Introduction
The neural bases of cognitive processing are not identical in children and adults. Therefore, when working toward treating brain disorders in children, such as childhood epilepsy, it is essential to account for these differences rather than making generalizations from adult studies. For example, semantic processing of sentences recruits more bilateral brain activation in children compared to left lateralized activation in adults (Brauer & Friederici, Reference Brauer and Friederici2007), and increased activation of left middle temporal gyrus and inferior parietal lobule is found with increasing age throughout childhood (Chou et al., Reference Chou, Booth, Bitan, Burman, Bigio, Cone and Cao2006). This type of lateralization-of-function difference in children becomes especially important when considering treatment options such as surgical excision of affected brain tissue.
Although there are numerous investigations of the neural representation of language in children, the neural underpinnings of memory in childhood is not well characterized. Here we specifically focused on relational memory, or the ability to learn and remember relations among multiple items, which is heavily pertinent to one's interactions in daily life and recruits hippocampal activity in adults (Hanlon et al., Reference Hanlon, Weisend, Huang, Lee, Moses, Paulson and Cañive2003; Leirer et al., Reference Leirer, Wienbruch, Paul-Jordanov, Kolassa, Elbert and Kolassa2010; Moses et al., 2008; Moses et al., 2009). Relational memory is essential for remembering people's names, navigating through streets, planning for future events, and underlies episodic and autobiographical forms of memory. This study investigated differences in the organization of relational memory in children and adults, and how they relate to behavioral performance.
Research has shown that the hippocampi are dynamic structures changing in size until at least 25 years of age (Gogtay et al., 2006), however, they appear to be relatively mature structurally in early childhood compared to the surrounding cortex (Alvarado & Bachvalier, Reference Alvarado and Bachvalier2000; Benes, Reference Benes1989, Benes, Turtle, Kahn, & Farol, Reference Benes, Turtle, Khan and Farol1994; Giedd et al., Reference Giedd, Snell, Lange, Rajapakse, Casey, Kozuch and Rapoport1996; Kretschmann, Kammradt, Krauthausen, Sauer, & Wingert, Reference Kretschmann, Kammradt, Krauthausen, Sauer and Wingert1986; Sowell, Delis, Stiles, & Jernigan, Reference Sowell, Delis, Stiles and Jernigan2001; Utsunomiya, Takano, Okazaki, & Mitsudome, Reference Utsunomiya, Takano, Okazaki and Mitsudome1999). The hippocampi are critical brain structures for relational memory (Cohen & Eichenbaum, Reference Cohen and Eichenbaum1993). In adults and children, bilateral damage to the hippocampus leads to severe global amnesia, in which there may be deficits for remembering relational information learned a certain time before the insult, as well as an inability to form new relational memories (Broman, Rose, Hotson, & Casey, Reference Broman, Rose, Hotson and Casey1997; Kartsounis, Rudge, & Stevens, Reference Kartsounis, Rudge and Stevens1995; Ostergaard, Reference Ostergaard1987; Rempel-Clower, Zola, Squire, & Amaral, Reference Rempel-Clower, Zola, Squire and Amaral1996; Victor & Agamanolis, 1990; Zola-Morgan, Squire, & Amaral, Reference Zola-Morgan, Squire and Amaral1986). Performance on relational memory tasks does not reach adult levels until later in childhood: 7 years old for spatial navigation (Overman, Pate, Moore, & Peuster, Reference Overman, Pate, Moore and Peuster1996), and 9–10 years old for explicit memory (Hömberg, Bickmann, & Müller, Reference Hömberg, Bickmann and Müller1993). This is likely due to immature hippocampal-cortical connectivity, which continues to develop until early adulthood (Giedd et al., Reference Giedd, Snell, Lange, Rajapakse, Casey, Kozuch and Rapoport1996; Sowell et al., Reference Sowell, Delis, Stiles and Jernigan2001). Thus, we will look at laterality in the context of development, as lateralization of hippocampal activation may be less in children versus adults due to the developing connectivity with the surrounding cortical regions. In support of the hypothesis that memory function will be less lateralized in children, evidence from neuropsychological testing shows that children with temporal lobe epilepsy do not show adult-like patterns of lateralization of relational memory functions (Gonzalez, Anderson, Wood, Mitchell, & Harvey, Reference Gonzalez, Anderson, Wood, Mitchell and Harvey2007).
