Introduction
Trace fossils generally provide meaningful information about the paleoenvironment in which the trace-producing animals lived, and they also shed light on the paleoecology of these organisms (e.g., Bromley, Reference Bromley1996; Buatois and Mángano, Reference Buatois and Mángano2011). On the other hand, the paleobiology of trace fossils is much less understood because, in most cases, it is difficult to identify the trace-maker. However, for some ichnogenera (e.g., Macaronichnus, Rosselia, Schaubcylindrichnus, Tasselia), possible trace-makers can be identified by detailed observations of fossil specimens combined with the investigation of modern analogous or counterpart traces (Clifton and Thompson, Reference Clifton and Thompson1978; Nara, Reference Nara1995, Reference Nara2006; Seike, Reference Seike2007, Reference Seike2008; Olivero and López Cabrera, Reference Olivero and López Cabrera2010; Seike et al., Reference Seike, Yanagishima, Nara and Sasaki2011), thus making it possible to increase our understanding of the paleobiology of trace fossils. Herein, we describe the ichnogenus Phymatoderma and a star-shaped horizontal trace fossils from the same lithologic unit (Neogene Misaki Formation, central Japan), and compare these trace fossils to modern analogous biogenic structures to identify the trace-maker.
Phymatoderma is composed of distinctive, sub-horizontal burrow systems comprising clusters of radiating tunnels that are filled with pellets (Fu, Reference Fu1991; Seilacher, Reference Seilacher2007; Miller, Reference Miller2011). Phymatoderma has been regarded as a product of a deposit-feeding worm-like animal because of the presence of the pelletal infill, which is generally interpreted as fecal origin (Seilacher, Reference Seilacher2007). In addition, sedimentologic and geochemical studies have revealed that the Phymatoderma producer ingested surface sediments and subsequently excreted fecal pellets into the subsurface burrow (Miller and Aalto, Reference Miller and Aalto1998; Miller and Vokes, Reference Miller and Vokes1998; Izumi, Reference Izumi2012). Despite such understanding of the paleoecology, the actual Phymatoderma-producing animal has not yet been identified (Miller, Reference Miller2011).
Kotake (Reference Kotake1990, Reference Kotake1991) described the fecal pellet-filled ‘giant Chondrites’ from the Pliocene deep-marine Shiramazu Formation, central Japan, and suggested an abyssal echiuran worm as a possible trace-maker of ‘giant Chondrites’ based on indirect, circumstantial evidence. Subsequently, the pellet-filled ‘giant Chondrites’ was reinterpreted as Phymatoderma (Izumi, Reference Izumi2013). Although the conclusion of Kotake (Reference Kotake1990) about the possible trace-maker is highly probable, there is no direct trace-fossil evidence that clearly indicates echiuran activities. In this context, newly found star-shaped trace fossils from the deep-sea Misaki Formation may represent significant specimens that substantiate abyssal echiuran feeding activities, which have been demonstrated by sea-floor photography (Ohta, Reference Ohta1984; Herring, Reference Herring2002), and support Kotake’s (Reference Kotake1990) hypothesis. Therefore, in the present study, Phymatoderma and the star-shaped trace fossils found from the Misaki Formation are described and discussed in detail.
Geologic setting and depositional environment
Miocene–Pliocene marine deposits ascribed to the Misaki Formation are exposed in the southern part of the Miura Peninsula, central Japan (Fig. 1). The Misaki Formation, which is unconformably overlain by the Pliocene Hatsuse Formation (Fig. 1.2), generally consists of fine-grained, light grayish-yellow siltstone and basaltic, lapilli-dominant volcaniclastic beds of pebble-to-sand size, which are known as scoria beds, with tuff intercalations (Lee and Ogawa, Reference Lee and Ogawa.1998). The fine-grained, light-colored siltstone is massive and forms a background that has been interpreted as hemipelagite deposits (Soh et al., Reference Soh, Taira, Ogawa, Taniguchi, Pickering and Stow1989). The beds of volcaniclastic sandstone and conglomerate may reach a maximum thickness of several tens of centimeters, and occur as turbidite, bottom current, and fall deposits (Stow et al., Reference Stow, Taira, Ogawa, Soh, Taniguchi and Pickering1998). The Hatsuse Formation is composed of scoriaceous lapilli and pumiceous beds of calc-alkaline composition (Taniguchi et al., Reference Taniguchi, Ogawa and Soh1991).
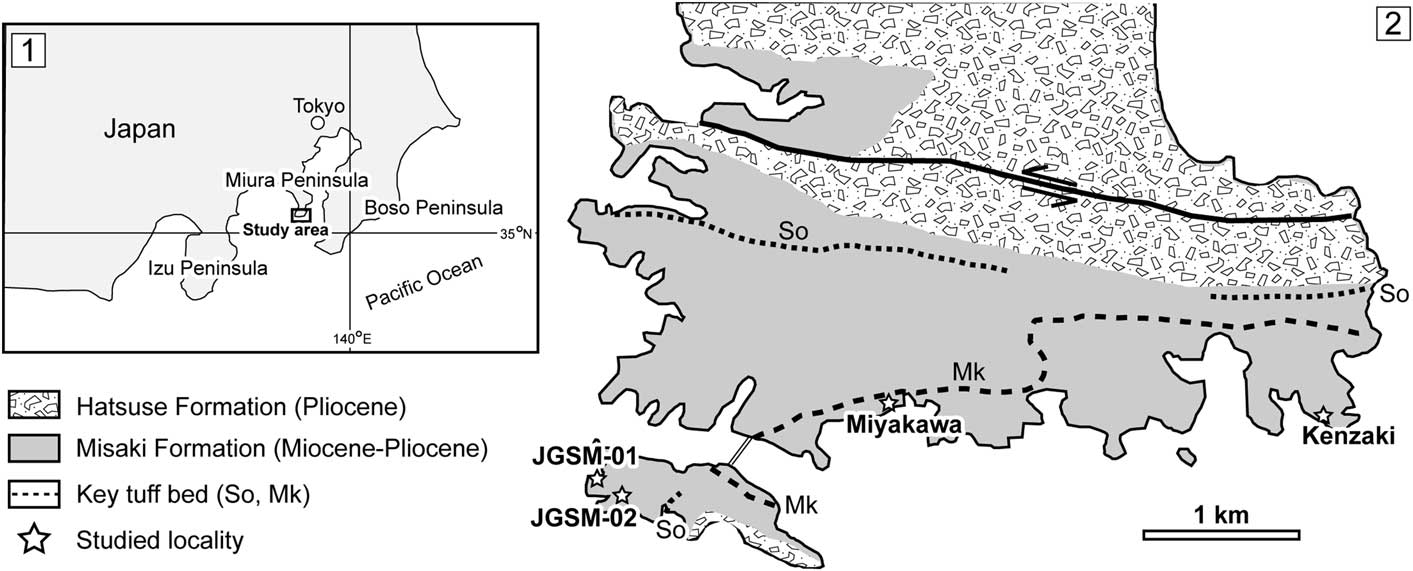
Figure 1 (1) Map of Japan showing the study area (southern part of the Miura Peninsula, central Japan). (2) Simplified geologic map of the southern part of the Miura Peninsula showing detailed localities of the four study sites (white stars). Modified from Yamamoto et al. (Reference Yamamoto, Nidaira, Ohta and Ogawa2009). GPS coordinates of each locality — JGSM-01: 35°08'09.7''N, 139°36'40.7''E; JGSM-02: 35°08'01.1''N, 139°36'43.5''E; Miyakawa: 35°08'28.2''N, 139°38'10.9''E; Kenzaki: 35°08'26.7''N, 139°40'32.6''E.
