Introduction
Tropical savannas are characterized by a continuous herbaceous layer, with C4 grasses being one of the most representative groups and the main fuel load for savanna fires (Trollope, Reference Trollope1982; Veldman et al., Reference Veldman, Overbeck, Negreiros, Mahy, Le Stradic, Fernandes, Durigan, Buisson, Putz and Bond2015). Fire is a common disturbance in these ecosystems (Bond and Van Wilgen, Reference Bond and Van Wilgen1996) and plays an extremely important role in maintaining structure, diversity and vegetation physiognomy (Bond and Keeley, Reference Bond and Keeley2005). Furthermore, grasses vary greatly in morphological and physiological traits (Sarmiento, Reference Sarmiento1992) and are therefore highly resilient to fire (Bond, Reference Bond, Cowling, Richardson and Pierce2004).
Fire-prone species, including grasses, have protected buds and a storage reserve that allow some species to resprout immediately after a fire event (‘resprouters’; Clarke et al., Reference Clarke, Lawes, Midgley, Lamont, Ojeda, Burrows, Enright and Knox2013; Pausas et al., Reference Pausas, Lamont, Paula, Appezzato-da-Glória and Fidelis2018), and others to persist by seedling recruitment (‘seeders’; Whelan, Reference Whelan1995; Bond and Van Wilgen, Reference Bond and Van Wilgen1996). Grasses have different strategies that allow them to dominate in fire-prone ecosystems, being one of the most flammable components of the herbaceous layer (Simpson et al., Reference Simpson, Ripley, Christin, Belcher, Lehmann, Thomas and Osborne2016). For example, the C4 photosynthetic pathway facilitates the accumulation of biomass, inducing high photosynthetic rates and efficient use of nutrients (Osborne, Reference Osborne2008; Edwards, Reference Edwards2012). Moreover, grasses are the most resilient group to fire in tropical savannas, being able to resprout immediately after fire (Bond, Reference Bond, Cowling, Richardson and Pierce2004) since they allocate reserves (e.g. fructans) mostly to their roots (Moraes et al., Reference Moraes, Chatterton, Harrison, Filgueiras and Figueiredo-Ribeiro2013). Therefore, species from fire-prone ecosystems must have the ability to persist after fire events by resprouting or they have seed traits that respond to fire-related germination cues by resisting or promoting seedling emergence (Ramos et al., Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016; Zirondi et al., Reference Zirondi, Silveira and Fidelis2019).
Little is known about germination traits of grasses (but see Aires et al., Reference Aires, Sato and Miranda2014; Ramos et al., Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016). For some grass species, seedling recruitment is low despite high seed production (Foster, Reference Foster2001; Foster and Tilman, Reference Foster and Tilman2003; Foster et al., Reference Foster, Murphy, Keller, Aschenbach, Questad and Kindscher2007). Dormancy, environmental factors (such as water, light and fire), competition and longevity can affect the establishment of grass species by seeds (Cole et al., Reference Cole, Lunt and Koen2005; Commander et al., Reference Commander, Golos, Miller and Merritt2017). In tropical savannas, grasses usually produce a high proportion of empty diaspores (Carmona et al., Reference Carmona, Martins and Favero1998; Wright et al., Reference Wright, Zuur and Chan2014; Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016), and they generally have physiological dormancy (Baskin and Baskin, Reference Baskin and Baskin2014; Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016; Erickson et al., Reference Erickson, Shackelford, Dixon, Turner and Merritt2016; Commander et al., Reference Commander, Golos, Miller and Merritt2017), which can be broken through exposure to high temperatures (see Ramos et al., Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016; Commander et al., Reference Commander, Golos, Miller and Merritt2017).
Species from fire-prone ecosystems may have different strategies related to germination traits (Keeley et al., Reference Keeley, Pausas, Rundel, Bond and Bradstock2011; Lamont and He, Reference Lamont and He2017) that enable them to persist in the vegetation community. Seed dormancy and germination stimulation, for example, can be affected directly and indirectly by fire. During the fire, seeds will be exposed to heat shocks (Moreira et al., Reference Moreira, Tormo, Estrelles and Pausas2010) and smoke (direct effect – see Moreira et al., Reference Moreira, Tormo, Estrelles and Pausas2010; Stradic et al., Reference Stradic, Silveira, Buisson, Cazelles, Carvalho, Fernandes and Ird2015). Additionally, after the fire, seeds in the soil seed bank are exposed to daily temperature fluctuation as a result of the fire opening gaps in the vegetation (indirect effect – see Santana et al., Reference Santana, Baeza and Blanes2013; Daibes et al., Reference Daibes, Zupo, Silveira and Fidelis2017). According to Ramos et al. (Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016), grasses with dormant seeds tolerate exposure to high temperatures better than non-dormant seeds, with some species resisting 110°C for 5 min. Moreover, temperature fluctuation can break the dormancy of some grass species (Kolb et al., Reference Kolb, Aparecida, Pilon and Durigan2016).