To study the neural organization of relational memory in children, we selected the transverse patterning (TP) task. The TP task is structurally analogous to the childhood game “rock-paper-scissors,” but uses three novel abstract stimuli (represented here as A, B, and C) presented in overlapping pairs (A beats B, B beats C, C beats A; or A+B−, B+C−, C+A−). The TP task requires participants to learn the relations among all the stimuli to solve the task (Driscoll, Howard, Prusky, Rudy, & Sutherland, Reference Driscoll, Howard, Prusky, Rudy and Sutherland2005). Performance on the TP task can be contrasted with an elemental control task, which uses non-overlapping pairs of novel stimuli (D+E−, F+G−, H+I−). The elemental control task can be solved by learning about individual items, and does not require memory for the relations among the stimuli. Specifically, participants can learn that stimuli D, F, and H always win, and do not need to learn about the relations of these stimuli with their partners. Evidence from human and non-human animal lesion studies demonstrates that TP relies on the hippocampal system, however, the elemental control task can be solved despite bilateral hippocampal lesions (Alvarado & Rudy, Reference Alvarado and Rudy1995; Driscoll et al., Reference Driscoll, Howard, Prusky, Rudy and Sutherland2005; Rickard & Grafman Reference Rickard and Grafman1998; Rickard, Verfaellie, & Grafman, Reference Rickard, Verfaellie and Grafman2006, but see Reed & Squire, 1999; Saksida, Busey, Buckmaster, & Murray, Reference Saksida, Busey, Buckmaster and Murray2007).
Previous neuroimaging work has shown that TP elicits robust hippocampal activation in adults that is detectable with magnetoencephalography (MEG) (Hanlon et al., Reference Hanlon, Weisend, Huang, Lee, Moses, Paulson and Cañive2003, Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005, Reference Hanlon, Houck, Lundy, Euler, Weisend, Thoma and Tesche2011; Leirer et al., Reference Leirer, Wienbruch, Paul-Jordanov, Kolassa, Elbert and Kolassa2010; Mills, Lalancette, Moses, Taylor, & Quraan, Reference Mills, Lalancette, Moses, Taylor and Quraan2012; Moses et al., Reference Moses, Ryan, Bardouille, Kovacevis, Hanlon and McIntosh2009; Quraan, Moses, Hung, Mills, & Taylor, Reference Quraan, Moses, Hung, Mills and Taylor2011) and functional magnetic resonance imaging (fMRI; Meltzer, Nigishi, & Constable, Reference Meltzer, Nigishi and Constable2008; Rowland, Griego, Spieker, Cortes, & Holcomb, Reference Rowland, Griego, Spieker, Cortes and Holcomb2010). Children as young as 4.5 years of age have demonstrated the ability to successfully complete TP (Rudy, Keith, & Georgen, Reference Rudy, Keith and Georgen1993): however, patterns of neural activation elicited by TP have not yet been examined in children.
We used MEG to investigate hippocampal activation elicited by TP in both adults and children, and to contrast two competing hypotheses regarding differences in neural activity profiles between adults and children: the compensation and maturation hypotheses. For adults, we expected to find predominantly right hippocampal activation during TP, as in previous work (Hanlon et al., Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005, Reference Hanlon, Houck, Lundy, Euler, Weisend, Thoma and Tesche2011; Mills et al., Reference Mills, Lalancette, Moses, Taylor and Quraan2012; Moses et al., Reference Moses, Ryan, Bardouille, Kovacevis, Hanlon and McIntosh2009). For children, the compensation hypothesis would argue that less lateralization would lead to better TP performance, as the left hippocampus would provide more processing resources to support an immature right hippocampal-cortical network. This compensation hypothesis is derived from studies of the organization of relational memory in pathological conditions. For example, Hanlon et al. (Reference Hanlon, Weisend, Huang, Lee, Moses, Paulson and Cañive2003) found that a patient with damage to the right hippocampus had increased left hippocampal activation, which may have allowed for behavioral compensation of the impaired right hippocampus. Additionally, Hanlon et al. (Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005) demonstrated that increased left hippocampal activation in schizophrenia patients (who have hippocampal dysfunction) led to higher performance accuracy on the TP task. In contrast, this relationship was not found in a control population, who showed predominantly right hippocampal activation. Evidence for the compensation hypothesis can also be found in other cognitive domains, such as language. For example, children with epilepsy who show less typical lateralization show superior verbal memory performance (Everts et al., Reference Everts, Harvey, Lillywhite, Wrennall, Abbott, Gonzalez and Aderson2010).