In the Misaki Formation, there are a few important and well-known key tuff marker beds (Fig. 1.2). Based on the age determination of the Mk and So key tuff beds combined with calcareous nannofossil age data, the depositional age of the Misaki Formation is approximately 9.75–4.50 Ma (Yoshida et al., Reference Yoshida, Shibuya, Torii and Sasajima1984; Kanie and Hattori, Reference Kanie and Hattori1991; Kanie et al., Reference Kanie, Okada, Sasahara and Tanaka1991). The Hatsuse Formation is assigned to stage CN10c (5.0–4.8 Ma; Berggren et al., Reference Berggren, Kent, Swisher and Aubry1995).
According to the benthic foraminiferal assemblage, the depositional setting of the Misaki Formation is interpreted to be a middle abyssal to abyssal environment with a water depth of 2000–3000 m (Akimoto et al., Reference Akimoto, Uchida and Oda1991; Kitazato, Reference Kitazato1997). In contrast, a much shallower environment, with a water depth of less than 200 m, has been estimated for the Hatsuse Formation (Kodama et al., Reference Kodama, Oka and Mitsunahi1980).
Materials and methods
Trace fossils from the Misaki Formation exposed along the southern part of the Miura Peninsula were investigated at four localities (JGSM-01, JGSM-02, Miyakawa, and Kenzaki; Fig. 1.2). Various types of trace fossils, such as Chondrites, Ophiomorpha, Planolites, Phycosiphon, Scolicia, and Zoophycos, were recognized, and some have been reported in previous studies (Lee and Ogawa, Reference Lee and Ogawa.1998). In the present study, Phymatoderma and star-shaped horizontal trace fossils were found for the first time from the Misaki Formation, and are described and discussed in detail.
Observations and measurements of the morphometric parameters of Phymatoderma and star-shaped trace fossils were primarily carried out in the field; however, some parameters of the star-shaped trace fossils were measured using the image-processing program ImageJ. In the laboratory, thin-section and SEM observations of the pelletal infill of Phymatoderma were performed to reveal the actual food source for the trace-maker. For SEM analysis, we used a field emission scanning electron microscope (FE-SEM; JSM-7000F, JEOL) at the Department of Earth and Planetary Science, Graduate School of Science, The University of Tokyo. In the preparation of material for microscopic analysis, small chips of Phymatoderma-bearing rocks were cut and attached to glass slides (28×48 mm), and were then polished with a graded series of carborundum.
Repository and institutional abbreviation
Trace-fossil specimens, including thin sections and polished sections, are housed at The University Museum, The University of Tokyo, Tokyo, Japan (UMUT-CW). Specimen list is available in the Supplemental Data.
Systematic ichnology
Ichnogenus Phymatoderma Brongniart, Reference Brongniart1849
Phymatoderma isp.
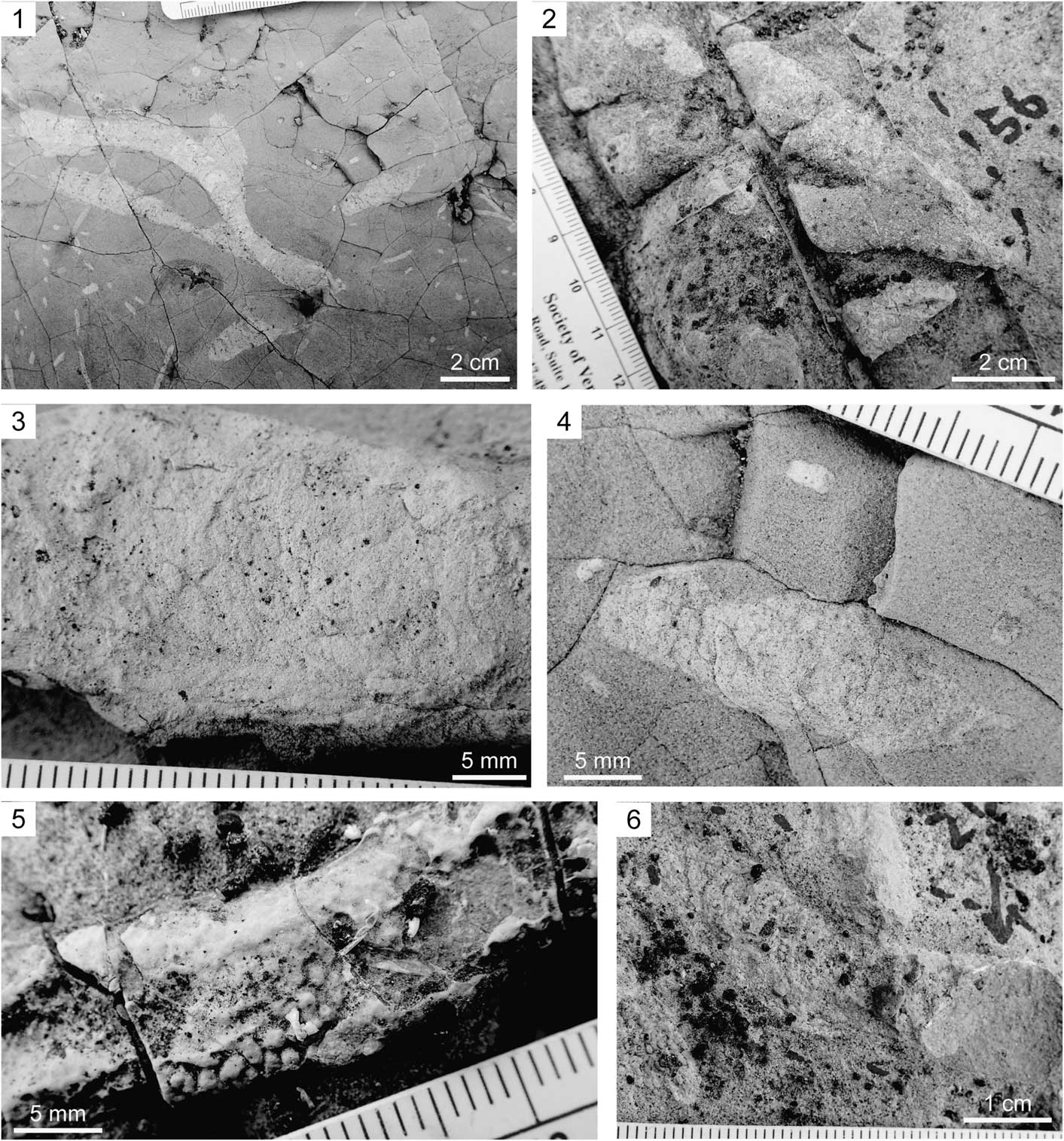
Figure 2 Field photographs of Phymatoderma from the Misaki Formation (all views nearly parallel to the bedding plane). (1) Overall view of Phymatoderma showing partly overlapped second-order branched tunnels; note the co-occurrence of Chondrites with much smaller diameters; JGSM-01 section. (2) Magnified photograph of Phymatoderma emphasizing the first-order branching; Kenzaki section. (3) Magnified view of the tunnel pelletal infill; Miyakawa section. (4) Magnified photograph of the tunnel showing well-preserved pelletal infill; JGSM-01 section. (5) Magnified view of the tunnel and pellets with three-dimensional preservation; Kenzaki section. (6) Magnified photograph showing meniscate structures; Kenzaki section.