Another important trait to evaluate is longevity and the capacity of seeds to resist high temperatures since most fires in the Cerrado occur during the dry season (Ramos-Neto and Pivello, Reference Ramos-Neto and Pivello2000; Pivello, Reference Pivello2011) and most grass species disperse during the rainy season (Munhoz and Felfili, Reference Munhoz and Felfili2007; Ramos et al., Reference Ramos, Diniz and Valls2014). Therefore, dormant seeds are assumed to be in the seed bank when the fire occurs. If seeds are still viable after the fire events, grass seedlings may be recruited when the first rains occur in the system. In general, native grass seeds have short longevity, reinforcing the formation of a transient soil seed bank for perennial species (Aires et al., Reference Aires, Sato and Miranda2014; de Andrade and Miranda, Reference de Andrade and Miranda2014), with many seeds losing their ability to germinate 1 year after dispersal (Thompson and Grime, Reference Thompson and Grime1979; Aires et al., Reference Aires, Sato and Miranda2014; Ramos et al., Reference Ramos, Diniz, Ooi, Borghetti and Valls2017).
To understand the mechanisms involved in the recruitment of native grasses of the Cerrado after the fire events, we aimed to understand the effects of fire on seed germination, conducting daily temperature fluctuation (indirect effect) and heat-shock (direct effect) treatments. Moreover, we analysed seed longevity of some grass species since longevity is a predictor of seed persistence in the soil (Thompson and Grime, Reference Thompson and Grime1979). The capacity to form a persistent soil seed bank implies a high potential among new individuals to establish in post-fire communities. We hypothesized that grass species from the Cerrado will have fire-resistant seeds, and that for dormant seeds, both the daily temperature fluctuation and heat-shock treatments would be sufficient to break physiological dormancy. Furthermore, we expected grass seeds to have shorter longevity, which could be a limiting factor for seedling recruitment from the seed bank.
Materials and methods
Study area and seed collection
Seeds of native grasses were sampled in two different areas of the Cerrado: Estação Ecológica de Itirapina (EEI, Southeastern Brazil, 47°51′–47°48′ W and 22°11′–22° 15′ S, 2300 ha) and Reserva Natural Serra do Tombador (RNST, Central Brazil, 47°45′–47°51′ W and 13°35′–13°38′ S, 8900 ha). Both areas have a seasonal climate, with a well-marked dry season from May to September and a wet season from October to April. At the EEI, the average annual temperature is 22°C and the average annual precipitation is 1459 mm (Zanchetta et al., Reference Zanchetta, Delgado, Silva, Reis, Silva Da Luca, Fernandes, Dutra-Lutgens, Tannus, Pinheiro, Martins and Sawaya2006). The RNST shows average temperatures ranging from 22 to 25°C and annual precipitation from 1300 to 1500 mm (Fundação Grupo Boticário, 2011). The main vegetation type at the seed collection site was campo sujo (Table 1), which is a vegetation dominated by a rich herbaceous layer with scattered shrubs and small trees (Coutinho, Reference Coutinho1982). Two species were collected in both campo sujo (CS) and wet grasslands (WG; Table 1). The wet grasslands are dominated by a graminoid layer with Poaceae, Cyperaceae and Xyridaceae species, and the water table is closer to the soil surface (Cianciaruso and Batalha, Reference Cianciaruso and Batalha2008).
Table 1. Grass species sampled in the two sites (EEI: Estação Ecológica de Itirapina, Southeastern Brazil, an RNST: Reserva Natural Serra do Tombador, Central Brazil), in different vegetation types (CS: campo sujo, WG: wet grassland), with the date of collection and different treatments applied: TF – daily temperature fluctuation, HS – heath shock, and the species which had their longevity (L) tested

We collected seeds of ten native grass species in the two study areas (Table 1). Seeds were collected from different populations and individuals (>15 individuals) to guarantee genetic variability. They were sorted in the lab, and empty diaspores were counted (from a total of 100 seeds from each species; Table 2). Seeds were stored for no more than 3 months before germination trials, and only undamaged and full seeds were used. Seeds with less than 30% total germination were classified as having physiological dormancy (PD; Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016). We applied daily temperature fluctuation (TF) and heat-shock (HS) under dry conditions as our two treatments before putting seeds to germinate. We also tested seed longevity (see below for methods used). Furthermore, we tested seeds for longevity.
