distally hollow (Figure 1G). Pharynx long, extending through 6–7 segments, a large, conical tooth distinctly back from anterior margin (Figures 1A, 4A–C). Proventricle shorter than pharynx, extending through four segments, with about 35 muscle cell rows, without midline (Figures 1A, 4A, D). Pygidium rounded, with a digitiform median stylus and two long anal cirri, with about 15–23 articles.
JUVENILES AND REPRODUCTION
Several specimens contained developing juveniles inside (e.g. Figure 4A) and this allowed us to follow the process of development in juveniles. Smallest juveniles have only two segments (Figure 5A), only the first chaetiger with chaetae, palps absent, antennae unarticulated, two dorsal tentacular cirri with a single article, lacking any evidence of ventral tentacular cirri, only two compound chaetae per parapodium, and dorsal and ventral simple chaetae from chaetiger 1, dorsal cirri still unarticulated, with a single article; posterior part with several incipient segments, but without cirri or chaetae, pygidium with a distinct, disproportionately long stylus and two articulated anal cirri. In longer specimens with four segments (Figure 5B), only one or two chaetigers, antennae are developed but still unarticulated, dorsal cirri oval, unarticulated, with one anterior segment, probably the peristomium, bearing a single pair of minute, oval, cirri. The pharynx and proventricle are not differentiated yet, but there is an anterior internal dark, barrel-shaped area which will presumably develop into the pharynx and proventricle (Figure 5A, B). Palps are not developed yet, and the prostomium has four eyes and two ventral eyespots. At the stage of 6–7 chaetigers (Figure 5C–E), palps are still absent, the appendages now have few articles (2–4) with the distal one longer and having some distal ‘hairs’; a detailed examination of what looks like a single pair of tentacular cirri, reveals an incipient ventral tentacular cirrus with a single, oval article; the pharynx and the proventricle are developing and differentiated, but still very small (through only one segment each) and without pharyngeal tooth; the pharynx develops in the prostomium and peristomium and the proventricle in the first chaetiger; the number of compound chaetae per parapodium is 5 in anterior, 3–4 in midbody, 2 in the last one; the dorsal simple chaetae start in chaetiger 1 and the ventral in chaetiger 1 or 2. These specimens with few segments have densely ciliated prostomium and peristomium. Specimens with 11 to 12 chaetigers have developing palps, a median antenna with six articles, and lateral antennae with three articles. The pharynx is not well developed and still spans a single chaetiger, the proventricle developing and spans two chaetigers (Figure 5F, G). Specimens of 16–18 chaetigers have developed palps, totally separated from each other or slightly fused basally, proventricle well developed, through 2–3 segments (4–5th or 3–6th), two pairs of tentacular cirri, dorsal simple chaeta from chaetiger 1, ventral simple chaeta from chaetiger 5, about 7 compound chaetae on anterior parapodia, 6 in midbody, 2 in last parapodia (Figure 5H, I).
Females were observed with either eggs or juveniles, but none with both. Developing larvae are usually packed together closely, filling the coelomic cavity of the reproductive segments, and might have obscured any developing eggs if more than one cohort was present simultaneously. Initially juveniles emerge rapidly from the anterior reproductive segments (Figure 4E) so that the mother appears to have a ball of juveniles attached to her. Later the remaining larvae start emerging from more posterior segments with either the head or tail first (Figures 2D, E, 4F). One female survived for two days after giving birth, but was at this point moribund. The female in Figure 4E showed signs of moribundity within hours of giving birth, but she also had extensive damage to the anterior of her body, which may have affected her ability to recover. Several females known to have recently given birth were broken and lacked the posterior half of the body. Several females were observed giving birth to juveniles as young as those in Figure 5A, B. This may have been a stress response to handling and refrigeration.
ETYMOLOGY
Unzima is the word for ‘being pregnant’ in the isiXhosa language.
