I. INTRODUCTION
Synthetic anhydrous analogues of the silicate framework minerals leucite (KAlSi2O6) and pollucite (CsAlSi2O6) can be prepared with the general formulae A 2BSi5O12 and ACSi2O6; where A is a monovalent alkali metal cation, B is a divalent cation and C is a trivalent cation. These structures all have the same topology with B and C cations partially substituting onto tetrahedrally coordinated sites (T-sites) in the silicate framework and charge balancing A cations sitting in extra-framework channels. The A cations can be replaced by ion exchange, Cs containing silicate framework minerals are of potential technological interest as storage media for radioactive Cs from nuclear waste (Gatta et al., Reference Gatta, Rotiroti, Fisch, Kadiyski and Armbruster2008).
We have used X-ray and neutron powder diffraction to determine and Rietveld refine the ambient temperature crystal structures of leucite analogues with the general formulae A 2BSi5O12 and ACSi2O6. Crystal structures have been refined in the Ia ![]() $\bar{3}$d cubic and I4 1/a tetragonal space groups (A = K, Rb, Cs; B = Mg, Mn, Co, Cu, Zn; C = Fe3+; Bell et al., Reference Bell, Henderson, Redfern, Cernik, Champness, Fitch and Kohn1994a, Reference Bell, Knight, Henderson and Fitch2010; Bell and Henderson, Reference Bell and Henderson1994a,Reference Bell and Hendersonb, Reference Bell and Henderson2018). These structures all have disordered T-site cations and also have A cation sites fully occupied with either K, Rb or Cs. Crystal structures have also been refined at ambient temperature for P2 1/c monoclinic crystal structures of leucite analogues with the general formulae A 2BSi5O12 (A = K, B = Mg, Fe2+, Co, Zn; Bell et al., Reference Bell, Henderson, Redfern, Cernik, Champness, Fitch and Kohn1994a; Bell and Henderson, Reference Bell and Henderson2018) and also for Pbca orthorhombic (A = Rb; B = Mg, Mn, Ni, Cd; Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009, Reference Bell and Henderson2016) and (A = Cs; B = Mg, Mn, Co, Ni, Cu, Zn, Cd; Bell et al., Reference Bell, Redfern, Henderson and Kohn1994b, Reference Bell, Knight, Henderson and Fitch2010; Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009) These structures all have ordered T-site cations and also have A cation sites fully occupied with either K, Rb or Cs. Cs2ZnSi5O12 undergoes a reversible phase transition from Pbca to Pa-3 on heating to 566 K (Bell and Henderson, Reference Bell and Henderson2012). K2MgSi5O12 undergoes a phase transition from P2 1/c to Pbca on heating to 622 K (Redfern and Henderson, Reference Redfern and Henderson1996).
$\bar{3}$d cubic and I4 1/a tetragonal space groups (A = K, Rb, Cs; B = Mg, Mn, Co, Cu, Zn; C = Fe3+; Bell et al., Reference Bell, Henderson, Redfern, Cernik, Champness, Fitch and Kohn1994a, Reference Bell, Knight, Henderson and Fitch2010; Bell and Henderson, Reference Bell and Henderson1994a,Reference Bell and Hendersonb, Reference Bell and Henderson2018). These structures all have disordered T-site cations and also have A cation sites fully occupied with either K, Rb or Cs. Crystal structures have also been refined at ambient temperature for P2 1/c monoclinic crystal structures of leucite analogues with the general formulae A 2BSi5O12 (A = K, B = Mg, Fe2+, Co, Zn; Bell et al., Reference Bell, Henderson, Redfern, Cernik, Champness, Fitch and Kohn1994a; Bell and Henderson, Reference Bell and Henderson2018) and also for Pbca orthorhombic (A = Rb; B = Mg, Mn, Ni, Cd; Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009, Reference Bell and Henderson2016) and (A = Cs; B = Mg, Mn, Co, Ni, Cu, Zn, Cd; Bell et al., Reference Bell, Redfern, Henderson and Kohn1994b, Reference Bell, Knight, Henderson and Fitch2010; Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009) These structures all have ordered T-site cations and also have A cation sites fully occupied with either K, Rb or Cs. Cs2ZnSi5O12 undergoes a reversible phase transition from Pbca to Pa-3 on heating to 566 K (Bell and Henderson, Reference Bell and Henderson2012). K2MgSi5O12 undergoes a phase transition from P2 1/c to Pbca on heating to 622 K (Redfern and Henderson, Reference Redfern and Henderson1996).