Table 2. Percentage of initial viable and empty seeds of native grasses of Cerrado
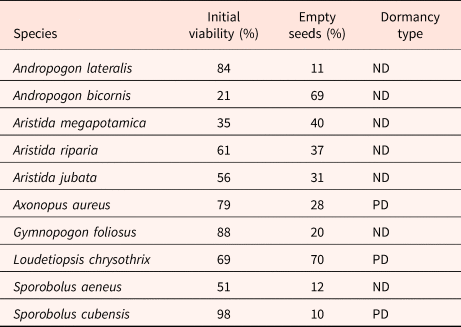
Classification of species in non-dormant (ND) and dormant species (Physiological Dormancy – PD).
Daily temperature fluctuation experiments (TF)
To evaluate the effects of daily temperature fluctuation experienced by seeds dispersed after the fire, 4 replicates of 25 seeds of each species were placed in germination chambers which simulated the temperature cycle throughout a single day (ranging from overnight lows of 19°C to peaks of 55°C with steps in temperature changes from low to high occurring every hour) for 45 days, and including a light regime of 12/12 h. Seeds were placed in Petri dishes with filter paper, but they were not watered, because we wanted to simulate the conditions experienced by seeds dispersed after fire before the rainy season since most of the fires in the Cerrado occur during the dry season. These temperatures were previously measured in the field for 90 days, and we used the average temperature of each hour of the day [for more information, see Daibes et al. (Reference Daibes, Zupo, Silveira and Fidelis2017)]. Each replicate was placed in a different chamber to avoid pseudoreplication. Four replicates, with 25 seeds of each species, were not submitted to treatment (control) and were instead left for the same period, sequestered inside brown paper bags, under natural light conditions and room temperature oscillating between 24 and 26°C.
Heat-shock experiments (HS)
Only nine of the ten grass species were used for this experiment, due to the amount of available seeds (for more details, see Table 1). For each species, we submitted 5 replicates of 20 seeds to the following heat-shock treatments: 100°C for 1 min, 100°C for 3 min and 200°C for 1 min; and we had one control, which was not exposed to the heat shocks. We chose these temperatures following the studies of Miranda et al. (Reference Miranda, Miranda, Dias and de Souza Dias1993), which measured fire temperatures in different Cerrado vegetation types. Usually, fires in the Cerrado are fast, of low intensity and temperatures, consuming most of the aboveground biomass (Miranda et al., Reference Miranda, Miranda, Dias and de Souza Dias1993; Rissi et al., Reference Rissi, Baeza, Gorgone-Barbosa, Zupo and Fidelis2017). Heat shocks were performed in a pre-heated electronic muffle, and each replicate was placed separately to avoid pseudoreplication.
Longevity
Seeds of seven grass species (Table 1) were stored in paper and ziplock bags at room temperature for 6 and 12 months after collection and were subsequently put to germinate. We used 4 replicates of 25 seeds for each species.
Germination procedures
After treatments (temperature fluctuation, heat shocks and longevity), seeds were placed in Petri dishes with two filter papers saturated with distilled water. These seeds were put to germinate in germination chambers (TECNAL – model TE-4013, germination chambers with photoperiod and ramp and level coordinator), with constant temperature (27°C), a light regime of 12/12 h (Fichino et al., Reference Fichino, Dombroski, Pivello and Fidelis2016), and mean radiation activity of 82 lum. ft−2. We performed observations three times a week for 30 days. Seeds that showed radicle or cotyledons were considered to be germinated and were removed from the Petri dishes. At the end of the germination trials, ungerminated seeds were submitted to the tetrazolium test (1%) to verify their viability (Lakon, Reference Lakon1949). These seeds were soaked in the tetrazolium solution in dark glass vials wrapped in aluminium foil and kept at 25–27°C for 24 h. Subsequently, the seeds were dissected, and those with tissue staining were considered viable seeds. For the species studied, 24 h under controlled conditions were ideal for staining of the embryo. The viability of the seeds that were not submitted to any type of treatments (control) was considered the initial viability (Table 2). The percentage of germination was considered in relation to the total number of seeds used for each experiment, and the percentage of viability was related only to seeds with embryos. Finally, in species with high dormancy levels, viable embryos may not be detected by the tetrazolium tests (Ooi et al., Reference Ooi, Auld and Whelan2004).