REMARKS
Syllis unzima sp. nov. is characterized by having unidentate compound chaetae with long spines on margin, a characteristic colour pattern, and its reproduction by viviparity. This species is closely related to other species of the genus such as S. vivipara from the Mediterranean Sea, Atlantic Ocean and Caribbean Sea, S. prolifera, that seems to inhabit circumtropical and temperate areas, S. rubicunda Aguado et al., Reference Aguado, San Martín and Nishi2006, recorded on Japanese coasts, S. antoniae Salcedo-Oropeza, San Martín & Solís-Weiss, Reference Salcedo-Oropeza, San Martín and Solís-Weiss2012, from the Pacific coast of México, S. busseltonensis (Hartmann-Schröder, Reference Hartmann-Schröder, Hartmann-Schröder and Hartman1982) from Australia and S. prolixa Ehlers, Reference Ehlers1901, from South America. All these have the same kind of aciculae, similar chaetal arrangement and a pharyngeal tooth slightly back from anterior margin. Except for S. vivipara and S. prolixa, all have bidentate falcigers. Syllis prolifera and S. rubicunda have distinctly bidentate falcigers, with both teeth similar in size and spinulation shorter and uniform, bidentate dorsal simple chaetae and a peristomium extending forward, partially covering the prostomium. Additionally, S. rubicunda has broad dorsal cirri and a distinctive red or orange colour pattern (see Aguado et al., (2006) for S. rubicunda; Salcedo-Oropeza et al. (Reference Salcedo-Oropeza, San Martín and Solís-Weiss2012) for S. antoniae; Hartmann-Schröder (Reference Hartmann-Schröder, Hartmann-Schröder and Hartman1982) and Licher (Reference Licher1999) for Syllis busseltonensis, and Fauvel (Reference Fauvel1923) and San Martín (Reference San Martín and Ramos2003) for Syllis prolifera). The most similar species is S. vivipara, with similar chaetae (although the spines on the margin are shorter) and viviparous reproduction. However, S. vivipara has no distinct colour pattern and inhabits a different habitat (algae and dead corals) (Krohn, Reference Krohn1869; Licher, Reference Licher1999). In addition, they differ with respect to size; according to Goodrich (Reference Goodrich1900) and Fauvel (Reference Fauvel1923), S. vivipara is 20 mm long while recently examined specimens are only 1.6 mm long (San Martín, Reference San Martín and Ramos2003). Goodrich's specimens have been lost and the size of these larger specimens cannot be confirmed. Syllis parturiens Haswell, Reference Haswell1920, another viviparous species from Australia (Haswell, Reference Haswell1920) and Gulf of Eilat (Red Sea) (Ben-Eliahu, Reference Ben-Eliahu1977), has longer bidentate blades, with shorter spines on the margin of the compound chaetae and the aciculae are acuminate. Syllis prolixa has similar compound chaetae, but the spines on margin are not so long, and it has a distinct different colour pattern, with one median reddish transversal band and two short laterals on each segment (Ehlers, Reference Ehlers1901; Hartmann-Schröder, Reference Hartmann-Schröder1962); recently, one of the authors (G.S.M.) re-examined some specimens of this species from Perú (unpublished data) and confirmed these differences; this species reproduces by stolons (Ehlers, Reference Ehlers1901).
HABITAT AND HOST ASSOCIATIONS
Syllis unzima sp. nov. was found on cultured H. scabra (Figure 6) in a closed experimental re-circulating system. Production tanks were lined with shade cloth and 5 cm of calcium carbonate sand substrate sourced from a commercial sand dune quarry (SSB Mining, Macassar, South Africa). Holothuria scabra were fed once daily with a commercially available abalone weaning diet Abfeed® (1 mm pellet) (Marifeed Pty Ltd, Hermanus, South Africa) at approximately 1% body weight. Syllis unzima sp. nov. was observed on the ventral surface of H. scabra within the three ambulacrae and inter-ambulacrae (Figure 6). When threatened, it was observed to move rapidly across the body and crawl into the mouth or cloacal openings (Figure 6). The infestation of S. unzima sp. nov. was restricted to slow-growing ‘runt’ juvenile H. scabra (mean weight 9.7 ± 4.3 g) in four production tanks stocked at high density (825–1208 g m−2). Within these tanks, an average of 22.1 ± 1.7% (mean ±SD) of the juveniles were infested with between 2 and 7 individuals of S. unzima sp. nov. per host (mean 3.8±1.0). The overall prevalence was low, at 12.3 %, as larger animals (67.7 ± 30.9 g) stocked at more conservative densities (355–675 g m−2) in the remaining 16 tanks were unaffected. Two additional specimens were also found; one on an oyster (Crassostrea gigas) in a closed culture facility and another on foliose coralline algae within the effluent outflow path from an abalone (H. midae) farm, respectively.