In this paper, we report the Rietveld (Rietveld, Reference Rietveld1969) refinements of the Pbca crystal structures of three more tectosilicate, cation-ordered leucite analogues with both Rb and Cs on the A cation sites; these have the stoichiometry of RbCsX 2+Si5O12 (X = Mg, Ni, Cd). The Pbca structure has two different sites for A cations, this study is to discover whether these sites have Rb and Cs cation order?
II. EXPERIMENTAL
A. sample synthesis
Rb2X 2+Si5O12 and Cs2X 2+Si5O12 were prepared from appropriate stoichiometric mixtures of Rb2CO3, Cs2CO3, SiO2, and XO (X = Mg, Ni, Cd; Bell et al., Reference Bell, Redfern, Henderson and Kohn1994b; Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009, Reference Bell and Henderson2016) and then 50:50 mixtures of the Rb and Cs samples were made for each of the three pairs of samples with the same X 2+ cation. For X = Ni the starting materials were both glasses, the mixture was sealed in a gold capsule and heated in a furnace at 1173 K for 7 days. For X = Cd and Mg the starting materials were both crystalline, the mixtures were sealed in a gold capsule (X = Cd) or a Pd-Ag alloy capsule (X = Mg) and these were heated in a furnace at 1173 K for 6 h.
B. X-ray powder diffraction data collection
After heating the samples were removed from the metal capsules, ground with a mortar and pestle and then mounted on low-background silicon wafers with a drop of acetone prior to ambient temperature X-ray powder diffraction. Data were collected for the X = Mg and Cd samples with a PANalytical X'Pert Pro MPD using CuKα X-rays, a graphite monochromator and a 2.122°2θ wide 100 channel X'Celerator area detector. For X = Mg data were collected in eight scans lasting 61 h in total which were summed together. These data were collected over the range 10°–100°2θ with a step width of 0.0167°2θ and an effective counting time of 5150 s per point. The beam size was defined with a 20 mm mask, fixed antiscatter (¼°) and divergence (⅛°) slits. For X = Cd data were collected in eight scans lasting 64 h in total which were summed together. These data were collected over the range 10°–100°2θ with a step width of 0.0167°2θ and an effective counting time of 5570 s per point. The beam size was defined with a 15 mm mask, fixed antiscatter (¼°) and divergence (⅛°) slits. For the X = Ni sample data were collected with a PANalytical Empyrean diffractometer using Co Kα X-rays with an iron β-filter and a 3.3473°2θ wide 255 channel PIXCEL-3D area detector. Data were collected in two scans over 28 h. These data were collected over the range 15°–100°2θ with a step width of 0.0131°2θ and an effective counting time of 2487 s per point, the beam size was defined with a 20 mm mask, fixed divergence antiscatter (¼°) slit and automatic divergence slit with a 20 mm long beam footprint. These diffracted intensities for the X = Ni sample were summed and then converted from automatic divergence slit mode to fixed divergence slit mode in High Score Plus (PANalytical, 2009) prior to data analysis. No smoothing or α 2 stripping was done on any of these data. Both diffractometers were calibrated with an external NIST 640e silicon standard.