Data analyses
To analyse differences in germination and viability percentages for each treatment (TF, HS and L) and species, we used generalized linear models (GLMs) with a quasi-binomial error distribution (values in percentage of germinated seeds and their viability). Post hoc function in the Tukey's test was used to compare the effect of the TF, HS and L treatments with the control of each treatment. We analysed seed germination and seed viability separately, using the same model for both. For seed germination and seed viability, treatments (TF, HS and L) were used as a fixed effect. All analyses were performed using the R 3.2.5 software (R Development Core Team, 2016) with the packages vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, Mcglinn, Minchin, O'hara, Simpson, Solymos, Henry, Stevens, Szoecs and Maintainer2019), lme4 (Bates et al., Reference Bates, Mächler, Bolker and Walker2015), multcomp (Hothorn et al., Reference Hothorn, Bretz and Westfall2008), lattice (Sarkat, Reference Sarkar2008) and ggplot2 (Wickham, Reference Wickham2009).
Results
In general, species showed low percentages of empty diaspores, with only two species having no embryo in more than 50% of their seeds: Andropogon bicornis (69%) and Loudetiopsis chrysothrix (70%; Table 2). Moreover, two species had seed viability lower than 50%: An. bicornis (21%) and Aristida megapotamica (35%; Table 2). Among the study species, only three presented physiological dormancy: Axonopus aureus, L. chrysothrix and Sporobolus cubensis (Table 2).
Seed germination of most species was not affected by daily temperature fluctuations (Fig. 1; Supplementary Table S1). However, species with dormant seeds had higher germination percentages when this treatment was applied. Ax. aureus showed an increase in relation to the control (45%, P = 0.01; Fig. 1) as did S. cubensis (57%) and L. chrysothrix (25%) (P = 0.006 and P = 0.001, respectively; Fig. 1). The viability of all study species was not affected by temperature fluctuation (Fig. 1; P > 0.05).