DISTRIBUTION
South-west and west coast of South Africa: east and west Walker Bay, and in Kleinzee.
Syllis amicarmillaris sp. nov.
(Figures 7–11)

Fig. 7. Syllis amicarmillaris, sp. nov.: (A) anterior end, dorsal view; (B) midbody parapodium, lateral view; (C–D) long and short alternate dorsal cirri, midbody. Holotype (SAM A60387). Scale bars: A, 375 µm; B–D, 18 µm.

Fig. 8. Syllis amicarmillaris, sp. nov.: (A) compound chaetae, anterior parapodium; (B) aciculae, anterior parapodium; (C) pseudosimple chaetae, midbody; (D) compound chaetae, midbody; (E) aciculae, midbody; (F) compound chaetae, posterior parapodium; (G) dorsal simple chaeta; (H) ventral simple chaeta; (I) acicula, posterior parapodium. Holotype (SAM A60387). Scale bars: A–I, 20 µm.

Fig. 9. Syllis amicarmillaris, sp. nov. scanning electron microscopy: (A) anterior end, dorsal view; (B) midbody dorso-lateral view; (C) posterior end, ventral view (MNCN16.01/14733).
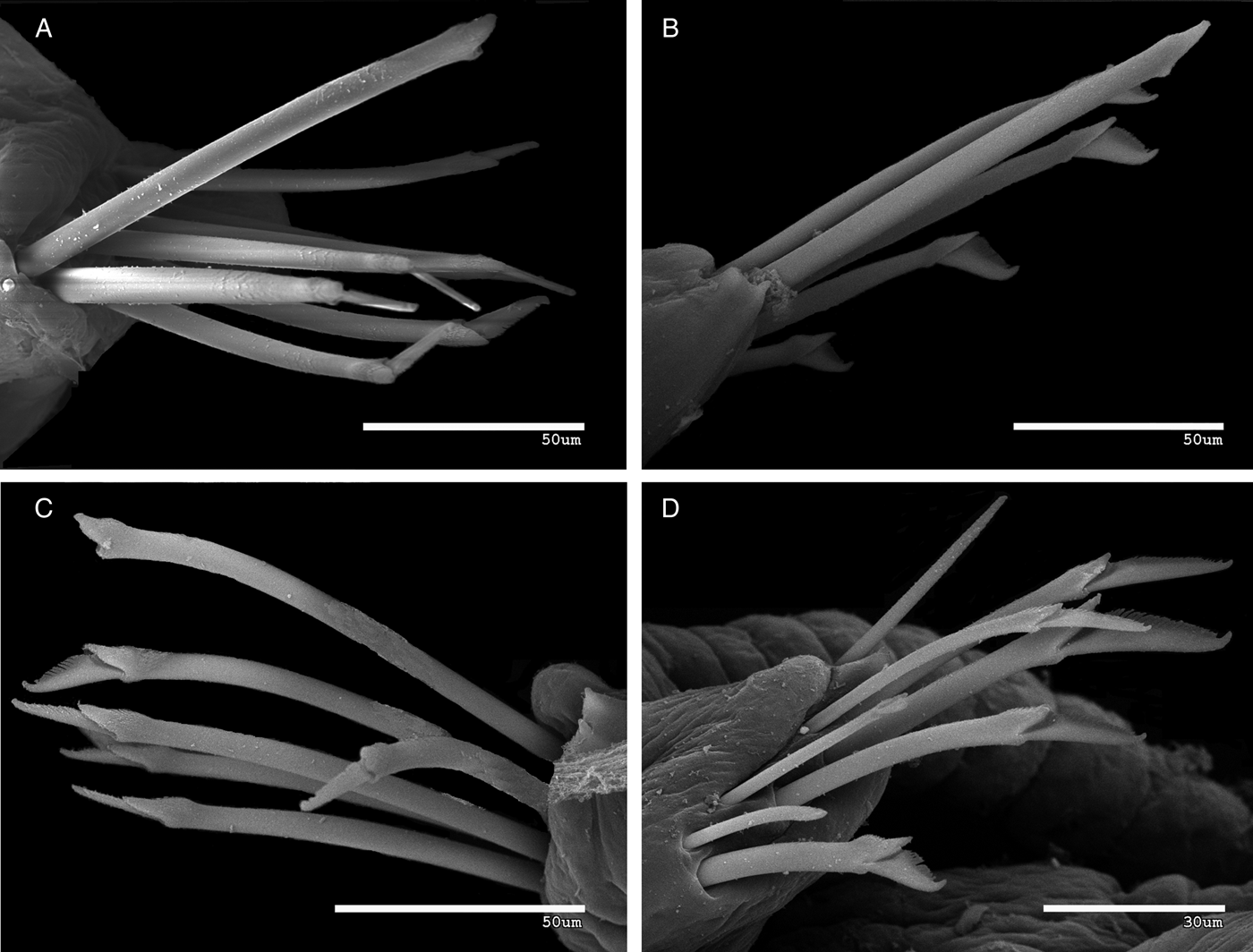
Fig. 10. Syllis amicarmillaris, sp. nov. scanning electron microscopy. Chaetal fascicles: (A–C) midbody; (D) posterior parapodium (MNCN 16.01/14733).