C. X-ray powder diffraction data analysis
Analyses of the summed powder diffraction data for each sample showed that all samples were single-phase and isostructural with the Pbca structure of Cs2CdSi5O12 (Bell et al., Reference Bell, Redfern, Henderson and Kohn1994b). Rietveld refinements were done using FULLPROF (Rodríguez-Carvajal, Reference Rodríguez-Carvajal1993), using the structures of Rb2XSi5O12 (Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009, Reference Bell and Henderson2016) as starting models but with half the Rb replaced by Cs. Backgrounds were fitted by linear interpolation between a set of background points with refinable heights. The Thompson-Cox-Hastings Pseudo-Voigt function (van Laar and Yelon, Reference van Laar and Yelon1984), convoluted with asymmetry because of axial divergence (Finger et al., Reference Finger, Cox and Jephcoat1994), was used to model the profile shape. For X = Mg data over the range 10°–71.5°2θ were used for data analysis as there were no visible Bragg reflections above 71.5°2θ. For X = Cd the whole 10°– 100°2θ range was used and for X = Ni the whole 15°–100°2θ range was used. Soft distance restraints were used on the Si-O and X-O distances. Si-O distances were restrained at 1.61 ± 0.01 Å; this distance is intermediate over the typical distance for tetrahedrally coordinated Si-O in framework silicates (1.59–1.63 Å; International Tables for X-ray Crystallography, 1985, Vol. III). Ni-O and Mg-O distances were restrained at 1.89 ± 0.01 Å; these distances are similar to those determined for X = Ni (Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2016), K2MgSi5O12 (Bell et al., Reference Bell, Henderson, Redfern, Cernik, Champness, Fitch and Kohn1994a) and Cs2MgSi5O12 (Bell and Henderson, Reference Bell and Henderson2009) leucites. Cd-O distances were restrained at 2.23 ± 0.01 Å; these distances are similar to those determined for X = Cd (Bell et al., Reference Bell, Redfern, Henderson and Kohn1994b; Bell and Henderson, Reference Bell and Henderson2009, Reference Bell and Henderson2012) leucites.
In these Rietveld refinements all atoms were located on the Pbca 8c Wyckoff general position. There are two distinct extraframework alkali metal cation sites for Rb and Cs. There are six T-sites, one for divalent X 2+ cations and five for silicon cations, there are also twelve oxygen anion sites. Isotropic atomic displacement parameters were constrained to be the same for all sites occupied by silicon. Isotropic atomic displacement parameters were also constrained to be the same for all sites occupied by oxygen. As there is only one X 2+ cation site then this site had no constraint on the isotropic atomic displacement parameter. Initial refinements had 50% Rb and 50% Cs on each of the two extraframework alkali metal cation sites, these site occupancies were allowed to refine but were constrained so that the sum of the occupancies for each element over the two sites was 100%. Isotropic atomic displacement parameters were constrained to be the same for Rb and Cs on the same alkali metal cation site but different to the isotropic atomic displacement parameters for Rb and Cs on the other alkali metal cation site. VESTA (Momma and Izumi, Reference Momma and Izumi2011) was used to plot crystal structures.
III. RESULTS AND DISCUSSION
Crystal structures have been refined for RbCsX 2+Si5O12 (X = Mg, Ni, Cd) leucite analogues from X-ray powder diffraction data. All are isostructural with their Pbca Rb2XSi5O12 and Cs2XSi5O12 analogues, with divalent X cations ordered onto separate T-sites than those occupied by Si. Table I shows the comparison of the refined lattice parameters and A cation site occupancies for RbCsX 2+Si5O12 compared to the published values for Rb2XSi5O12 (Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009, Reference Bell and Henderson2016) and Cs2XSi5O12 (Bell and Henderson, Reference Bell and Henderson1996, Reference Bell and Henderson2009; Bell et al., Reference Bell, Redfern, Henderson and Kohn1994b). Table II similarly shows refined interatomic distance and angles; the mean X-O and Si-O distances are close to the constraint distances, the mean O-X-O and O-Si-O angles are close to the ideal tetrahedral angle of 109.47°. Table III similarly shows the tetrahedral angle variances for the X and Si sites (Robinson et al., Reference Robinson, Gibbs and Ribbe1971) in the silicate framework structures.
Table I. Refined lattice parameters and A site occupancies.

Table II. Refined interatomic distances and angles.

Table III. Tetrahedral angle variance.

Tetrahedral angle variance [σ 2, deg2]: σ 2 = Σ(θ-109.47)2/5 (Robinson et al., Reference Robinson, Gibbs and Ribbe1971) where θ is the O-T-O tetrahedral angle. Mean variance and standard deviation is given for the five Si tetrahedral sites in each structure.