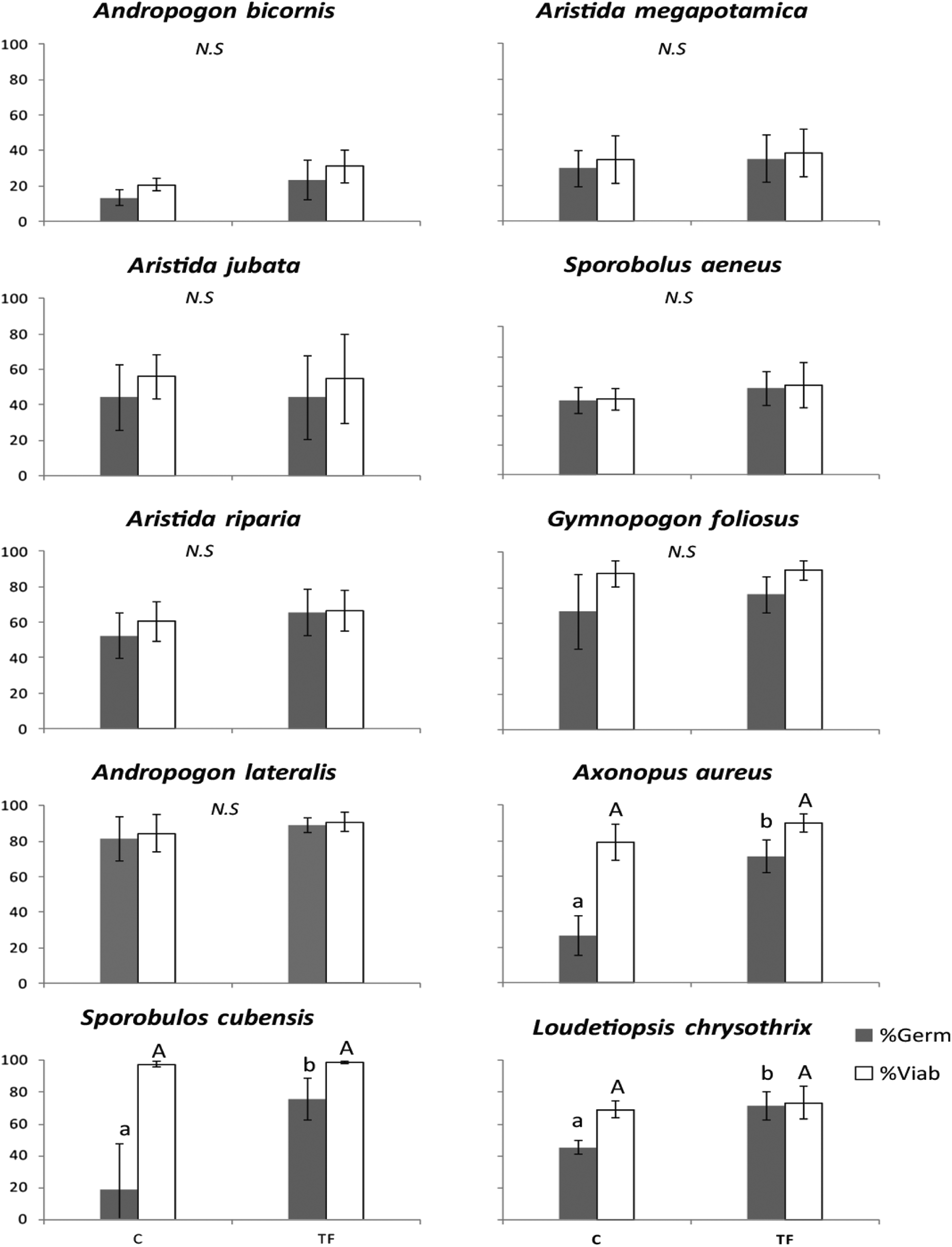
Fig. 1. Effects of daily temperature fluctuation on seeds germination (grey bar) and viability (white bar) of ten native grass species (mean ± SE) when exposed to the different treatments: C (control, no exposure to temperature fluctuation) and TF (temperature fluctuation). Different lowercase letters mean significant differences among treatments for seed germination and different capital letters mean significant differences among for seed viability (P ≤ 0.05).
Ar. megapotamica and Gymnopogon foliosus seeds resisted high temperatures, their germination and viability percentages remaining unaffected by the tested temperatures (Fig. 2; P > 0.05). All species resisted up to 100°C for 1 min, neither germination nor viability being affected by the treatments (Fig. 2; P > 0.05). However, the exposure to 100°C for 3 min led to a 62% decrease in germination (P = 0.004; Fig. 2) and a 66% decrease in viability (P = 0.005; Fig. 2) of Andropogon lateralis seeds in comparison to the control. Seeds of Sporobolus aeneus also showed decreased germination and viability when exposed to 100°C for 3 min (P = 0.02 and P = 0.05, respectively; Fig. 2).
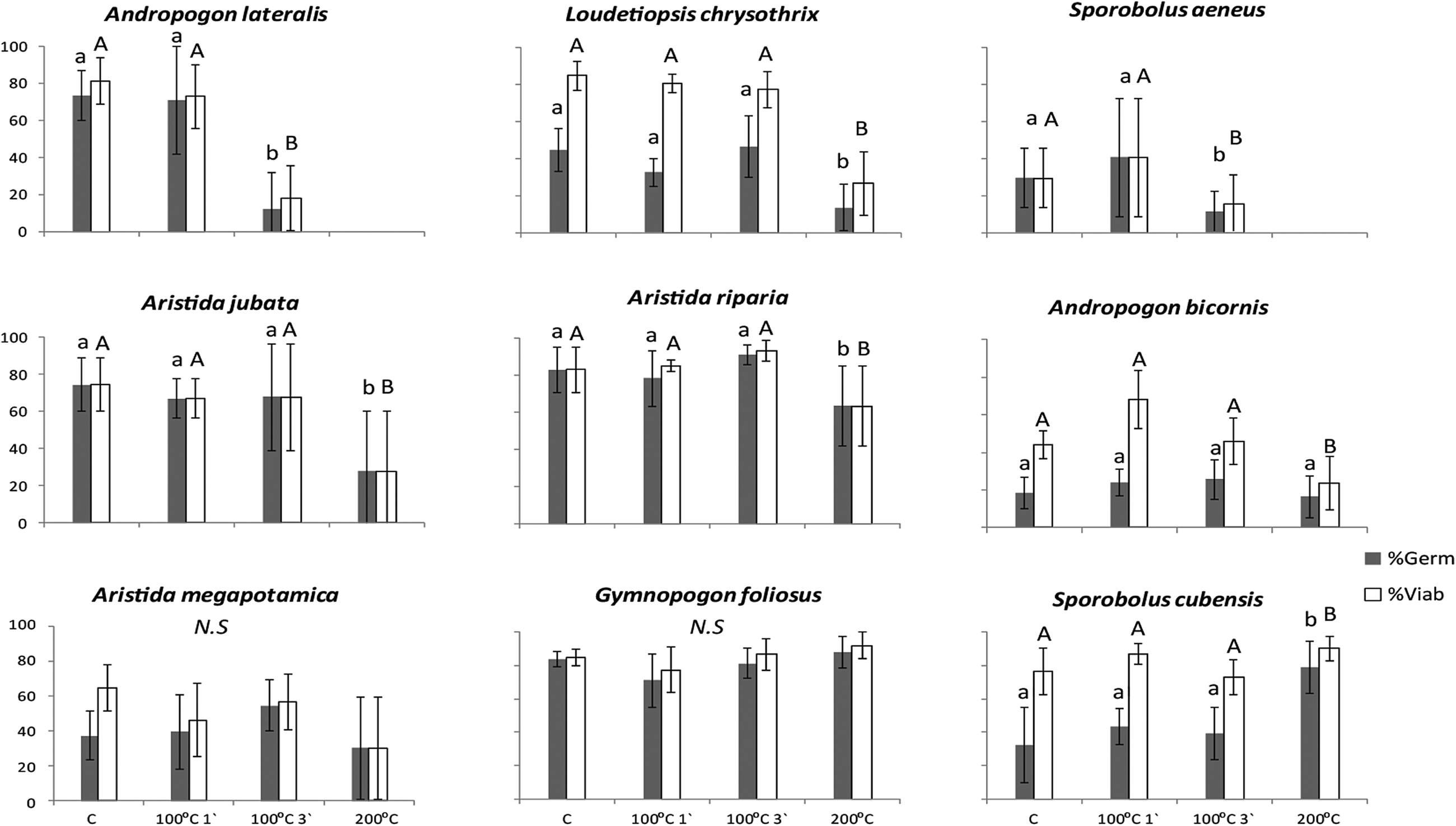
Fig. 2. Germination percentage (mean ± SE) of native grass species of Cerrado, according to the different heat-shock treatments: control (C), 100°C 1′, 100°C 3′ and 200°C 1′. Different letters mean significant differences among treatments for seed germination and different capital letters mean significant differences among for seed viability (P ≤ 0.05).