Fig. 11. Syllis amicarmillaris, sp. nov.: (A) whole, gravid female; (B) anterior end; (C) pharynx with tooth; (D) proventricle.
TYPE MATERIAL
Holotype: entire animal with slide of parapodia (coordinates: 34°36′6″S 19°20′12″E; upper intertidal within effluent outflow from on-shore abalone farm) (SAM A60387); coll. C.A. Simon, 21 May 2012.
Paratypes: 1 entire specimen (SAM A60388), 2 entire specimens (MNCN 16.01/14710), 1 whole specimen on SEM stub (MNCN16.01/14733); (Coordinates: 34°36′6″S 19°20′12″E, in upper intertidal within effluent outflow from on-shore abalone farm), all collected by C.A. Simon, 21 May 2012; 1 entire juvenile (MNCN 16.01/14711) (coordinates: 33°1′17″S 17°58′20″E, surface of abalone shells in baskets hanging from long lines in bay); coll. C.A. Simon, 24 October 2012.
DESCRIPTION
Holotype complete, 32 mm long, 0.47 mm wide, 181 chaetigers. Body elongate, cylindrical, without colour pattern (Figure 11A, B). Prostomium oval (Figure 7A). Two pairs of eyes in trapezoidal arrangement (Figures 7A, 11A, B). Palps triangular, as long as prostomium. Antennae, tentacular and dorsal cirri short and thick, distinctly articulated, fusiform (Figures 7A–C, 9A–C, 11A, B). Median antenna slightly longer than prostomium and palps together, with about 19 articles, arising slightly back from posterior pair of eyes; lateral antennae shorter than median one, with about 16 articles, originating in front of anterior pair of eyes (Figures 7A, 11B). Peristomium well differentiated, shorter than subsequent segments (Figures 7A, 11B); two pairs of tentacular cirri, dorsal pair similar to median antenna, with about 13 articles, ventral pair slightly shorter, with about 9–10 articles. Dorsal cirri shorter than body width, fusiform (Figures 7A–D, 9A–C, 11A, B), with espiralized hyaline inclusions on articles (Figure 7B) alternating in length in midbody (about 12–19 articles) (Figures 7A, C, D, 9B, 11A, D). Ventral cirri short, digitiform, not extending beyond parapodial lobes (Figure 7B). Parapodial lobes slightly distally bilobed (Figures 7B, 10D). About 12–14 compound chaetae per parapodium on anterior segments, 6–8 in midbody, up to 5 chaetae per parapodium in most posterior chaetigers. All falcigers heterogomph, bidentate, with small proximal tooth, and thin, moderately long spines on margin, all similar throughout, but some dorso-ventral and antero-posterior gradation in length (Figures. 7B, 8A, D, F, 10A–C); blades of anterior falcigers longer (between 27 and 40 µm) and thinner (Figure 8A) and progressively diminishing in length (Figure 8D, F) (22–35 µm in midbody; around 15–30 µm on posterior parapodium). Except anterior and posterior parapodia, one (sometimes two) dorsal thick simple chaeta by loss of blade and enlargement of shaft (Figures 7B, 8C, 10A–C). Solitary dorsal simple chaeta on posterior chaetigers, slender, bidentate, (Figures 8G, 10D); ventral simple chaetae thin, sinuose, bidentate (Figures 8H, 10D). Up to five aciculae in anterior parapodia (Figure 8B), three in midbody (Figure 8E) and solitary, thicker, acuminate (Figure 8I) in mid-posterior and posterior parapodia. Pharynx long, extending over 9–10 segments, with small, conical tooth on anterior margin (Figures 7A, 11A–C). Proventricle similar in length to pharynx or even longer, extending through 10 segments, with about 45–50 muscle cell rows (Figures 7A, 11A, D). Pygidium rounded, with a digitiform median stylus and two long anal cirri, with about 18 articles.
