INTRODUCTION
Until 15 years ago, little was known about infections of humans with Oesophagostomum species. Not more than some 53 cases of human oesophagostomiasis had been reported across the globe. Oesophagostomum infection in man was considered to be a rare zoonosis and a variety of monkey species were suspected to be the normal host (Baylet and Paillet, 1959; Haaf and van Soest, 1964; Barrowclough and Crome, 1979). Adult Oesophagostomum worms were once recovered from stool samples that were supposed to contain adult hookworms in prisoners in northern Nigeria (Leiper, 1911; Johnson, 1913). In 1991, following the discovery that the human population in northern Togo excreted eggs of Oesophagostomum bifurcum, it was concluded that O. bifurcum is a locally common parasite of humans not requiring any animal reservoir to complete its life-cycle (Polderman et al. 1991).
Details about the life-cycle and route of transmission of Oesophagostomum infection in humans are lacking but, based on experimental infection of monkeys and with related species of sheep, it is assumed that humans are infected by ingestion of L3-larvae through the oral route (Eberhard et al. 2001; Dash, 1973). Ingested larvae pass through 2 stages of development, an intestinal stage occurring in the intestinal lumen and a histotropic stage normally occurring in the gut wall. The adult Oesophagostomum worms live as separate sexes in the intestinal lumen of the definitive host and lay eggs that are excreted with host's faeces and, if deposited onto soil with appropriate temperature and humidity, they hatch and develop into infective forms (filariform larvae) within 3–7 days (Dash, 1973; Barutzki and Gothe, 1998; Fossing, 1995; Pit et al. 2000). In the subsequent histotropic stage of infection, L3-larvae invade the colon wall, but tissues of extra-intestinal organs such as the lungs, liver and muscles of the trunk and abdominal walls may occasionally be affected as well. The palpable and painful protruding mass formed as a result of intense tissue reactions around a nodule containing juvenile Oesophagostomum worms is called a ‘Tumour de Dapaong’ named after the provincial capital of the northern Togolese province where these characteristic lesions were originally described (Gigase et al. 1987). Some larvae manage to escape from the nodules to return to the intestinal lumen and mature into adult male and female worms for the commencement of egg production.
Systematic and area-wide surveys conducted in northern Ghana and Togo confirmed that O. bifurcum is a common parasite in these regions with an estimated 25% of the indigenous population infected and a million at risk (Pit et al. 1999a). The area along the Ghana-Togo border was shown to be the most intense focus of infection with high village prevalences of both O. bifurcum and hookworm infection (Storey et al. 2000; Yelifari et al. 2005). It is unclear why the distribution of O. bifurcum infection is limited to this area alone and why infection is associated with females and specific age groups.
This paper primarily presents a detailed analysis and interpretation of the age and gender-specific differences in the prevalence and intensity of infection with O. bifurcum compared to those of hookworm. A major reason for such analysis is that the route of transmission for O. bifurcum infection is still obscure. Oral infection with L3-larvae as demonstrated for O. bifurcum infections in monkeys would seem possible but current epidemiological data seem to suggest otherwise in humans. Association with hookworm infection has appeared to be the rule in previous observations and may suggest percutaneous rather than oral transmission (Krepel, Baeta and Polderman, 1992; Pit et al. 1999a; Yelifari et al. 2005). The paper also examines other potential causes of aggregations of infection in this population.
POPULATIONS, MATERIALS AND METHOD
The study area is located in the northeast part of Ghana in the Bawku east district of the Upper East Region and covers about 60 km2. The area is subsahelian with savannah grassland; the principal crops cultivated in the area are maize and sorghum. The rainy season lasts from May to September with approx. 800–1000 mm of annual rainfall followed by a somewhat longer dry season. The principal tribes of the area are the Bimoba (65%) and Kusasi (29%). Three other minority tribes: Mamprusi (2%), Fulani (2%) and Busanga (2%) also live in the area. Extended families live in scattered compounds, housing an average of 14 persons per compound (range 1–180). The compounds are grouped in villages but the delineation between one village and another is vague. The localization of the study area with its widely dispersed compounds is shown in Fig. 1.

Fig. 1. Map of the study area showing the distribution of villages and compounds in the area. The numbers indicate different villages. Small dots-compound not examined in this survey.