By contrast, the maturation hypothesis suggests that, since laterality tends to increase with age, more right lateralized hippocampal activation in children would lead to better performance, as this is what is typically found in adults, and, therefore, would be a sign of a more mature and efficient brain. Research from other domains indicates that children who showed more adult-like patterns of lateralization, in this case right hemisphere lateralization, between the ages of 5 and 7 years also showed superior navigation performance (Tsujii, Yamammoto, Masuda, & Wantabe, Reference Tsujii, Yamamto, Masuda and Watanabe2009). Additional evidence from language lateralization research using diffusion tensor imaging (DTI), fMRI, and MEG suggests that areas of the brain become more specialized as a function of age, changing throughout childhood and adolescence (Chou et al., Reference Chou, Booth, Bitan, Burman, Bigio, Cone and Cao2006; Holland et al., Reference Holland, Plante, Weber Byars, Strawsburg, Schmithorst and Ball2001; Ressel, Wilke, Lidzba, Lutzenberger, & Krägeloh-Mann, Reference Ressel, Wilke, Lidzba, Lutzenberger and Krägeloh-Mann2008; Szaflarski, Holland, Schmithorst, & Byars, Reference Szaflarski, Holland, Schmithorst and Byars2006) and that this is associated with superior behavioral performance (Everts et al., Reference Everts, Lidzba, Wilke, Keifer, Mordansini, Schroth and Steinlin2009; Lebel & Beaulieu, Reference Lebel and Beaulieu2009). Therefore, it is possible that children who show a more adult-like pattern of right hippocampal lateralization during the TP task will show better performance.
Thus, successful behavioral performance in children that is associated with greater left/bilateral hippocampal activation would lend support to the compensation hypothesis and suggest that increased recruitment of the left hippocampus is used to support an immature right hippocampus. However, successful behavioral performance in children that is associated instead with greater right hippocampal activation would provide evidence for the maturation hypothesis. More generally, this research will contribute to our understanding of how the neural bases of memory in children differ from those of adults.
Materials and Methods
Participants
Participants were 22 children (11 female; M = 15 years; range, 11–18 years), who were divided into a younger group of 10 children aged 11–14 (4 female; M = 12.9 years; SE = 1.17), and an older group of 12 children aged 15–18 (7 female; M = 16.8 years; SE = 1.18). All children had normal development according to parental report, and had normal structural MRIs. Standardized abbreviated intelligence testing was administered using two subtests (vocabulary and matrix reasoning) of the Wechsler Abbreviated Scale of Intelligence (Wechsler, Reference Wechsler1999); the mean IQ of the participants was 116. Sixteen adults were also recruited; one person's data was excluded due to poor data quality. Fifteen university-educated adults were included in the final analyses (9 female; M = 24 years; range, 21–31 years; SE = 1.6). The study was approved by the Hospital for Sick Children Research Ethics Board and all participants (and their parents, if applicable) provided informed consent.