ETYMOLOGY
The name refers to the similarity to the species S. amica in the chaetae, and S. armillaris in the general aspect of body.
REMARKS
Syllis amicarmillaris sp. nov. is characterized by having an elongated body, with relatively short, fusiform dorsal cirri and the presence of one, sometimes two, pseudosimple chaeta on midbody parapodia by loss of blade and enlargement of shaft. The general aspect of body of S. amicarmillaris sp. nov., is quite similar to S. armillaris and S. hyalina Grube, 1863, two apparently cosmopolitan species (San Martín, Reference San Martín and Ramos2003), but the chaetae are different, with the latter species lacking the typical pseudosimple chaetae by loss of blades and enlargement of shafts. Only four other species of the genus have these chaetae: S. amica from North Atlantic and Mediterranean Sea, apparently cosmopolitan and also recorded on the KwaZulu–Natal coast of South Africa; S. ferrani Alós & San Martín, 1987, from western Mediterranean, S. elongata (Johnson, 1901) from north-east and central east Pacific, and S. magdalena Wesenberg-Lund, Reference Wesenberg-Lund1962, from Chile and Perú. Syllis amica has more elongated dorsal cirri and shorter proventricle, compound chaetae with shorter blades and the posterior aciculae are distally rounded. Syllis ferrani has a distinct colour pattern, ‘variegata’ type, the midbody dorsal cirri are similar to those of S. amicarmillaris sp. nov. but the difference in length between long and short cirri is more marked, and the anterior dorsal cirri are elongated, and the compound chaetae are different, with rounded tips and shorter spines on margin. Syllis magdalena is strongly pigmented with chocolate-brown, the dorsal cirri are more elongated, and the compound chaetae have shorter blades (Hartmann-Schröder, Reference Hartmann-Schröder1962; Wesenberg-Lund, Reference Wesenberg-Lund1962). Finally, S. elongata also has short, fusiform dorsal cirri, but they are much shorter, with fewer articles, and the compound chaetae have much shorter blades, unidentate. Some recently examined specimens of S. elongata from Perú (Aguirre & San Martín, unpublished data) show these differences, and also have slender transversal dark stripes on dorsum of anterior segments. See also Licher (Reference Licher1999) and San Martín (Reference San Martín and Ramos2003) for comparison.
Loss of blades and enlargement of shafts also occur in species of other genera, and it is probably an adaptation to live in very hard substrates such as dead corals, coralligenous concretions, oyster beds, etc. Other examples are Opisthosyllis japonica Imajima, 1966 (Japan and Australia), Opisthosyllis simpliseta Hartmann-Schröder, 1981 (Australia) (see San Martín et al., Reference San Martín, Hutchings and Aguado2008), and Parasphaerosyllis malimali Capa, San Martín & López, Reference Capa, San Martín and López2001, from Central East Pacific (Capa et al., 2001).
HABITAT
Common in sediment within the effluent outflow path from abalone (H. midae) farms and less so on the surface of abalone grown in off-shore longline culture system.
DISTRIBUTION
South-west and west coast of South Africa: west and east Walker Bay, and in Saldanha Bay.
DISCUSSION
Facultative associations with cultured Holothuria scabra
The Syllidae are widely distributed across a range of marine environments from the intertidal zone to the abyssal plains, however they exhibit maximum abundance and diversity in shallow waters (Glasby, Reference Glasby, Beesley, Ross and Glasby2000; San Martín, Reference San Martín and Ramos2003; Aguado et al., Reference Aguado, San Martín and Nishi2006). While Syllis unzima sp. nov. and Syllis amicarmillaris sp. nov. were both first found in intertidal waters of the south and southwestern coasts of South Africa on algae and soft sediments, respectively, they also appear to be relatively flexible in their habitats and are able to form facultative associations with cultured marine invertebrates in both open-ocean and land-based aquaculture systems after entrance via incoming seawater pumping systems. Cultured marine invertebrates such as bivalves, gastropods and holothurians may offer advantageous conditions to syllids, as they represent a high density of potentially suitable hosts and possibly increased access to food.