Most species showed a decrease in both germination and viability when exposed to 200°C. Two species did not germinate at all at this temperature, and all their seeds were dead after the treatment (An. lateralis and S. aeneus; Fig. 2). L. chrysothrix, Aristida jubata and Aristida riparia showed a significant decrease in germination and viability percentages (Fig. 2), while An. bicornis showed a 21% decrease in viability (P < 0.001); however, germination percentages among these species did not differ from the control when seeds were exposed to 200°C. Lastly, one species, S. cubensis, had its dormancy broken when seeds were exposed to 200°C and germination increased by 48% (P = 0.006; Fig. 2).
The germination and viability of seeds stored for 6 months were generally unaffected by time (P > 0.05; Table 3). However, Ar. megapotamica showed a 50% increase in germination and viability after 6 months (P < 0.001 and P = 0.006, respectively; Table 3). Conversely, seeds of Ar. riparia showed lower germination and viability percentages after 6 months (P = 0.014 and P = 0.003, respectively; Table 3).
Table 3. Germination and viability percentages (mean ± SD) for seeds from different native grass species of Cerrado just after seed collection (0) and 6 and 12 months after storage

* P ≤ 0.05, **P ≤ 0.001.
After 1 year of storage, germination and viability of the seeds of G. foliosus were the same as at the beginning of the experiments (P > 0.05; Table 3). However, some species showed a significant decrease in both germination and viability, such as An. lateralis (34% in germination percentage and 29% in viability), Ar. riparia (24% in germination and 23% in viability) and S. aeneus (19% in germination and 18% in viability; Table 3). Seeds of Ar. megapotamica increased germination (66%, P < 0.001) and viability relative to the beginning of the experiments (60%, P = 0.003; Table 3), as did L. chrysothrix seeds, which showed higher germination and viability percentages after 1 year of storage (P ≤ 0.05; Table 3).