A. RbCsMg2+Si5O12 structure
Figures 1 and 2, respectively, show the Rietveld difference and the VESTA crystal structure plots for the refined crystal structure of RbCsMg2+Si5O12, in Figure 2 light blue (Cs+) and pink (Rb+) shadings show the occupancies of the two A cation sites. Table I shows that this crystal structure has a unit cell volume which is approximately intermediate between those for Rb2Mg2+Si5O12 and Cs2Mg2+Si5O12. The refined occupancies of the two extraframework A sites are: Rb1 0.63(3), Cs1 0.37(3); Rb2 0.37(3), Cs2 0.63(3); there is some partial, but not complete, ordering of these occupancies. The greater occupancy of the larger Cs+ cation on the extraframework A2 cation site is reflected in the larger A2-O distance compared to A1-O (see Table II). Table II also shows that the mean Si-O-Si and Si-O-X angles are similar for all X = Mg structures. Table III shows that the MgO4 tetrahedron in the silicate framework structure for RbCsMg2+Si5O12 is less distorted than those for Rb2Mg2+Si5O12 and Cs2Mg2+Si5O12.

Figure 1. (color online) Rietveld difference plot for RbCsMg2+Si5O12. Blue circles represent observed data points, red line represents calculated data points, green line represents difference curve and purple crosses represent positions of Bragg reflections. R-factors for this refinement were:- R p = 1.2305, Rwp = 1.5794, R exp = 1.6182, χ 2 = 0.9526. Miller indices are shown for some of the stronger peaks in the powder diffraction plot.

Figure 2. (color online) VESTA structure plot for RbCsMg2+Si5O12, viewed down [111] showing a channel for extraframework Rb+ and Cs+ cations. SiO4 tetrahedra are shown in blue, MgO4 tetrahedra are shown in orange with O2− anions are shown in red. Pink (Rb+) and light blue (Cs+) shadings show the occupancies of the two A cation sites.
B. RbCsNi2+Si5O12 structure
Figures 3 and 4, respectively, show the Rietveld difference and the VESTA crystal structure plots for the refined crystal structure of RbCsNi2+Si5O12, in Figure 4 light blue (Cs+) and pink (Rb+) shadings show the occupancies of the two A cation sites. Table I shows that this crystal structure also has a unit cell volume which is approximately intermediate between those for Rb2Ni2+Si5O12 and Cs2Ni2+Si5O12. The refined occupancies of the two extraframework A sites are: Rb1 0.49(6), Cs1 0.51(6); Rb2 0.51(6), Cs2 0.49(6). Within error limits there is complete disorder of these occupancies. For X = Ni the A-O distances for each of the two extraframework A cation sites are the same within error limits reflecting the disorder of Rb and Cs over these two sites. Table II also shows that the mean Si-O-Si angles are similar for all X = Ni structures but the corresponding Si-O-X angles are all smaller. The Si-O-X angle for Rb2Ni2+Si5O12 is smaller than those for RbCsNi2+Si5O12 and Cs2Ni2+Si5O12. Table III shows that the NiO4 tetrahedron in the silicate framework structure for RbCsNi2+Si5O12 is slightly less distorted than those for Rb2Ni2+Si5O12 and Cs2Ni2+Si5O12.