Figure 3 Field photographs of Phymatoderma tunnels extending in a slightly upward direction; vertical cross-sectional view; JGSM-01 section. (1) Overall view. (2) Close-up photograph of the tunnels displaying an upward direction; pellets are relatively well preserved and consist of white-colored sediments with minor amounts of black scoria grains; note the partial overlap of tunnel 1 and tunnel 2; dotted lines represent the contour of the tunnels.

Figure 4 Field photograph of the composite Phymatoderma, which was reburrowed with Chondrites (white arrows); vertical cross-sectional view; Miyakawa section; dotted line represents the contour of the tunnel.
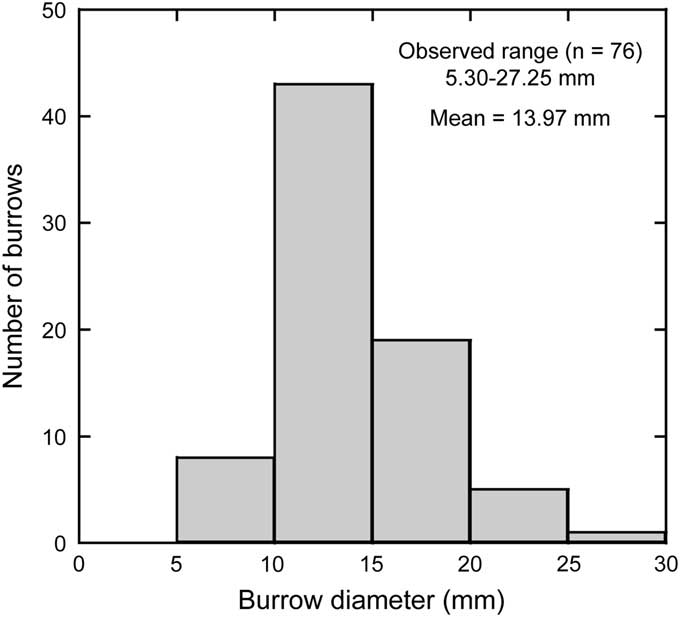
Figure 5 Size distribution of Phymatoderma from the Misaki Formation.
Specimens
Approximately 80 specimens studied in the field in JGSM-01, Miyakawa, and Kenzaki, twelve specimens collected (UMUT-CW31690, 31692, 31693, 31697–31705), two thin sections (UMUT-CW31694, 31695) and two polished sections (UMUT-CW31691, 31696).
Description
A burrow system composed of horizontal to sub-horizontal, straight to slightly curved tunnels with an ellipsoidal cross-section. The tunnels are mostly parallel to the bedding planes, representing first-order, and less commonly second-order branches (Fig. 2.1, 2.2), which overlap in some cases (Fig. 2.1). Although rare, in the vertical cross-sectional view, the tunnels extend slightly upward in direction (Fig. 3). Each tunnel is stuffed with ellipsoidal pellets, which generally show white to light-gray color with minor amounts of black scoria grains (Figs. 2.3–2.5, 3). In some specimens, the tunnels were reburrowed with Chondrites (Fig. 4). The tunnels are highly compressed in most cases; however, three-dimensional preservation is evident in some tunnels and pellets at Kenzaki section (Fig. 2.5). The tunnel diameters, which were measured nearly parallel to the bedding plane, range from 5.30–27.25 mm, with a mean value of 13.97 mm (n = 76; Fig. 5). The branching angle is basically constant in a single specimen (ca. 25–40°; Fig. 2.1, 2.2). Both the tunnels and pellets have no linings. In some specimens, pellets are organized in meniscate structures (Fig. 2.6). However, in other tunnels, such meniscate structures are either nonvisible or only weakly visible (Fig. 2.3).
Remarks
Although these trace fossils might be ascribed to Phymatoderma granulata von Schloteim, Reference von Schloteim1822 or Phymatoderma melvillensis Uchman and Gaździcki, Reference Uchman and Gaździcki2010, the exact classification is difficult. Tunnels filled with pelleted sediments that show local meniscate structure is a diagnostic feature of P. melvillensis (Uchman and Gaździcki, Reference Uchman and Gaździcki2010), which can also be recognized for the present specimens (Fig. 2.6). On the other hand, Miller and Vokes (Reference Miller and Vokes1998) recognized withdrawal structures within the tunnels of P. granulata. However, the occasional occurrence of the primary successive branches in the specimens examined (Fig. 2.1) has never been observed in P. melvillensis (Uchman and Gaździcki, Reference Uchman and Gaździcki2010; Mazumdar et al., Reference Mazumdar, Joshi and Kocherla2011; Izumi and Uchman, Reference Izumi and Uchman2015); instead, these features are commonly recognized in P. granulata (Izumi, Reference Izumi2012). Therefore, specimens with preserved better branching patterns are required to resolve the current ambiguous classification.
