Junctional ectopic tachycardia is an arrhythmia that presents either as a congenital idiopathic disease or, more often, as a transient phenomenon after surgery for CHD.Reference Erickson 1 Junctional ectopic tachycardia is defined as a narrow QRS complex with atrioventricular dissociation or retrograde atrial conduction. Post-operative junctional ectopic tachycardia is associated with surgical injury and is thought to be due to abnormal automaticity of the atrioventricular node and the bundle of His. Junctional ectopic tachycardia often occurs after surgical repair for tetralogy of Fallot, ventricular septal defects, atrioventricular septal defects, and atrial partitioning for transposition of the great arteries.Reference Erickson 1 , Reference Moak, Arias and Kaltman 2 Post-operative junctional ectopic tachycardia has an incidence of 1.4–15.3%,Reference Moak, Arias and Kaltman 2 , Reference Kow, Ernest and Lawrence 3 and usually appears within the first 72 hours after open-heart surgery for CHD.Reference Erickson 1 To our knowledge, although there are a few reports about post-operative junctional ectopic tachycardia occurring several years after open-heart surgery, there are no reports of junctional ectopic tachycardia caused by sepsis. In our patient, junctional ectopic tachycardia was successfully treated using low-dose landiolol hydrochloride, an ultra-short-acting β-blocker.
Case report
A 4-year-old girl, previously diagnosed with asplenia, a univentricular heart, and total anomalous pulmonary venous return, presented for treatment. She was born at week 38 of gestation, and had a birth weight of 3360 g. She underwent pulmonary artery banding at the age of 1 month, followed by a bi-directional Glenn procedure and total anomalous pulmonary venous return repair at the age of 5 months. Extra-cardiac Fontan palliation was then performed at the age of 19 months. She did not have post-operative arrhythmia, and electrocardiography at the age of 29 months indicated sinus rhythm. When she was 50-months old, she developed a fever on day 7 of a cold. The next morning, she required admission to an affiliated general hospital due to hyperthermia and tachycardia. Although her body temperature was 37.7°C after admission, her heart rate was 190 bpm; a P-wave was not present on her electrocardiogram. The attending paediatrician suspected paroxysmal supraventricular tachycardia and ordered an intravenous bolus injection of adenosine to control the tachycardia. Despite three intravenous bolus injections of adenosine, the tachycardia persisted. Therefore, the patient was transferred to our hospital. At the time of her arrival at our hospital, tachycardia was present and we performed electrical cardioversion. Although the tachycardia was initially terminated, it immediately re-initiated. An echocardiogram did not reveal cardiac dysfunction. Electrocardiography performed during the course of intravenous bolus adenosine therapy showed persistent tachycardia with ventriculoatrial dissociation, which led to a diagnosis of junctional ectopic tachycardia (Fig 1a). The patient’s laboratory results indicated a serum C-reactive protein level of 5.49 mg/dl and a procalcitonin level of 39.19 ng/ml. Based on her blood culture results, she was diagnosed with sepsis caused by Streptococcus pneumoniae.
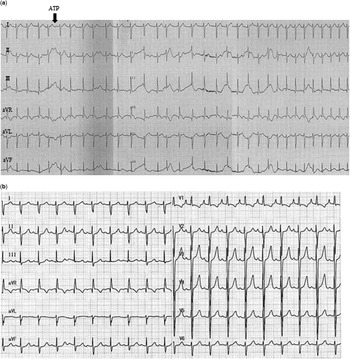
Figure 1 Patient electrocardiograms showing that ( a ) the intravenous bolus injection of adenosine created atrioventricular block with ventriculoatrial dissociation without terminating tachycardia, which led to a diagnosis of junctional ectopic tachycardia. ( b ) After treatment with landiolol hydrochloride, a sinus rhythm was restored with a heart rate of 112 bpm.