Initially, all compounds of the area were mapped with GPS (Global Positioning System) and identification numbers assigned to them. All the inhabitants of the compounds were registered and assigned a unique identity number. The latitudes and longitudes of all compounds and of the middle of all villages were mapped. Data from 24 villages with approximately 17500 inhabitants living in 1227 compounds were recorded.
Following mapping and census, 10% of the registered population was randomly sampled through a cluster sampling procedure with the compound as the sampling unit. When the sum of all inhabitants of the sampled compounds in a village fell short of the planned 10% of the registered village population, additional compounds were added. In the selected compounds, all inhabitants were included in the study. Due to time constraints, 3 villages were not examined and consequently, 1245 subjects in the selected compounds were asked to provide a stool sample for parasitological examination. Samples were received from 1087 subjects, resulting in a compliance of 87·3%. Samples from 76 persons were so small that they had to be discarded. Data presented thus include stool samples from 1011 persons.
Basic demographic data were collected from the selected compound including the number of persons present in the compound, age, gender and tribal background. The presence of animals and hygienic status of compounds were also noted. A compound was considered clean if the floor was properly swept and cleaned and the entrance was kept clean without weeds.
Stool samples were collected and processed on the same day. From each sample, a single 25 mg Kato smear was prepared and examined under ×100 magnification within 15–30 min of preparation. Since eggs of O. bifurcum and hookworm are morphologically similar and cannot be differentiated by the Kato procedure, differential diagnosis was made by coproculture. From each stool sample, 3 cultures were prepared as previously described (Ziem et al. 2005). In short, portions of 6 g of stool were mixed with equal volumes of vermiculite and the mixture was divided into 3 Petri dishes. The coprocultures were kept moist at room temperature (25–35 °C) and checked daily for the presence of maggots that were manually removed if present. Two of the cultures were examined microscopically by 2 different microscopists and the third one served as a spare culture, to be used in case maggots or fungi spoiled the other cultures. After a culture period of 5–7 days, the culture fluid was ‘harvested’ into a conical tube and examined after 2 h. Aliquots of 100 μl of the sediment were examined under the microscope using ×100 magnifications for the presence of L3-larvae. The differentiation between L3-larvae of O. bifurcum, hookworm and Strongyloides stercoralis was based on criteria described by Blotkamp et al. (1993). Larval counts are presented as the total numbers of larvae found in 2 cultures; in the few instances where only 1 culture could be read, the count of that culture was multiplied by a factor of 2.
Data analysis
The demographic and parasitological data were cleaned and entered in a computer using EpiInfo version 6 (CDC, Atlanta, GA, USA). Statistical analysis was performed using SPSS statistical package, version 11.1 (SPSS Inc., Chicago, IL, USA) and S-PLUS 6.2 Professional Edition (Insightful corp., Seattle WA, USA).
The distribution of infection in the area was described both at the individual level and at the village level. For the total number of individuals examined, (n=1011), O. bifurcum and hookworm infections were expressed as age-, gender- and tribe-specific prevalences and the intensity of infection was calculated as geometric mean egg count (gmec) and geometric mean larval count (gmlc) of positive individuals. Differences of infection between groups were analysed with non-parametric (Kruskal-Wallis) tests and a P-value less than 0·05 was considered significant.
Analysis of clustering of infections at the village and compound levels was done for the presence of O. bifurcum infection (larval count ≥1 larva), the presence of heavy O. bifurcum infections (>10 larvae cultured) and for heavy hookworm infections (>100 larvae). Infections can be evenly distributed or clustered in compounds. The number of compounds with a given number of O. bifurcum and hookworm-infected inhabitants was described according to the number of inhabitants examined. The observed distribution of infected inhabitants per compound is compared to the expected number of individuals, assuming a simple binomial distribution using a test for household aggregation described by Walter (1974) and Conway et al. (1995).
O. bifurcum infection over the area was stratified according to village prevalence of infection and tribal composition within a village. A village was considered a low prevalence village if O. bifurcum prevalence was less than 50% and a high prevalence village if the prevalence was 50% or more. Villages inhabited by Bimoba, Kusasi or both tribes, were categorized as Bimoba-, Kusasi- and mixed-villages respectively. The distribution of O. bifurcum infection according to prevalence and tribe was compared and represented on a map using S-PLUS 6.2 professional.