Indeterminate star-shaped trace fossil
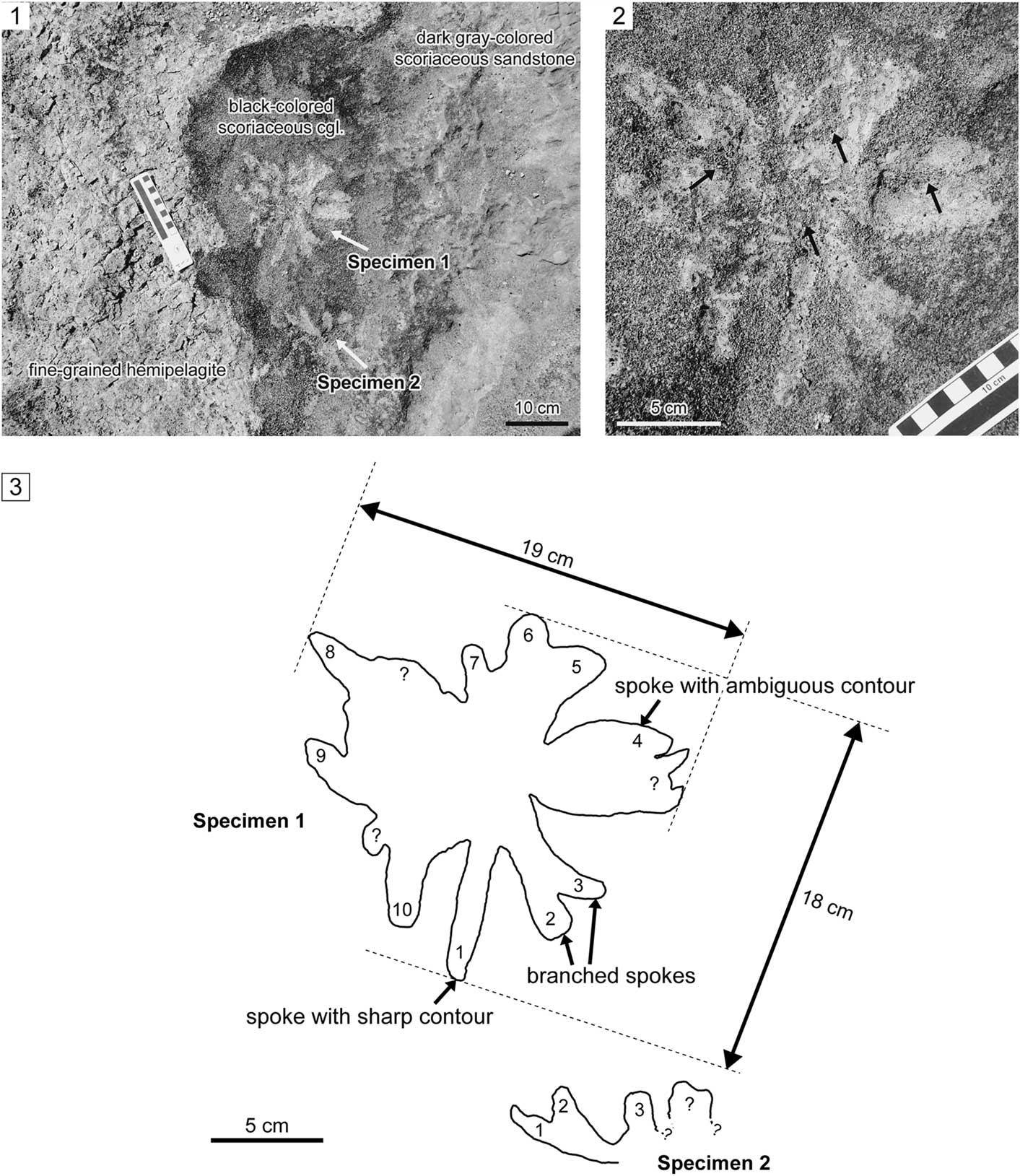
Figure 6 Star-shaped horizontal trace fossils from the Misaki Formation; view parallel to the bedding plane; JGSM-02 section. (1) General view of the two specimens. (2) Magnified view of the relatively well-preserved specimen (specimen 1); note that the spokes are mainly filled with light-gray to yellowish, fine-grained sediments (hemipelagite), with minor amounts of coarser scoria grains (arrows), which might have been derived from the overlying scoriaceous sandstone. (3) Sketch of specimen contours; each obvious spoke is numbered serially.
Specimens
Two specimens studied in the field in JGSM-02.
Description
The nearly complete specimen (specimen 1; Fig. 6) is a relatively large star-shaped (i.e., rosetted) horizontal trace fossil, which consists of a set of many spokes radiating from the center (Fig. 6.2). The specimens occur horizontally in the bedding plane (top surface) of the black scoriaceous conglomerate (Fig. 7). The overall size of specimen 1 is 18 × 19 cm (Fig. 6.3). Some spokes show sharp contours; however, other spokes amalgamate with contiguous spokes, which leads to ambiguous contours (Fig. 6.3). In some cases, several spokes overlap near the center, resulting in branched morphology (Fig. 6.2, 6.3). Spokes are filled with light-gray to yellowish, fine-grained sediments and covered with scarce black, coarse scoria grains (Fig. 6.2). Infilling fine-grained sediments are homogenous (Fig. 6.2). Lithologic composition and color of the main infill of the spokes are remarkably different from the surrounding host scoriaceous conglomerate, and instead resemble and reflect the underlying fine-grained hemipelagite (Figs. 6.1, 7). The exact number of spokes cannot be determined because of the overlapping and/or amalgamation of the spokes, but at least ten spokes can be clearly recognized in specimen 1 (Fig. 6.3). At least three spokes are preserved in the case of the incomplete specimen (specimen 2; Fig. 6.3). The spoke diameters, which were measured nearly parallel to the bedding plane, range from 11.49–20.96 mm for specimen 1 (mean value=15.94 mm; n=10) and 12.40–16.20 mm for specimen 2 (mean value=13.77 mm; n=3). Morphometric parameters are also summarized in Table 1.
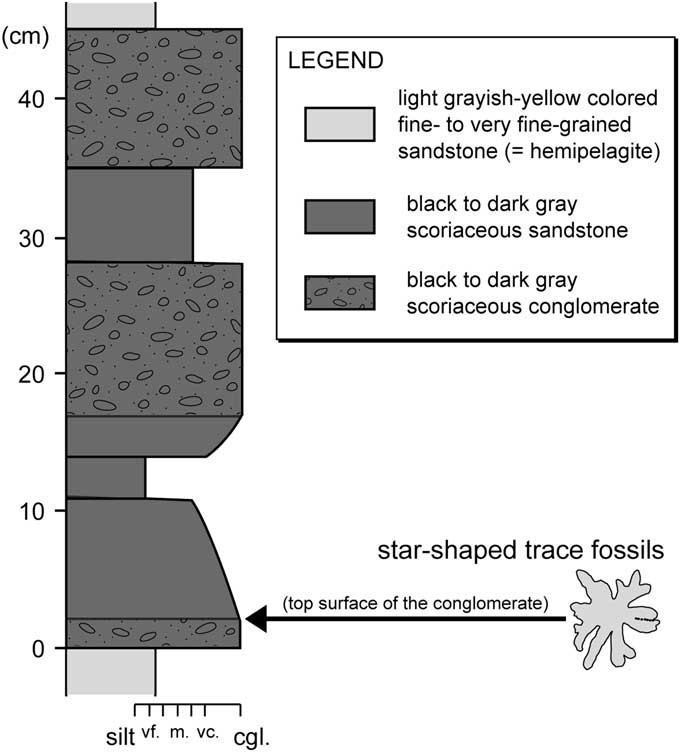
Figure 7 Lithologic column near the occurrence horizon of the star-shaped trace fossils. Note that the star-shaped trace fossils occur horizontally in the bedding plane (top surface) of the black scoriaceous conglomerate, which is indicated by an arrow.