Polychaetes forming symbiotic or facultative associations with other organisms usually prefer hosts that are relatively large and able to provide good shelter; consequently their location on the host often correlates to the most protected areas (Martin & Britayev, Reference Martin and Britayev1998). In burrowing holothurians this tends to be inside the host burrows and for starfish, sea urchins and ophiuroids, the oral/ventral surface in contact with the sediment (Martin & Britayev, Reference Martin and Britayev1998). This information correlates well with the position of S. unzima sp. nov. on H. scabra and with its sheltering behaviour, which was also observed in the polychaete Gastrolepidia clavigera (Schmarda, 1861) on its tropical holothurian hosts (Britayev & Lyskin, Reference Britayev and Lyskin2002). However, the presence of this worm only on smaller cucumbers in the current study suggests that other factors, such as the high stocking density, may also have influenced infestation levels.
The location of the S. unzima sp. nov. on the sea cucumber H. scabra may not only be associated with shelter or physical protection but may also be linked to feeding behaviour, as suggested by Hendler & Meyer (Reference Hendler and Meyer1982). Fauchald & Jumars (Reference Fauchald and Jumars1979) postulated that all members of the subfamily Syllinae are free-living carnivores feeding on hydroids, bryozoans and other colonial invertebrates, using their pharyngeal tooth to pierce the body surface of their host and suck out the contents with the help of the proventricle. However, the Syllidae demonstrate remarkable morphofunctional diversity in their feeding habits and a diverse range of trophic guilds including carnivores, herbivores and detritivores have since been reported based on the study of intestinal and faecal contents (Giangrande et al., Reference Giangrande, Licciano and Pagliara2000). The current study could not find conclusive evidence to suggest that S. unzima sp. nov. is feeding directly on H. scabra. Given the location of the worms on H. scabra, it is, however, possible that they are actually ‘stealing’ food from their hosts rather than feeding off them. A number of syllids considered as commensals have been observed in locations that allow them to gather food from the alimentary tract of their hosts. For example, Branchiosyllis exilis (Gravier, 1900) positions itself at the proximal end of the ventral surface of its brittle star host Ophiocoma echinata (Lamarck, 1816), directly in the path of food boluses carried by the tube feet to the mouth (Hendler & Mayer, 1982; Martin & Britayev, Reference Martin and Britayev1998). On the other hand, Haplosyllis chamaeleon (Laubier, Reference Laubier1960) has been observed with its anterior end inside the gastric cavity of polyps of the gorgonian Paramuricea clavata (Risso, 1826), potentially feeding on the polyps or polyp stomach contents, and thus living as a parasite or kleptoparasite rather than as a commensal (Laubier, Reference Laubier1960; Martin & Britayev, Reference Martin and Britayev1998; Martin et al., Reference Martin, Núñez, Riera and Gil2002).
Vivipary and larval development
Although brood protection is common among polychaetes (Giangrande, Reference Giangrande1997), vivipary, an extreme form of brood protection, is much less common. Previous reviews of reproduction in polychaetes by Schroeder & Hermans (Reference Schroeder, Hermans, Giese and Pearse1975), Wilson (Reference Wilson1991) and Giangrande (Reference Giangrande1997) recorded 19 viviparous species in 13, nine and nine families respectively, with only some common to all reviews. Our literature review confirmed 26 viviparous species belonging to nine families and five orders (Supplementary Material). With eight confirmed (including S. unzima sp. nov.) and two unconfirmed reports, Syllidae has the greatest representation (Exogoninae and Syllinae), followed by Cirratullidae (four species) and Spionidae and Sabellidae (three species each).