Ethical considerations
Ethical clearance for the study was obtained from the Ghana Health Service in Bolgatanga and a witnessed informed consent procedure was followed. Both the head of compound and selected subjects were verbally asked for their consent; another team member kept record of proper adherence to this verbal procedure. After the survey, all persons examined were treated with 400 mg albendazole. The project was recommended by the Danish Central Scientific-Ethical Committee of Denmark.
RESULTS
For a total of 1011 subjects (505 males and 506 females) from whom stool samples were received, 996 (98·5%) samples were examined by Kato and all samples were examined by coproculture. When examined by Kato, hookworm-like eggs were detected in 872 (87·6%) subjects. Egg counts ranged up to 45720 eggs/g (epg) and the geometric mean egg count (gmec) of positive individuals was 1018 epg. Eggs of Taenia spp., Hymenolepis nana and Schistosoma mansoni were detected in 8 (0·8%), 2 (0·2%) and 10 (1·0%) cases, respectively, but ova of Ascaris spp. and Trichuris spp. were never detected. Table 1 shows the prevalence and intensity of infection by sex and age group determined by the Kato method. The distribution of infection was similar across all age classes from the age of 5 years and above, but slightly lower in children less than 5 years. Across all age classes, the proportion of males with hookworm-like eggs in their stool was similar to that of females (88·9% vs 86·2%) but the gmec for males was significantly higher than that for females (1142 vs 906 epg; P=0·031) (Table 1).

Upon coproculture, third-stage larvae of hookworm and O. bifurcum were detected in 879 (86·9%) (Table 2) and 536 (53·0%) subjects respectively (Table 3). In 167 (16·5%) subjects, L3-larvae of Strongyloides stercoralis were detected. Gender-specific analysis showed that hookworm prevalence was similar for males and females (87·1% vs 86·8%) but the intensity of infection in males was significantly higher than that in females (97 vs 75 larvae; P=0·015, Table 2). Across all age groups, hookworm prevalence did not significantly differ for males and females, except in the age group of 10 to 14 years, where both prevalence (97·0% vs 86·0%; P=0·033) and larval counts (gmlc 107 vs 63 larvae, P=0·016) were significantly higher for boys than for girls (Table 2). In the case of O. bifurcum, significantly more infections were detected in females than in males (57·1% vs 48·9%; P=0·009). This gender difference was mainly attributed to a higher prevalence of infection in adult females of 20 years and above compared to males of the same age group (63·8% vs 43·7%; P<0·001). No significant gender-related difference was, however, detected in subjects below 20 years of age (Table 3).


At the individual level, a strong association was found between hookworm and O. bifurcum infections. A cross-tabulation of hookworm infection with that of O. bifurcum gave a significant association (OR=11·4; 95% CI 6·5–19·8, P<0·001). The relative risk was 5·2, which implies that when infected with hookworm, the individual has 5-fold risk of being infected with O. bifurcum. The prevalence of O. bifurcum infection was particularly increased among individuals with higher hookworm larval counts as illustrated in Fig. 2. This was true both for males and for females.

Fig. 2. Association of Oesophagostomum bifurcum-prevalence with intensity of hookworm infection. Solid bars indicate prevalence of O. bifurcum infection in males and hatched bars indicate that in females. The numbers on top of the bars indicate the number of subjects examined.
When patterns of O. bifurcum and hookworm infections were analysed in relation to compounds neither Oesophagostomum nor hookworm infections appeared evenly distributed over the area. Significant clustering of infection was seen in certain compounds for the presence of O. bifurcum, of ‘heavy O. bifurcum infections’ (>10 larvae cultured) and of ‘heavy hookworm infections’ (>100 larvae cultured) (P<0·001). Plotting the location of highly infected compounds showed that these were not randomly distributed over the area: some parts of the apparently homogeneous environment of the study area were definitely more heavily infected with O. bifurcum than other parts (Fig. 3). A similar but somewhat less-pronounced pattern was obtained when plotting the compounds with heavy and light hookworm infections.

Fig. 3. Map showing the geographical distribution of the compounds included in the study and the prevalence of Oesophagostomum bifurcum infection in compounds examined by coproculture. Open circle-compounds in which 3 or more persons were examined but were not infected with O. bifurcum. Open triangles-compounds in which 3 or more persons were examined but less than 50% were infected with O. bifurcum. Closed triangles-compounds in which 3 or more inhabitants were examined and more than 50% were infected with O. bifurcum. Thin lines-local dirt roads. Thick lines-throughway roads.