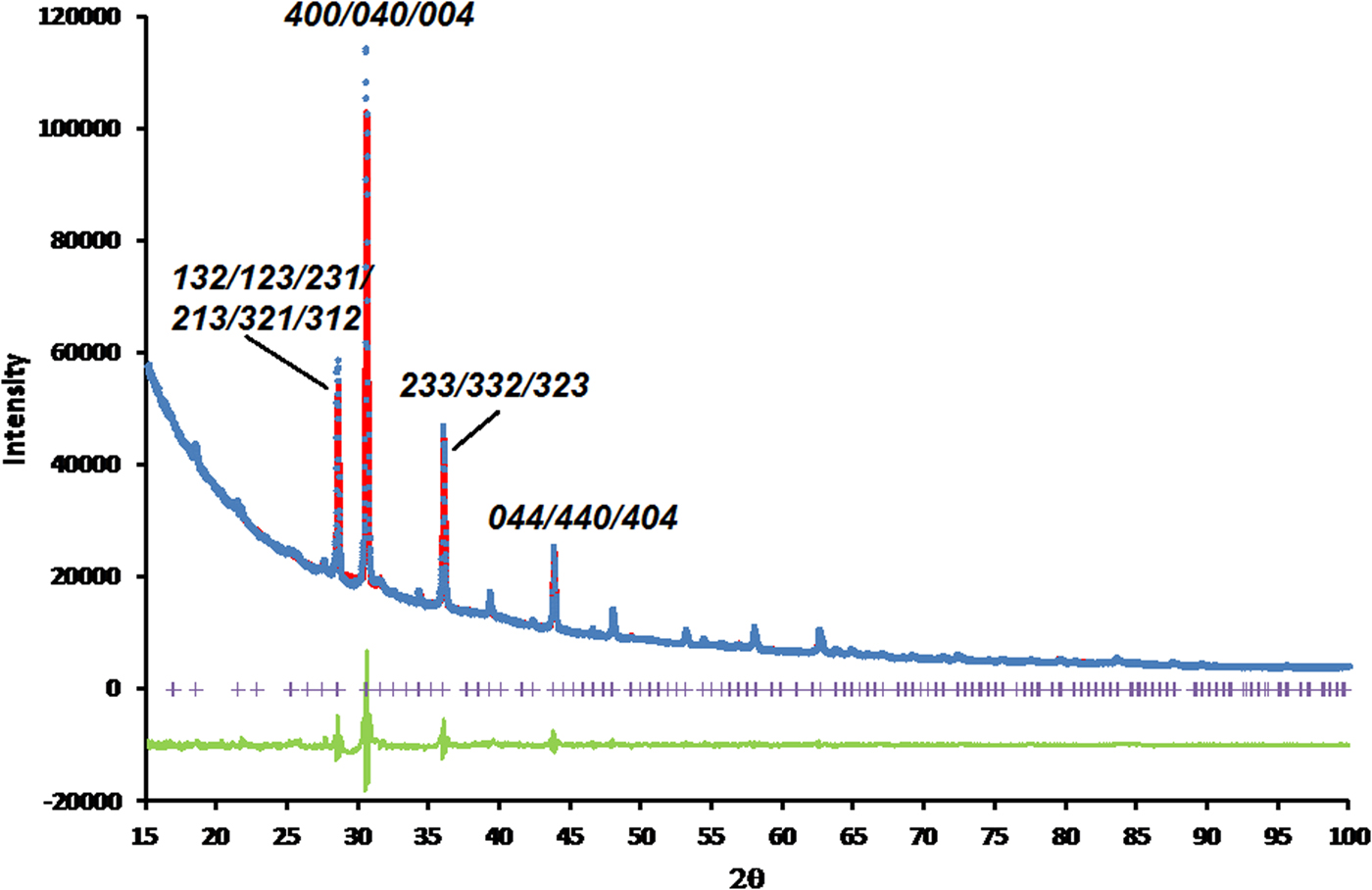
Figure 3. (Color online) Rietveld difference plot for RbCsNi2+Si5O12. Blue circles represent observed data points, red line represents calculated data points, green line represents difference curve and purple crosses represent positions of Bragg reflections. R-factors for this refinement were:- R p = 1.6058, Rwp = 2.7720, R exp = 0.8916, χ 2 = 9.6659. Miller indices are shown for some of the stronger peaks in the powder diffraction plot.

Figure 4. (color online) VESTA structure plot for RbCsNi2+Si5O12, viewed down [111] showing a channel for extraframework Rb+ and Cs+ cations. SiO4 tetrahedra are shown in blue, NiO4 tetrahedra are shown in grey with O2− anions are shown in red. Pink (Rb+) and light blue (Cs+) shadings show the occupancies of the two A cation sites.