Discussion
Dormancy prevents germination of seeds of many different species in unpredictable environments where conditions favourable for recruitment are only brief. Germination of physiologically dormant (PD) seeds is directly influenced by environmental cues such as temperature, light or water availability (Baskin and Baskin, Reference Baskin and Baskin2004) and is the most common type of dormancy in tropical savannas (Dayrell et al., Reference Dayrell, Garcia, Negreiros, Baskin, Baskin and Silveira2016), particularly prevalent in grass species (Carmona et al., Reference Carmona, Martins and Favero1998; Musso et al., Reference Musso, Miranda, Aires, Bastos, Soares and Loureiro2014; Kolb et al., Reference Kolb, Aparecida, Pilon and Durigan2016). Understanding how these mechanisms interact with fire cues to promote seed germination is crucial to the understanding of post-fire plant regeneration in these ecosystems.
In fire-prone ecosystems, fire events are responsible for maintaining and influencing the structure and diversity of plant communities (Bond and Keeley, Reference Bond and Keeley2005), opening gaps within the vegetation (Fidelis et al., Reference Fidelis, Blanco, Müller, Pillar and Pfadenhauer2012), and creating new sites for species to establish (Grubb, Reference Grubb1977). Conditions in the gaps may differ from the rest of the system, with greater daily temperature fluctuation in the soil (Fidelis and Blanco, Reference Fidelis and Blanco2014; Santana et al., Reference Santana, Baeza and Blanes2013; Daibes et al., Reference Daibes, Zupo, Silveira and Fidelis2017), which may directly influence seed recruitment. In this study, three species presented physiological dormancy: Ax. aureus, L. chrysothrix and S. cubensis. All three species had their dormancy broken by daily temperature fluctuation. In the Cerrado, gaps in vegetation cause differences in fire temperature on the surface, creating important safe-sites for seed germination and survival (Daibes et al., Reference Daibes, Gorgone-Barbosa, Silveira and Fidelis2018). Thus, our study shows that temperature fluctuation is an essential mechanism for the germination of some grass species in Cerrado, confirming that fire heterogeneity is important for seedling regeneration.
On the other hand, daily temperature fluctuation did not affect germination and viability percentages of non-dormant species, indicating that their seeds tolerate a wide range of temperature in the soil. Some studies using Cerrado grasses showed that alternating two temperatures (e.g. 15/25°C or 20/30°C) affected seed viability negatively (Musso et al., Reference Musso, Miranda, Aires, Bastos, Soares and Loureiro2014; Stradic et al., Reference Stradic, Silveira, Buisson, Cazelles, Carvalho, Fernandes and Ird2015). In contrast to the above-cited studies, which used only two temperature regimes and a single step each diurnal period, our study used temperature curves that simulated those measured in the field. This may influence the differences we found for non-dormant species. No effect on seed viability of non-dormant seeds, but also demonstrated that that daily temperature fluctuation affects positively seed germination of dormant seeds.
Fire itself influences seed germination by increasing temperatures during fire events, which could have positive (break of dormancy, see Keeley et al., Reference Keeley, Pausas, Rundel, Bond and Bradstock2011) and negative (kill seeds) effects on seed germination. We found one species, Sporobolus cubensis, whose dormancy was broken when exposed to 200°C, indicating not only that seeds of this species can survive fire but also that the high temperatures produced by fire enhance their germination. Native grass species resprout and flower rapidly after fire, releasing their seeds while gaps in the vegetation still exist (Fidelis et al., pers. comm.). Thus, the seedling establishment of this species occurs through recruitment from the soil seed bank that accumulates after each fire event. This strategy is probably also used by other grass species with fire-related cues for germination in Cerrado areas, such as Mesosetum ferrugineum – this species’ dormancy was broken after exposure to high temperatures (Ramos et al., Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016), and it resprouted and flowered vigorously after fire (Fidelis et al., pers. comm.). These seeds may present higher contents of heat-shock proteins (Wehmeyer et al., Reference Wehmeyer, Hernandez, Finkelstein and Vierling1996), which could be an advantage in fire-prone ecosystems, being a response to fire events (adaptive traits; Keeley et al., Reference Keeley, Pausas, Rundel, Bond and Bradstock2011; Lamont and He, Reference Lamont and He2017) since 200°C can be reached only during fires.