Table 1 Morphometric parameters of the studied star-shaped horizontal trace fossils from the Misaki Formation; SD = standard deviation; mm = millimeters.

* Measured in the lab using an image-processing program called ImageJ
Remarks
The ichnogenus Dactyloidites Hall, 1866 has a similar rosetted morphology (Fürsich and Bromley, Reference Fürsich and Bromley1985; Gibert et al., Reference Gibert, de, Martinell and Domènech1995; Uchman and Pervesler, Reference Uchman and Pervesler2007). The diagnostic feature of Dactyloidites is the vertical radial spreite structure having a central shaft (Fürsich and Bromley, Reference Fürsich and Bromley1985), although spreite are occasional (Wilmsen and Niebhur, Reference Wilmsen and Niebuhr2014). However, the present specimens are clearly horizontal surface traces that lack vertical shafts and spreiten structures (Fig. 6), thus they are not ascribed to Dactyloidites. Alternatively, the present specimens may also be similar to the ichnogenus Glockerichnus Pickerill, Reference Pickerill1982, which is a star-shaped sole trail with numerous ribs radiating from the center. However, the rare occurrence of the star-shaped trace fossil from the Misaki Formation prevents accurate classification.
Microscopic observation of the pelletal infill of Phymatoderma
Dark-brown to black amorphous organic debris of various sizes are recognized in thin sections and are abundant within the pelletal infill of Phymatoderma (Fig. 8.1), along with inorganic mineral particles that are the main component. SEM observations revealed the presence of various types of microfossils (e.g., diatoms, radiolaria) in the pelletal infill (Fig. 8.2–8.6). In most cases, the diatom tests within the pellets were mostly discrete test fragments (Fig. 8.2). Although much less common, relatively well-preserved diatoms such as Stephanopyxsis sp. (Fig. 8.3) and Paralia sp. (Fig. 8.4) were occasionally observed. The preservation of radiolaria was generally better than that of diatoms (Fig. 8.5, 8.6).
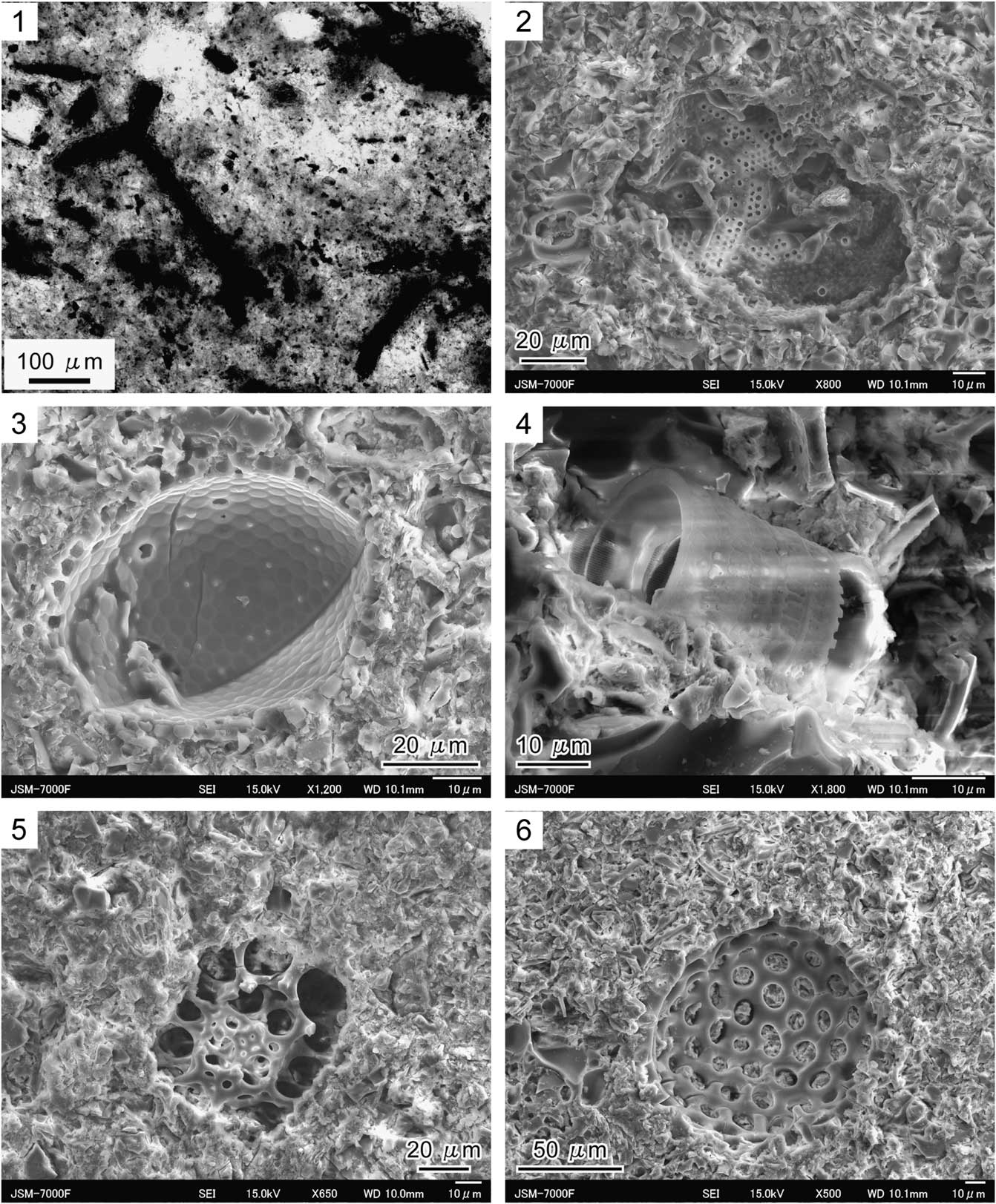
Figure 8 Photomicrographs of the pelletal infill of Phymatoderma from the Misaki Formation; thin-section (1) and SEM (2–6) images; samples UMUT-CW31694 and UMUT-CW31696 from Miyakawa section were used for thin-section and SEM observations, respectively. (1) Photomicrograph emphasizing the presence of amorphous dark-gray to black organic debris. (2) Fragment of diatom microfossil. (3) Diatom genus Stephanopyxsis. (4) Diatom genus Paralia. (5, 6) Indeterminate radiolarian.