Vivipary ensures maximal survival of offspring through low larval and juvenile mortality (Chia, Reference Chia1974) as the propagules are protected when they are most vulnerable. Thus vivipary is likely to occur when conditions either limit fecundity or increase the vulnerability of exposed larvae. Viviparous polychaetes therefore fall into three categories (Table 1 and Supplementary Material). Firstly; small, interstitial animals that cannot store many eggs (Olive, Reference Olive and Conway Morris1985; Aguado & San Martín, Reference Aguado and San Martín2006). Eighteen of the 25 reviewed species are 10 mm and smaller, and 15 are 5 mm and smaller. Secondly; species inhabiting cold environments where brood care and direct development may eliminate protracted planktonic development (see review by Pearse et al., Reference Pearse, Mooi, Lockhart, Brandt, Krupnik, Lang and Miller2009). This is exemplified by Boccardia natrix (Söderström, 1920) which, at 70 mm, is the largest viviparous species considered here and has been recorded in cold climates. Similarly, vivipary has only been reported for Parexogone hebes (Webster & Benedict, 1884) in Canada, the coldest extreme of its distribution range (Pocklington & Hutcheson, Reference Pocklington and Hutcheson1983). This may be an adaptation to cold rather than to small body size (Franke, Reference Franke1999), but these specimens may actually represent a different biological species to those found in Europe. Thirdly, species living in water of low or variable salinity (cf. Olive, Reference Olive and Conway Morris1985; Barnes et al., Reference Barnes, Calow, Olive, Golding and Spicer2001), which creates a harsh osmotic environment for larvae. Examples include Hediste limnicola (Johnson, 1903), the second largest species reviewed here, Chaetozone vivipara (Christie, 1984), and the spionids Streblospio benedicti Webster 1879 and Rhynchospio glutea (Ehlers 1897). Vivipary in Caobangia Giard, 1893 species may be an adaptation to its small adult size, freshwater environment and shell-boring habit by minimizing loss of larvae while dispersing between hosts and enhancing the development of a local population once settled. Syllis vivipara does not fall comfortably into any of these categories; it is 20 mm long (see Fauvel, Reference Fauvel1923) but lives in environments that are neither extremely cold nor low or variable in salinity.
Table 1. Vivipary in valid species of Syllidae.

S, semelparous; I, iteroparous; *, 2 larvae of slightly different sizes are shown in Russell, Reference Russell1995, figure 2G; # schizogamous reproduction according to Wilson, et al., Reference Wilson, Hutchings and Glasby2003.
Reviews can provide minimum insight into patterns of vivipary in this family because so little information is available (Garwood, Reference Garwood1991; Franke, Reference Franke1999). These reviews have, however, raised the still unanswered questions of whether and how the eggs are fertilized (Goodrich, Reference Goodrich1900; Franke, Reference Franke1999) as well as questions related to the synchrony of embryonic development and number of reproductive events per year, which could be further influenced by body size and the birth processes.
Small marine invertebrates are iteroparous, producing several small broods of asynchronously, and directly, developing embryos, compared to larger species that are usually semelparous, producing large broods of synchronously developing planktonic offspring (Olive, Reference Olive and Conway Morris1985). But what about variability within a group of species with the same developmental mode, but with a large range of body and brood sizes? There is an order of a magnitude difference in size between the smallest and largest known viviparous syllids. Their broods range from 1 or 2 to more than 44 juveniles and there is a single, confirmed, report of the mother's death soon after spawning (Table 1). While S. unzima sp. nov. mothers do survive for a few days after spawning, they may not recover. Given this variability, one could hypothesize that smaller species producing fewer asynchronously developing offspring at a time would be iteroparous, with the converse being true for the larger species. Unfortunately, this hypothesis cannot be tested with the available data. Asynchronous development of embryos, which suggests iteroparity, occurs in the small Parexogone meridionalis (Cognetti, 1955) and Syllis botosaneanui (Hartman-Schröder, 1973), and the large S. vivipara (sensu Goodrich, Reference Goodrich1900, Table 2). By contrast, the only species with large broods and synchronously developing larvae is the intermediately sized S. unzima sp. nov. Iteroparity may also depend on the birth process. Extensive damage to the body was reported in three species. The small Dentatisyllis mortoni Ding, Licher & Westheide, Reference Ding, Licher and Westheide1998 survived just a few days after juveniles broke through the body wall, sometimes causing extensive tears in the process (Ding et al., Reference Ding, Licher and Westheide1998). Similarly, in the larger S. vivipara (sensu Goodrich, Reference Goodrich1900) and S. unzima sp. nov., juveniles emerge by breaking the posterior of the parent's body. However, whether mothers could recover and regenerate was not determined. By contrast, the small P. hebes has distinctive segmental apertures with caps, from which the juveniles emerge (Pocklington & Hutcheson, Reference Pocklington and Hutcheson1983), suggesting that the mother might survive the birthing process more readily than in the previous examples. No information on post-birth survival of mothers, and, by extension, the number of reproductive events per lifetime, is available for the rest of the species.