In our study, all species with dormant and non-dormant seeds resisted temperatures up to 100°C for 1 min. Fire-prone species can be stimulated to germinate after fire, or they can be tolerant, their seeds’ viability and capacity to germinate remaining unchanged (Paula and Pausas, Reference Paula and Pausas2008; Fichino et al., Reference Fichino, Dombroski, Pivello and Fidelis2016). In open savannas, fire is usually fast, with low temperatures at the surface and even lower temperatures in the first 3 cm of the soil (Miranda et al., Reference Miranda, Miranda, Dias and de Souza Dias1993; Rissi et al., Reference Rissi, Baeza, Gorgone-Barbosa, Zupo and Fidelis2017; Schmidt et al., Reference Schmidt, Fidelis, Miranda and Ticktin2017; Daibes et al., Reference Daibes, Gorgone-Barbosa, Silveira and Fidelis2018). Therefore, seeds may be able to survive the passage of such fire in these savannas, as long as they are not directly damaged by the flames. A high percentage of survival among the seeds would ensure the maintenance of the soil seed bank, and it would enable seedling recruitment from the seed bank in post-fire environments.
However, seeds of the species An. lateralis, L. chrysothrix and S. aeneus, which were collected in both wet grasslands and in open savanna (campo sujo), showed lower fire resistance. This result suggests that the environmental conditions, in this case water availability, may also influence germination traits and responses to fire since the only two species that did not germinate and had viable seeds at 200°C were An. lateralis and S. aeneus. In Cerrado, fires in wet grasslands usually have a short residence time at 1 cm and a lower maximum temperature than fires in open savannas (Schmidt et al., Reference Schmidt, Fidelis, Miranda and Ticktin2017). Thus, the seeds of wet grassland species are usually not exposed to fires as hot as the ones in open savannas. Indeed, Ramos et al. (Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016) also found that grass species from wet grasslands had lower germination percentages and lower resistance to fire temperatures than grass species of open savannas (campo sujo). These findings suggest that the evolution of fire-resistant seeds may be related to habitat moisture (Ramos et al., Reference Ramos, Liaffa, Diniz, Munhoz, Ooi, Borghetti and Valls2016).
Most of the studied grass species have seeds with low longevity, indicated by a gradual decline in viability percentages among the stored seeds, in comparison to freshly collected seeds. In some grass species, however, both germination and viability percentages increased after storage for 6 and 12 months. According to Martin (Reference Martin1946), grass seeds have fully developed embryos and, therefore, cannot present morphological dormancy. Ar. megapotamica, for example, showed a 25% increase in seed viability in this study. Thus, discarding the hypothesis of the occurrence of morphological dormancy, the increased germination and viability in these species could indicate that our tetrazolium tests were not sufficient to identify viable embryos in freshly sampled seeds of species with high dormancy levels, as observed in other fire-prone ecosystems (Ooi et al., Reference Ooi, Auld and Whelan2004). These high levels of dormancy of this species might also have affected germination percentages (35%).
In summary, our study showed that the dormancy of Cerrado grass seeds was broken by exposure to daily temperature fluctuation and heat shock, two important fire-related germination cues in post-fire environments. In addition to the break of dormancy, most of the studied grass species had fire-tolerant seeds, confirming that resistance to fire is an important seed trait in Cerrado. These results reflect field conditions where dormant seeds are dispersed in the rainy season and remain in the soil for months before the onset of the next rainy season. The observed pattern can favour recolonization by grass species through seeds, which could be attributed to trade-offs between seed traits (resistance and break of dormancy) and environmental factors (high temperatures and temperature fluctuation). However, the longevity of these seeds suggests the formation of a transitory seed bank during a limited period per year, indicating a limiting factor in the success of native grass species’ regeneration from the seed bank.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S0960258520000094.
Acknowledgements
We thank L.F. Daibes, G. Damasceno and H.L. Zirondi for helping with seed collection. We also thank Desirée Ramos for her valuable suggestions to improve this manuscript. We thank Megan King for the English review of this manuscript. Finally, we thank the editor and the anonymous reviewers who helped us to improve this manuscript.
Financial support
This study was supported by FAPESP (2015/06743-0), CNPq (455183/2014-7), Fundação Grupo Boticário (0153_2011_PR) and Neotropical Grasslands Conservancy. M. Dairel received scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Financing code 001 and A. Fidelis receives productivity grant from CNPq (303988/2018-5).