Stimuli and Procedures
Participants were trained in the TP and elemental tasks before the MEG recording session until they reached a criterion of 80% correct responses. All participants performed the tasks in the MEG until they reached 200 correct trials at which point recording was terminated. The order of TP and elemental tasks was counterbalanced across participants in the pre-training session, and then subsequently performed during the MEG recording, in the same order as training. Three stimuli (A, B, and C) were used in the TP task, grouped into three overlapping stimuli pairs in which A was correct when paired with B, B was correct when paired with C and C was correct when paired with A (A+B−, B+C−, C+A−). Three stimulus pairs were used for the elemental task (D+E−, F+G−, H+I−) (Figure 1). Participants used their right index and middle finger to select the target (winning) stimulus of each pair. Participants were required to learn the relations of the stimuli through trial and error. Correct responses were followed by a cartoon of a happy face and the caption “good job,” and incorrect responses were followed by a cartoon of a frowning face and the caption “wrong.” Stimuli remained on the screen until participants responded. MEG recording continued until 150 correct responses were obtained or until 15 min had elapsed.

Fig. 1 Stimuli used for the transverse patterning (left) and elemental (right) tasks. + denotes wins, − denotes loses.
Data Collection
MEG recording was performed on a CTF 151-channel MEG system (MISL Inc., Coquitlam, BC, Canada) with a sampling rate of 625 Hz, in a magnetically shielded room at the Hospital for Sick Children. Indicator coils were placed on participants’ nasion and bilateral periauricular points to monitor head position within the MEG. We recorded head location before and after the recording session and if participants moved more than 5 mm the data were excluded. A structural MRI was also obtained for each participant at the Hospital for Sick Children using a 1.5 Tesla GE scanner (GE Medical Systems, Milwaukee, WI) and a T1 three-dimensional SPGR-pulse sequence. MRIs were used to co-register the MEG sources of activation with neuroanatomy.
Data Analyses
Only the correct responses were retained for analyses, and all participants had 200 correct trials per task. The MEG data were first parsed into trials via a photodiode signal which demarcated the start of each trial. Source localization was performed using an event-related vector beamformer (Quraan & Cheyne, Reference Quraan and Cheyne2010; Sekihara, Nagarajan, Poeppel, Marantz, & Miyashita, Reference Sekihara, Nagarajan, Poeppel, Marantz and Miyashita2001). The beamformer spatial filter estimated source activity as a pseudo-Z statistic across the whole brain on a grid size of 5 mm. Functional images for each subject were constructed for every sample over the range of 0 to 700 ms, using 5 ms steps (e.g., 200 ms, 205 ms, 210 ms). The files were then normalized to an MNI-152 template and converted into Analysis of Functional NeuroImages (AFNI) format.
Spatiotemporal changes across TP and elemental tasks were characterized using the partial least squares (PLS) multivariate approach (McIntosh, Bookstein, Haxby, & Grady, Reference McIntosh, Bookstein, Haxby and Grady1996; McIntosh & Lobaugh, Reference McIntosh and Lobaugh2004). Mean-centered PLS analysis was used in which values for different blocks are expressed relative to the overall mean. Mean-centered PLS is a data-driven approach that determines the task contrasts (or latent variables) that contribute to the most variance in the data. Latent variables (LVs) are composed of contrast coefficients that characterize relationships between the tasks. Patterns of differences as well as commonalities can be expressed in the resulting contrasts. Using this type of analysis, we can determine activation patterns unique to specific tasks.
A matrix was constructed containing the pseudo-Z value for every participant at each brain voxel at each time point as a raw in this matrix. The matrix was arranged with subjects and conditions (TP and elemental) as rows, and voxels across time-points as columns. Rows were stacked as subjects (for respective age-group) within conditions within age-groups. With n total participants and k condition blocks, there are n * k rows in the matrix. Columns were arranged as voxels within time-points. With m voxels and t time points, there are m * t columns in the matrix. An additional matrix contained grand mean pseudo-Z values, which were computed by averaging across each condition (TP and elemental) at each brain voxel at each time-point. These values from the grand mean matrix were subtracted from the corresponding tasks in the initial matrix resulting in a mean-centered matrix.
Singular value decomposition (SVD) was then applied. SVD yields three matrices, each of which provides unique information. The first, the “design latent variable” (LV), is composed of contrast coefficients that characterize the differences or commonalities between task blocks, collapsed across the entire brain volume and time epoch. The second matrix, the “brain LV,” contains the covariance of the design with the brain data for each brain voxel at each time point, indicating where and when the differences coded in the design LV are expressed. The third matrix contains the singular values, which are the multivariate covariance of the design and the brain data collapsed across the entire brain volume, time and all task blocks.