Possible association of infection with tribe, the number of compound inhabitants (compound size), the hygienic standards and the presence of various kinds of livestock within the compound were analysed and the significance level tested using the Kruskal-Wallis test (Table 4). There was a significant association of O. bifurcum infection-rates (but not of those of heavy hookworm infections) with tribal background. The Bimoba were more infected with O. bifurcum than the Kusasi (P<0·001). The other variables did not show any significant association with either infection. The association with occupation was not further pursued because over 96% of all families were farmers.

The map in Fig. 4 shows the geographical distribution of Bimoba- and Kusasi-villages and the geographical localization of villages with low (<50% of compound's inhabitants infected with O. bifurcum) and high (≥50% of the compound's inhabitants infected) prevalence of O. bifurcum infections in the area. The figure shows that many of the villages with high O. bifurcum prevalence are the Bimoba villages. Most of the villages in the area are either inhabited by Bimoba only or by Kusasi only, but in 6 of the villages a mixed population of Bimoba and Kusasi exists. Further analysis comparing the prevalence of O. bifurcum infection in Bimoba and Kusasi people living together in those 6 villages showed no tribal difference of O. bifurcum infection (Table 5).
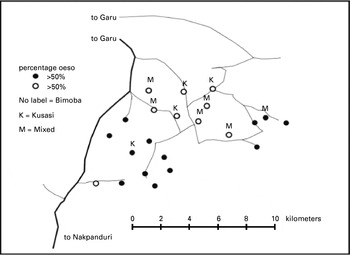
Fig. 4. Distribution of Oesophagostomum bifurcum infection by village and tribe.

DISCUSSION
Earlier research on O. bifurcum infections in the area indicated that important seasonal and year-to-year fluctuations occur (Pit et al. 2000). To minimize the influence of these variations, the survey and all stool examinations were completed in 2 months, between early September and late October 2001, shortly after the rainy season.
There is little experience in the use of stool cultures in parasitological surveys. In studying the variation of the number of larvae cultured, Pit et al. (1999b) concluded that the sensitivity of a single culture varied from 60% to 85% even when individual larval counts are low. Krepel et al. (1992) recognized a tendency towards log-normal distributions in the larval counts based on a fairly large number of subjects. The present study confirms these earlier observations. Larval counts obtained by coproculture can therefore be analysed in the same way as egg counts in Kato smears. The stool culture sensitivity compares very well with that of the Kato method: the prevalence of hookworm-like infection measured by the Kato method is comparable to that obtained with hookworm-stool culture. The use of geometric mean counts to describe the intensity of infections is also justified by the observation of approximated log-normality of the distributions of both egg counts in Kato smears and larval counts in stool cultures.
Necator americanus has been recognized to be the predominant hookworm species in the area (Brooker et al. 2002) and in the present study, too, N. americanus predominated, but Ancylostoma duodenale was found occasionally in a few villages. For unexplained reasons A. duodenale is more common in villages west of the present study area (de Gruijter et al. 2005b).
In earlier studies in northern Togo and Ghana, an association was found between infections with O. bifurcum and hookworm (Krepel et al. 1992; Pit et al. 1999a; Yelifari et al. 2005). The present study confirms this association. This association remains significant whether analysed for the population at large or for various subpopulations. It is highly unlikely that this is due to methodological errors since stool cultures were prepared in duplicate and were examined independently by 2 experienced microscopists. The clustering should therefore be attributed either to similarity of environmental factors favouring transmission or to similar methods of transmission for O. bifurcum and the predominant N. americanus. These observations would suggest percutaneous transmission of O. bifurcum in contrast to the general but not well-founded belief of oral transmission of O. bifurcum infections in humans. On the other hand, the difference in clustering of infection with hookworm in young males and O. bifurcum in adult females might suggest that a similar route of infection is unlikely.