Discussion
Comparison with modern analogues and likely trace-maker
To identify the animal that most likely produced the specific trace fossils, it is useful to compare them to modern analogous traces. The relatively large star-shaped horizontal trace fossil is closely similar to surface-feeding traces produced by echiuran worms, which have been recognized on modern deep-sea floor (Ohta, Reference Ohta1984; Herring, Reference Herring2002). Among the modern analogous star-shaped surface feeding traces, Type IV traces described by Ohta (Reference Ohta1984), whose possible producers are echiuran worms, are most similar to the present fossil specimens (Figs. 6, 9; see also Ohta, Reference Ohta1984, fig. 4).
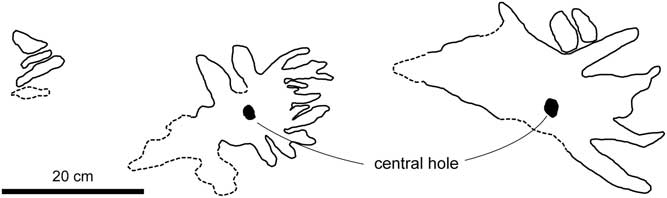
Figure 9 Sketch of modern star-shaped feeding traces produced by abyssal echiuran worms (type IV traces by Ohta, Reference Ohta1984, fig. 4, redrawn). Central holes are present (arrows); dotted lines represent the ambiguous contour of the traces. Note the general similarity of overall morphology between these traces and the star-shaped trace fossils from the Misaki Formation (Fig. 6).
Not only are overall morphologic similarities between the fossil specimens and modern analogues evident (Figs. 6, 9), but dimension and the number of spokes are also similar (discussed below; Table 2). Namely, lengths of spokes of modern type IV star-shaped traces range from 86–152 mm (Ohta, Reference Ohta1984, table 1). Although Ohta (Reference Ohta1984) did not describe the overall size of the type IV traces, the overall size ranges from ~17.2 cm-radius (=86 mm×2) to 30.4 cm radius (=152 mm×2). The star-shaped trace fossil from the Misaki Formation (specimen 1) is ~18 cm×19 cm (Fig. 6.3), which is probably within the range of Ohta’s (Reference Ohta1984) type IV traces. In addition, the spoke diameters of both modern and fossil specimens are also very similar (Table 2). In the case of fossil specimen 1 from the Misaki Formation, the spokes are 11.49–20.96 mm in diameter (Table 1), which is also within the range of the recorded spoke diameters of the modern type IV traces (10.70–26.50 mm; Ohta, Reference Ohta1984). Both fossil and modern star-shaped traces also have a comparable number of observed spokes (Table 2). At least 10 spokes can be distinguished in fossil specimen 1 (Fig. 6.3), and Ohta (Reference Ohta1984) observed 12–20 spokes in the type IV traces. Type IV traces have smaller numbers of spokes than other types of modern deep-sea star-shaped traces (type II, III; Ohta, Reference Ohta1984), but are quite similar to fossil specimen 1. Lastly, bathymetry of both modern and fossil traces is quite similar (Table 2), which occur in deep-water settings with water depth of more than 2000 m (Ohta, Reference Ohta1984; Akimoto et al., Reference Akimoto, Uchida and Oda1991; Kitazato, Reference Kitazato1997). These lines of evidence suggest that the star-shaped horizontal trace fossils from the Misaki Formation are the feeding traces produced by surface deposit-feeding activity of echiuran worms (Fig. 10).
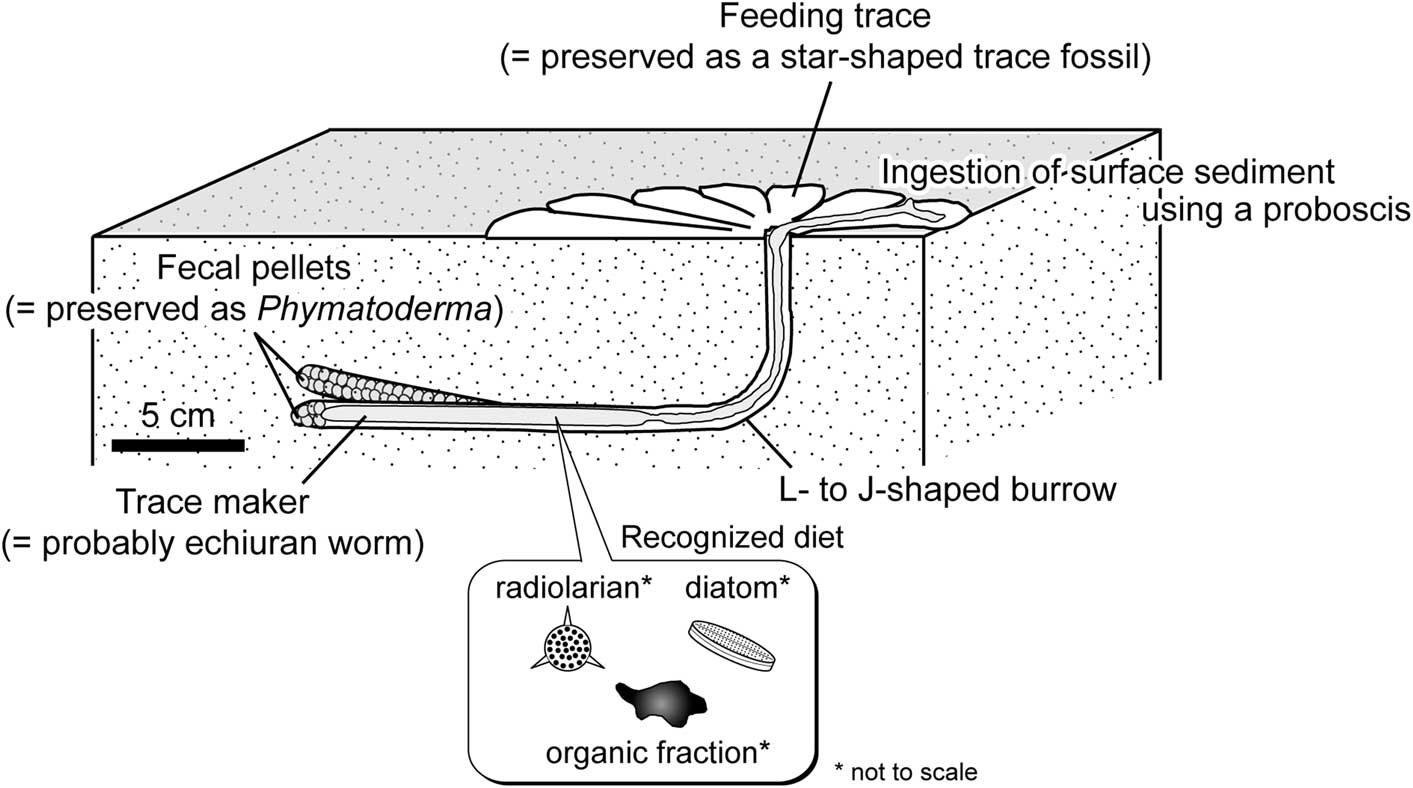
Figure 10 Schematic diagram reconstructing the formation process of Phymatoderma and the star-shaped trace fossils from the Misaki Formation. Both types of trace fossils were probably produced by abyssal echiuran worms. The star-shaped trace fossil may have been a surface-feeding trace produced by deposit-feeding activity, whereas Phymatoderma is interpreted to have been subsurface fecal pellets excreted in the L- or J-shaped burrow by the trace-maker. Echiuran feeding trace and burrow were drawn based on de Vaugelas (Reference de Vaugelas1989). Because the star-shaped feeding traces are surface biogenic structures, the preservation potential might be much lower than that of the subsurface fecal pellets. Microorganisms (e.g., diatom, radiolarian) and fresh organic fraction may have been the actual food source for the trace-maker.