C. RbCsCd2+Si5O12 structure
Figures 5 and 6, respectively, show the Rietveld difference and the VESTA crystal structure plots for the refined crystal structure of RbCsCd2+Si5O12, in Figure 6 light blue (Cs+) and pink (Rb+) shadings show the occupancies of the two A cation sites. Table I shows that this crystal structure has a unit cell volume larger than that for Rb2Cd2+Si5O12 (Bell and Henderson, Reference Bell and Henderson1996) and smaller than that for Cs2Cd2+Si5O12 (Bell et al., Reference Bell, Henderson, Redfern, Cernik, Champness, Fitch and Kohn1994a,Reference Bell, Redfern, Henderson and Kohnb). However, Table I shows that this crystal structure has a unit cell volume much closer to (but still smaller) than that for Cs2Cd2+Si5O12 than that for Rb2Cd2+Si5O12. The refined occupancies of the two extraframework A sites are: Rb1 0.52(2), Cs1 0.48(2); Rb2 0.48(2), Cs2 0.52(2). Within error limits there is complete disorder of these occupancies, similar to RbCsNi2+Si5O12. For X = Cd the A-O distances for each of the two extraframework A cation sites are also the same within error limits reflecting the disorder of Rb and Cs over these two sites. Table III shows that the CdO4 tetrahedra in the silicate framework structure for RbCsCd2+Si5O12 is less distorted than those for Rb2Cd2+Si5O12 and Cs2Cd2+Si5O12. As was also seen for X = Ni, all X = Cd structures have larger mean Si-O-Si angles than the corresponding mean Si-O-X angles.

Figure 5. (color online) Rietveld difference plot for RbCsCd2+Si5O12. Blue circles represent observed data points, red line represents calculated data points, green line represents difference curve and purple crosses represent positions of Bragg reflections. R-factors for this refinement were:- R p = 3.5399, Rwp = 4.5294, R exp = 3.0185, χ 2 = 2.2516. Miller indices are shown for some of the stronger peaks in the powder diffraction plot.

Figure 6. (color onlinr) VESTA structure plot for RbCsCd2+Si5O12, viewed down [111] showing a channel for extraframework Rb+ and Cs+ cations. SiO4 tetrahedra are shown in blue, CdO4 tetrahedra are shown in green with O2− anions are shown in red. Pink (Rb+) and light blue (Cs+) shadings show the occupancies of the two A cation sites.
D. comparison of RbCsX 2+Si5O12 structures
The refined structure for X = Mg shows that having two different extraframework cation species results in structural changes compared to the structures with only one type extraframework cation. There are different mean A-O distances for the two A sites and partial A site cation ordering. For all X = Mg structures (with both one and two different extraframework cation species) the mean Si-O-Si and Si-O-X angles are similar.
However the refined structures for both X = Ni and Cd have similar mean A-O distances for the two A sites and no A site cation ordering. For all X = Ni and Cd structures (with both one and two different extraframework cation species) the mean Si-O-Si angles are larger than the corresponding Si-O-X angles. We have reported (Bell and Henderson, Reference Bell and Henderson2018) that this is the usual relationship for ordered P2 1/c leucites and reflects the presence of weaker X-O bonds in the tetrahedral framework leading to greater degrees of framework collapse about the cavity cation sites.
The refined unit cell volumes for the X = Mg and X = Ni (see Table I) are approximately intermediate between the unit cell volumes for the corresponding structures with only one extraframework cation species. The unit cell volumes for the Rb2X 2+Si5O12 structures are smaller than the volumes for the RbCsX 2+Si5O12 structures by approximately the same amount that the corresponding unit cell volumes are larger for Cs2X 2+Si5O12 structures. However, for X = Cd (see Table I) the unit cell volume for the Rb2Cd2+Si5O12 structure is much smaller than that for the RbCsCd2+Si5O12 structure, and the unit cell volume for the RbCsCd2+Si5O12 structure is almost as large as the volume for the Cs2Cd2+Si5O12 structure. This relationship might suggest that the framework is close to being fully expanded for a mean cavity cation radius slightly lower than that for Cs.
IV. CONCLUSIONS
Crystal structures have been refined for RbCsX 2+Si5O12 (X = Mg, Ni, Cd) leucite analogues. These are isostructural with their Pbca Rb2XSi5O12 and Cs2XSi5O12 analogues. For X = Mg there is partial ordering of the Rb and Cs cations over the alkali metal cation A sites. However, for X = Ni and Cd these alkali metal cations are completely disordered.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715619000071.
ACKNOWLEDGEMENT
The authors wish to acknowledge the use of the EPSRC funded National Chemical Database Service hosted by the Royal Society of Chemistry.