The assumption of oral transmission of O. bifurcum in human populations has been extrapolated from the study of Oesophagostomum infections of sheep, pigs and monkeys. In fact, those studies equally showed that percutaneous infection with O. bifurcum is possible, but that only a very small proportion of larvae manages to infect the host through this route. It should also be remembered that L3-larvae if swallowed easily penetrate the intestinal wall to form the characteristic nodular lesions. Clearly, the larvae have the capacity to penetrate the host's mucosa. Finally, both Random Amplification of Polymorphic DNA (RAPD) and Amplified Fragment Length Polymorphism (AFLP) analysis and epidemiological observations have demonstrated that the human and simian strains of O. bifurcum are different (de Gruijter et al. 2004, de Gruijter et al. 2005b). Cross-infection cannot be excluded but it is not efficient from humans to monkeys (Eberhard et al. 2001) and does not seem to take place from monkeys and baboons to man. The evidence of lack of transmission from monkeys to man, the molecular differences of the human and simian strains of the parasite, the capacity of the L3-larvae to penetrate the colonic mucosa as well as the strong association of N. americanus and O. bifurcum infections, as demonstrated in this paper, all support the hypothesis of percutaneous transmission. Proof of the route of transmission remains to be established experimentally, but these observations recommend caution in accepting a theory of oral infection in humans on the basis of experiments in pigs, sheep and non-human primates.
To further understand the transmission of O. bifurcum in the study area, we analysed the prevalence and intensities of infection in different compartments of the population: in different age-, gender-, and tribal-groups and in relation to hygienic standards and sizes of the compounds. Finally we looked at patterns of clustering in terms of the longitudes and latitudes of the compounds and villages.
The hygienic standard of compounds and the number of people living together in one compound could both be factors to influence the level of infection. However, our data do not show such association. It must be admitted that quantification of hygienic standards is difficult to perform reliably. Standard questionnaires were used by field staff, and there was reasonable consistency in what were considered unhygienic and hygienic compounds. Likewise, higher population densities in a compound did not appear to favour transmission. Overall, therefore, the socio-economic observations fail to provide a better understanding of the route of transmission.
Significant clustering of helminth infections at the household level has previously been described (Forrester et al. 1988; Chan et al. 1994; Conway et al. 1995). Important conclusions with regards to the modes of transmission and factors influencing transmission were derived from such analysis. In the present study using similar statistical procedures, highly significant clustering could be demonstrated for the presence of O. bifurcum, ‘heavy O. bifurcum’ infections and similarly for ‘heavy hookworm infections’. Such procedures may only be used in otherwise homogenously infected geographical regions but here we have shown that O. bifurcum infection rates vary significantly across the study area. If analysis of clustering at the household level is repeated separately for villages with comparable prevalence of O. bifurcum infection, the compound-based clustering is much less pronounced. However, detailed analysis of different subgroups is always complicated, since the population sizes get smaller as the study population is split into more homogeneous compartments.
Bringing the epidemiological observations together, it appears that a major cause for variation in O. bifurcum infection is the location of a village within the endemic area. It remains unexplained why the specific localization of one village is much more favourable for transmission than that of another village.
O. bifurcum infection of monkeys is extremely common both in northern and southern Ghana, and (involving related species) elsewhere in Africa, Asia and Latin America as well. In contrast, the distribution of human Oesophagostomum infection in northern Ghana and Togo is restricted to a small geographical area (Pit et al. 1999a; Yelifari et al. 2005). South of latitude N 9.92, little or no infection is found. It is intriguing to note that the distribution of human Oesophagostomum infection is limited and would seem to be much more sensitive to minor variations in ecological conditions than the simian species. Indeed, the transmission biology of O. bifurcum in humans remains difficult to understand and appears to differ from the species infecting non-human primates.
The following are acknowledged for their assistance towards the success of the study. The DBL-Institute for Health Research and Development, Glaxo-Smith-Kline and Leiden University Medical Centre (LUMC) as well as the Gratama-Foundation in The Netherlands provided funds for the fieldwork. Bayita Albano, Daniel Laar, Mohammed Awel, Moses Kolan, Leonard Yelifari, Mathilda Abugri and Dutch students from LUMC and Ghanaian students from UDS made the fieldwork possible. Particular mention should be made of Diana Slats, Fieke Oostvogel, Bram Diederen, Gabrielle van Ramshorst, Erik Planken, Esther Hamoen, Edwin Yenli, Isaac Akanko, Ayanga Abu, Abdul-Latif and Daakpala Roland for their assistance. The Ghana Health Service in Bolgatanga and Bawku are gratefully acknowledged for their support.