Table 2. The sequence in which selected sense, digestive and locomotory organs appear in syllid larvae with different modes of development.

The numbers indicate size of the larvae in terms of the number of segments, 0, trochophore larva, 0.5, post-trochophore. N/A, not applicable. Blank cells indicate missing data. ≤ precedes the earliest stage observed with that particular character, but it is not known when it first appeared. #, information gleaned from diagrams.
Larval development has been described for several syllid species, but these descriptions are often incomplete (e.g. Okada, Reference Okada1930; Qian & Chia, Reference Qian and Chia1989; Pernet, Reference Pernet1998). However, available data highlight a high variability in the timing and sequence of the appearance of sensory, feeding and movement organs, and the only difference which could, with confidence, be attributed to larval developmental mode is in the appearance of the first pair of eyespots (Table 2), the first sensory organ to appear in all species. The earliest is during the trochophore or post-trochophore stage in free-spawners and species with abbreviated brood protection (either in the brooding sac attached to female stolons of autolytins or in the gelatinous masses of other genera) and a short pelagic stage. By contrast, in species without a pelagic phase, the eyespots may appear when the larva is just one segment long, to as late as at the ten-segment stage (in the autolytin, Proceraea rzavskyi, which was synonymized with Epigamia alexandri, after Nygren, Reference Nygren2004), with parasitic larvae. The remaining sensory organs considered here all appeared as early as the trochophore stage to as late as the eleven-segment stage. In most species all organs except the palps had appeared at the four-segment stage. The palps appeared from as early as the four-, to as late as the twelve-, segment stages (in S. amica). This variability is exemplified in the Syllinae where the brooded S. unzima sp. nov. larvae develop most of their sensory organs as early as, or earlier than, the free-spawning species. Furthermore, these organs also appear earlier in S. unzima sp. nov. than they do in the externally brooding Exogoninae (Table 2). Thus it is clear that the timing of the appearance of most of the sensory organs is not directly related to larval or juvenile release.
The initial appearance of digestive organs also appears unrelated to larval developmental mode. Although the pharynx and proventricle anlages (the rudiments of the organs) appear relatively early, the timing of the differentiation of these organs varies considerably (Table 2). In the externally brooding Exogoninae, this seems to depend on the size at emergence, while it appears to be delayed in Myrianida Milne Edwards, 1845, S. variegata and S. unzima sp. nov. irrespective of timing of emergence. The appearance of the parapodia seems to be determined by the size at which the larvae become independent rather than developmental mode per se, or possibly by between sub-family differences. Thus they appear just before or when the externally brooded larvae are released. By contrast, they appear at the one- or two-segment stage in the free-spawning Syllis species and S. unzima sp. nov., suggesting that for them the timing of the appearance may be genus specific, rather than related to timing of emergence.
Although several reviews have been written about reproduction in the syllids (e.g. Garwood, Reference Garwood1991; Franke, Reference Franke1999), none have delved deeply into larval development. In this comparison, Heacox's (Reference Heacox1980) observation that larval development in syllids is similar to each other, but with greater similarity within the Syllinae, is supported, but it also highlights a little more variability than previously suggested. More information is needed to conduct more comprehensive comparisons or analyses of larval development and vivipary in this family.
ACKNOWLEDGEMENTS
The authors would like to thank the staff at the abalone farms in Walker Bay for allowing access to their farms, the oyster farmers on the west coast for donating oysters and abalone shells respectively, and Lina Mjindi for suggesting the name for Syllis unzima. We also thank the two referees whose suggestions significantly improved the paper.
FINANCIAL SUPPORT
C.S. received funding from the National Research Foundation (Thuthuka Programme, grant number: TTK20100726000013413) and the University of Stellenbosch, Subcommittee B. G.R. is supported by a Biotechnology and Biological Sciences Research Council (BBSRC) Industrial Case Studentship.
Supplementary Material
The supplementary material referred to in this paper can be found online at journals.cambridge.org/mbi.