The treatment regimen that followed was designed to treat both the junctional ectopic tachycardia and the sepsis. The treatment options were discussed with the child’s parents and they provided their informed consent for the child’s treatment, including the off-label use of recommended drugs. For junctional ectopic tachycardia, we initiated an infusion of landiolol (1.0 μg/kg minute) in conjunction with thermal control and sedation; the landiolol dosage was later increased to 3.0 μg/(kg minute). Within 1 hour, her heart rate had decreased to 140 bpm; however, the patient became hypotensive with a systolic pressure of 79 mmHg, which necessitated the administration of phenylephrine hydrochloride, an α-1 agonist. Thereafter, the patient’s hypotension resolved without an increase in heart rate. After 15 hours of landiolol administration, the patient converted to a sinus rhythm with an heart rate of 112 bpm (Fig 1b). Junctional ectopic tachycardia did not recur, and the sepsis showed immediate improvement after the patient began effective antibiotic and immunoglobulin therapy.
Discussion
This is the first report of junctional ectopic tachycardia caused by sepsis, occurring 31 months after open-heart surgery in a child with CHD. Post-operative junctional ectopic tachycardia usually appears within the first 72 hours following open-heart surgery for CHD.Reference Erickson 1 Post-operative junctional ectopic tachycardia is known to result from an abnormal automaticity of the atrioventricular node and the bundle of His that is associated with surgical injury. In our patient, however, we surmise that junctional ectopic tachycardia, which developed a few years after surgery, was due to some factor other than surgical injury. First, the patient had heterotaxy syndrome. Bae et alReference Bae, Noh and Choi 4 reported that patients with heterotaxy syndrome are at risk for developing junctional tachycardia outside the immediate post-operative period. Therefore, the baseline rhythm of this patient may be easily converted to tachycardia, despite not having evidence of sinus node dysfunction or atrioventricular node dysfunction. Second, post-operative junctional ectopic tachycardia has also been shown to be related to inflammation associated with viral myocarditis. Maiers et alReference Maiers and Ebenroth 5 reported a case of junctional ectopic tachycardia occurring after viral myocarditis, and suggested that inflammation of the atrioventricular node and His bundle, following myocarditis, may result in junctional ectopic tachycardia. Although our patient did not develop myocarditis, systemic inflammation resulting from the sepsis may have impacted the atrioventricular node and His bundle, leading to junctional ectopic tachycardia.
The treatment of junctional ectopic tachycardia is not well-established in children. Although past reports have demonstrated that amiodarone and procainamide are effective for the treatment of post-operative junctional ectopic tachycardia, the use of these medicines in patients who are at high risk for sudden changes in haemodynamics and electrolyte abnormalities when the patient has sepsis is difficult. Landiolol is an ultra-short-acting β-adrenergic blocker, developed in Japan, which has been demonstrated to be effective for the treatment of junctional ectopic tachycardia (Table 1);Reference Saiki, Nakagawa, Ishido, Masutani and Senzaki 6 – Reference Maehata, Nishigaki, Kawahira, Yanase and Sugita 9 however, a recommended dose of this drug has not yet been established in children. The doses used in our patient and in past reports have ranged from 1 to 40 μg/(kg minute). Landiolol was previously shown to be effective for restoring sinus rhythm in 11 of 13 patients (85%); bradycardia was observed in one patient who was started on a 40 µg/kg minute infusion of landiolol.Reference Tokunaga, Hiramatsu and Kanemoto 7 The other patients in the study did not demonstrate any side-effects;Reference Tokunaga, Hiramatsu and Kanemoto 7 therefore, we recommend a starting dose of 1 μg/(kg minute), without a bolus infusion. In addition, Hagiwara et alReference Hagiwara, Iwasaka, Maeda and Noguchi 10 reported that landiolol has protective effects in an lipopolysaccharide-induced systemic inflammation model, demonstrating that landiolol inhibits the release of inflammatory mediators. Therefore, we believe that landiolol is a good choice for the initial treatment of junctional ectopic tachycardia caused by sepsis.
Table 1 Previously published reports describing the efficacy of landiolol in patients with junctional ectopic tachycardia (JET).

In conclusion, we treated a child with a history of open-heart surgery, performed 31 months previously, who developed junctional ectopic tachycardia associated with sepsis. The administration of landiolol was quite effective for the treatment of junctional ectopic tachycardia.
Acknowledgement
We thank Dr. Kouich Nakau, Dr. Aya Kajihama and Dr. Mio Taketazu for helpful discussions, assistance with treatment, and critically reviewing our manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The family of the patient described in this report provided their informed consent for the treatment of the child.