Table 2 Comparison of the present star-shaped trace fossils with their modern analogous echiuran feeding traces; mm = millimeters; cm = centimeters.

*Fossil specimen: Specimen 1 from the Misaki Formation described by this study.
*Modern analogue: type IV traces described by Ohta (Reference Ohta1984).
**Longer axis of the central hole, which was measured using fig. 4 in Ohta (Reference Ohta1984).
***Shorter axis of the central hole, which was measured using fig. 4 in Ohta (Reference Ohta1984).
The pellets of Phymatoderma have been interpreted as fecal pellets that were excreted by a surface deposit-feeding producer and were stuffed within the burrow (Miller and Aalto, Reference Miller and Aalto1998; Miller and Vokes, Reference Miller and Vokes1998; Izumi, Reference Izumi2012). Considering that both Phymatoderma and star-shaped trace fossils occur in the same lithologic unit (Misaki Formation), it is reasonable to presume that Phymatoderma from the Misaki Formation represents the fecal pellets excreted by deposit-feeding echiuran worms (Fig. 10). Abyssal echiuran worms typically produce L-shaped burrows (de Vaugelas, Reference de Vaugelas1989), and the fecal pellets resulting from deposit-feeding activity on the sediment surface may be excreted and retained in the burrow (Ohta, Reference Ohta1984). Such a mode of excretion (e.g., fecal pellets stuffed within the burrow) has been known also for the shallow-water echiuran worm Echiurus (see Elders, Reference Elders1975); thus, this may be considered a common excretion mode of echiurans. Some of the subsurface fecal pellets excreted by ancient deep-sea echiuran worms might have been preserved in Phymatoderma, as illustrated in Figure 10. Vertical cross-sectional views of Phymatoderma from the Misaki Formation occasionally show upward branching (Fig. 3), which implies that the trace-maker of Phymatoderma produced J-shaped burrow in some cases (Fig. 10). J-shaped burrows made by echiuran worms have been observed in Maxmuelleria lankesteri (see Nickell et al., Reference Nickell, Atkinson, Hughes, Ansell and Smith1994, fig. 2c), although this species lives in the muddy sediments of shallow-marine environments (Nickel et al., 1994). In addition, it is worthwhile to compare the sizes of Phymatoderma from the Misaki Formation with those of the central holes of Ohta’s (Reference Ohta1984) type IV traces, because the tunnel diameter of Phymatoderma and the central hole of the echiuran feeding trace might roughly correspond to the body width of the producers. Because of the lack of the original description by Ohta (Reference Ohta1984), the widths and lengths of the central holes of type IV traces (Ohta, Reference Ohta1984, fig. 4) were measured using an image-processing program (Image J). As a result, the width and length of the central hole range from 15.16–21.95 mm and 25.75–26.73 mm, respectively (Table 2), which are within the range of the tunnel diameter of Phymatoderma (5.30–27.25 mm; Fig. 5). This similarity in size may also support our interpretation that both star-shaped trace fossils and Phymatoderma from the Misaki Formation were feeding and fecal traces of ancient abyssal echiuran worms (Fig. 10).
On the basis of comprehensive studies of the pellet-filled Zoophycos from the Neogene deep-marine deposits exposed in the southern part of the Boso Peninsula, central Japan, Kotake (Reference Kotake1990, Reference Kotake1992, Reference Kotake1995) assumed that the abyssal echiuran worm was a possible trace-maker of Zoophycos. Indeed, Zoophycos was also observed from the Misaki Formation by previous authors (Lee and Ogawa, Reference Lee and Ogawa.1998) and during our fieldwork at the Kenzaki section. However, pellets are only weakly visible or nonvisible in the Misaki Zoophycos. Although this study cannot exclude the possibility that Zoophycos was also a fecal trace made by deep-sea echiuran worms, the overall complex morphology of pellet-filled Zoophycos (Kotake, Reference Kotake1989, Reference Kotake1992, Reference Kotake1995) does not resemble the previously known echiuran burrow morphology (de Vaugelas, Reference de Vaugelas1989; Nickell et al., Reference Nickell, Atkinson, Hughes, Ansell and Smith1994); thus, our interpretation that the deep-sea Phymatoderma was produced by echiuran worms (Fig. 10) seems more probable.
Chronologic comparisons
During the Phanerozoic, the oldest known body-fossil evidence of echiuran worms is Coprinoscolex ellogimus, which was described from the Middle Pennsylvanian Francis Creek Shale of the Mazon Creek area, northeastern Illinois (Jones and Thompson, Reference Jones and Thompson1977). The oldest known record of Phymatoderma is P. burkei from the Permian Teresina Formation of Brazil (Lima and Netto, Reference Lima and Netto2012). However, considering the records of ‘Chondrites granulatus’ that are now interpreted as Phymatoderma granulata (e.g., Fu, Reference Fu1991; Miller, Reference Miller2011; Izumi, Reference Izumi2012, Reference Izumi2013), the oldest known C. granulatus was found from the Carboniferous Limestone in Bristol (Simpson, Reference Simpson1957). The temporal coincidence of the oldest known Phanerozoic evidence of both echiuran body fossil and Phymatoderma may further reinforce the possibility that Phymatoderma is an echiuran fecal trace fossil (Fig. 10).