Statistical assessment for PLS is done using permutation tests and bootstrap estimation of standard errors for the brain LVs (Efron & Tibshirani, 1986; McIntosh et al., Reference McIntosh, Bookstein, Haxby and Grady1996; McIntosh & Lobaugh, Reference McIntosh and Lobaugh2004). The permutation test assesses whether the effect represented in a given LV, captured by the singular value, is sufficiently strong to be different from randomly assigned data. The standard error estimates from the bootstrap tests are used to assess the reliability of the nonzero values in significant LVs.
Additionally, bivariate correlations were performed to examine the relationship among age, behavioral task (TP and elemental) accuracy, and reaction time. Analysis of variance (ANOVA) was used to compare accuracy and reaction times across age groups.
Results
Behavioral Performance
Overall, increased age was correlated with superior accuracy on the TP (r = 0.40; p = .01), but not the elemental (r = 0.11; p = .53) task. Increased age was also correlated with faster reaction times for both tasks (r's > −0.33; p's < .04) (Table 1).
Table 1 Mean of accuracy and reaction time for TP and Elemental tasks for younger children, older children, and adults (with SDs in parentheses)
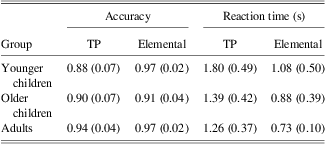
Note. TP = transverse patterning.
A mixed-factor repeated-measures ANOVA was used to examine performance on the transverse patterning and elemental tasks in the younger children, older children, and adult groups. Regardless of age, accuracy was higher for elemental than TP, illustrated by a main effect of task (F(1,34) = 87.6; p < .0001). Accuracy for TP improved across the age groups, whereas accuracy for the elemental task remained stable, illustrated by an interaction of task by age group (F(2,34) = 4.7; p = .02). There was no significant main effect of age group on accuracy (F(2,34) = 1.8; p = .18). TP reaction time was found to be a significant covariate for TP accuracy (F(1,34) = 3.99; p = .05); however, elemental reaction time was not a significant covariate for elemental accuracy (F(1,34) = 0.94; p = .34).
PLS Analysis of Brain Activation
Latent variable (LV)1: Main effect of task
Mean-centered PLS analysis comparing brain activation during performance of the TP and elemental tasks revealed a significant latent variable that showed a difference in hippocampal activation between the two tasks, which was consistent across all age groups (Figure 2), but most strongly expressed in the younger children.

Fig. 2 Latent variable 1 from partial least squares analysis expresses significant difference between activation during the transverse patterning (TP) and Elemental (ELE) tasks. These task differences were most robust in the 11–14 years group, although a similar pattern occurred in the other groups.
Left hippocampal activation [IJKafni coordinates: (11, 21, 6)] was greater for the TP versus the elemental task up to 490 ms following stimulus onset (Figure 3A). Specifically, when looking at differences in the amplitude of left hippocampal activity between the two tasks for each group (Figure 3B), robust task differences were found between 100 and 125 ms and approximately 205 ms following stimulus onset with all groups having higher amplitude activation during TP than elemental tasks. Inspection of the waveforms revealed that the younger children had the greatest difference in amplitude between tasks at both time points. Another robust task difference was found between 320 and 340 ms following stimulus onset; both groups of children had higher amplitudes of left hippocampal activation during the TP than the elemental task, but this was more strongly expressed in the youngest group. Conversely, a robust task difference was found at 540 ms following stimulus onset; the amplitude of left hippocampal activation was higher during the elemental than the TP task; this difference was observed in all age groups, and was greatest in the younger children.