Preservation potential
The occurrence of the star-shaped trace fossil is far rarer than that of Phymatoderma, in the case of the Misaki Formation. This may be related to the difference in preservation potential between surface and subsurface biogenic structures. As discussed above, the star-shaped trace fossils are interpreted as surface feeding traces produced by a probable echiuran worm (Fig. 10). On the other hand, fecal pellets excreted within the subsurface burrows may be preserved in Phymatoderma (Fig. 10), although Phymatoderma generally occupies a relatively shallow tier (e.g., Miller and Vokes, Reference Miller and Vokes1998).
It is reasonable to assume that the deeper a trace is produced below the surface, the higher its preservation potential; thus, surface traces have a very small chance of being preserved (Wetzel, Reference Wetzel2010). Despite the low preservation possibility of surface traces, it has been inferred that the preservation filter is switched off by non- or low-erosive event deposits (e.g., volcanic ash, distal turbidites) that prevent further bioturbation below (Wetzel, Reference Wetzel2010). This process enables surface traces to be observed on the sediment surface, as presented by previous authors (e.g., Ekdale and Berger, Reference Ekdale and Berger1978; Kitchell et al., Reference Kitchell, Kitchell, Johnson and Hunkins1978; Gaillard, Reference Gaillard1991; Wetzel, Reference Wetzel2008). In fact, the star-shaped horizontal trace fossils from the Misaki Formation can be observed just on the sediment surface (Fig. 6). The star-shaped trace fossils are overlain by volcaniclastic (scoriaceous) sandy sediments, which are interpreted to have been deposited quickly (Lee and Ogawa, Reference Lee and Ogawa.1998); and this may have prevented further erosion of surface traces by bioturbation.
Diets of the trace-maker
Potential food sources for deposit feeders are fresh organic fractions such as microbes, plankton, meiofauna, microalgae and non-living organic matter in ingested sediment particles (Lopez and Levinton, Reference Lopez and Levinton1987; Levinton, Reference Levinton1989; Mayer, Reference Mayer1989). Therefore, in the case of fossil fecal pellets excreted by deposit feeders, it is reasonable to assume that organic matter and/or microfossils preserved within the pellets were the actual diets of the producers. In particular, amorphous organic debris (phytodetritus) and various types of microfossils (coccoliths, dinoflagellates, and planktic foraminifer) have been actually observed within the pelletal infill of Phymatoderma (Miller and Vokes, Reference Miller and Vokes1998; Olivero et al., Reference Olivero, Ponce, López Cabrera and Martinioni2004; Miller, Reference Miller2011; Izumi, Reference Izumi2013; Izumi et al., Reference Izumi, Rodríguez-Tovar, Piñuela and García-Ramos2014). This study provides new evidence that amorphous organic debris and siliceous microfossils (e.g., diatoms and radiolaria) are preserved in the fecal pellets of Phymatoderma from the Neogene Misaki Formation (Fig. 8), strongly suggesting that such materials were the main dietary sources for the trace-maker (Fig. 10). Considering that calcareous nannofossils and foraminifers are recognized from the Misaki Formation (Kanie et al., Reference Kanie, Okada, Sasahara and Tanaka1991; Kitazato, Reference Kitazato1997), these microorganisms might have also been fed upon by the Phymatoderma-producer; however, microscopic observations by this study did not detect calcareous nannofossils or foraminifers. Among such various microorganisms, diatoms might have played an extremely significant role as a dietary source for the Phymatoderma-producer because diatoms have relatively higher blooming rate than other blooming phytoplankton such as calcareous nannoplankton (Margalef, Reference Margalef1979; Furnas, Reference Furnas1990; Hüneke and Henrich, Reference Hüneke and Henrich2011).
In general, phytodetrital organic matter is much more important than terrigenous organic matter for deep-sea benthos because there is usually little or infrequent flux of terrestrial organic debris to the deep-sea floor, whereas sinking phytodetritus is continuously delivered through the water column to the seafloor despite seasonal fluctuation (Alldredge and Cohen, Reference Alldredge and Cohen1987; Gage and Tyler, Reference Gage and Tyler1991; Kepkay, Reference Kepkay1994; Armstrong et al., Reference Armstrong, Lee, Hedges, Honjo and Wakeham2001; Klass and Archer, Reference Klass and Archer2002; Honjo et al., Reference Honjo, Manganini, Krishfield and Francois2008). In addition, various oceanographic studies have revealed that episodic inputs of organic matter due to phytoplankton bloom aggregation may be critical for sustaining abyssal benthic communities including mobile megabenthos (see Turner, Reference Turner2002 for review). These lines of evidence may also support our interpretation that diatoms are the most significant diet for the trace-maker.
Conclusions
Phymatoderma and the star-shaped horizontal trace fossil were discovered from the same lithologic unit (Neogene deep-marine Misaki Formation, central Japan). Compared with modern analogous surface-feeding traces and subsurface burrows produced by deep-sea echiuran worms, the star-shaped trace fossil and Phymatoderma found in the Misaki Formation may be interpreted as feeding and fecal traces of ancient abyssal echiurans, respectively. The difference in preservation potential between surface and subsurface traces may result in the abundant occurrence of Phymatoderma as compared to the relatively rare star-shaped trace fossil. Thin-section and SEM analyses of the pelletal infill of Phymatoderma provide evidence that the trace-maker fed on organic debris and microorganisms (diatoms and radiolaria). It may be assumed that other microorganisms, such as foraminifer and calcareous nannoplankton, were also the prey of the trace-maker, although these microfossils were not observed. Considering that the oldest known Phanerozoic echiuran body fossil and ‘Chondrites granulatus,’ which has been now interpreted as Phymatoderma granulata, have been found from Carboniferous marine strata, our interpretation that Phymatoderma contains echiuran fecal pellets is further reinforced.
Acknowledgments
We thank K. Endo (The University of Tokyo) for his supervision throughout the present study. We are also indebted to A. Okubo (The University of Tokyo) for her assistance with SEM analysis, M. Saito-Kato and K. Ogane (National Museum of Nature and Science, Tokyo) for their helpful information about microfossils and marine plankton, Y. Ito (The University Museum, The University of Tokyo) for his assistance with housing the trace-fossil specimen at the museum, and K. Tanabe, M. Gunji (The University Museum, The University of Tokyo), K. Seike and R. Goto (The University of Tokyo) for the crucial discussion and comments. This work was financially supported by a grant from the Japan Society for the Promotion of Science awarded to KI (24-8818). Thanks are also due to S. Hageman (Editor), G. Mángano (Associate Editor), and two reviewers (A. Uchman and anonymous reviewer), who improved the manuscript greatly during revision.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.8jr80