Fig. 3 (A) Thresholded at a bootstrap ratio greater than 3. Partial least squares bootstrap ratio plots across each brain voxel from latent variable 1 (LV1) showing a left hippocampal region of interest (ROI) at 120 ms following stimulus onset. Brain areas shown in red demonstrate regions of greater activation during the transverse patterning (TP) task versus the Elemental task, while areas shown in blue demonstrate greater activation during the Elemental task versus the TP task. (B) Group average of difference waveform for TP minus elemental for the left hippocampal ROI from LV1 [IJKafni coordinates: (11, 21, 6)] for all three groups. Circles denote a bootstrap ratio ≥ 2.5; Xs denote a bootstrap ratio ≤ −2.5.
Right hippocampal activation [IJKafni coordinates: (23, 19, 7)] was greater for TP versus elemental task at 80 and 505 ms during TP (Figure 4). Inspection of the waveforms reveals that at both time points, this effect was driven primarily by the younger children, followed by the older children. In contrast, activation was greater for the elemental versus the TP task between 150 and 175 ms (Figure 4B) in the older children and adults, with the opposite pattern (TP > elemental) expressed in the younger children.

Fig. 4 (A) Thresholded at a bootstrap ratio greater than 3. Partial least squares bootstrap ratio plots across each brain voxel from latent variable 1 (LV1) showing a right hippocampal region of interest (ROI) at 505 ms following stimulus onset. Brain areas shown in red demonstrate regions of greater activation during the transverse patterning (TP) task versus the Elemental task, while areas shown in blue demonstrate greater activation during the Elemental task versus the TP task. (B) Group average of difference waveforms for TP minus elemental for the right hippocampal ROI from LV1 [IJKafni coordinates: (23, 19, 7)] for all three groups. Circles denote a bootstrap ratio ≥ 2.5; Xs denote a bootstrap ratio ≤ −2.5
LV2: Interaction of task by group
Mean-centered PLS analysis revealed a second LV that represented an interaction between task and age group (Figure 5). A difference between TP and elemental tasks was present for all age groups, however, the younger children showed a distinct and opposite pattern of brain activation from the older children and adults.

Fig. 5 Latent variable 2 (LV2) expresses difference between activation during the transverse patterning (TP) and Elemental (ELE) tasks and between age groups. This LV distinguished brain activation patterns of younger children from that of the older children and the adults.
Robust differences in left hippocampal activation [IJKafni coordinates: (11, 19, 9)] were observed for the TP versus the elemental task between 440 and 500 ms (Figures 6). Examination of the waveforms revealed that for the majority of this time window, all groups had a higher amplitude of left hippocampal activation during the TP compared to the elemental task, with the older children having the largest amplitude difference between the two tasks.

Fig. 6 (A) Thresholded at a bootstrap ratio greater than 3. Partial least squares bootstrap ratio plots across each brain voxel from latent variable 2 (LV2) showing a left hippocampal region of interest (ROI) at 490 ms following stimulus onset. Brain areas shown in red demonstrate regions of stronger activation during the transverse patterning (TP) task versus the Elemental task for the older children and adult; and stronger activation during Elemental than TP for the younger children. Areas shown in blue demonstrate stronger activation during the Elemental task versus the TP task for the older children and adults; and demonstrate greater activation during TP versus Elemental for the younger children. (B) Group average of difference waveforms for TP minus elemental for the left hippocampal ROI from LV2 [IJKafni coordinates: (11, 19, 8)] for all three groups. Circles denote a bootstrap ratio ≥ 2.5; Xs denote a bootstrap ratio ≤ −2.5.
Robust task differences were also observed in right hippocampal activation [IJKafni coordinates: (21, 17, 7)] during 150–190 ms and 225–250 ms. Inspection of the waveforms revealed that, the younger children showed distinct patterns compared to the other two groups. A higher amplitude of activation was seen during the TP versus elemental task for the younger children between 150 and 190 ms, whereas the other groups showed higher amplitudes for the elemental task compared to TP (Figure 7). At 225–250 ms, the younger children had a higher amplitude of right hippocampal activation for the elemental task compared to the TP task, whereas the older children and adult groups had higher amplitudes for the TP compared to the elemental task.

Fig. 7 (A) Thresholded at a bootstrap ratio greater than 3. Partial least squares bootstrap ratio plots across each brain voxel from latent variable 2 (LV2) showing a right hippocampal region of interest (ROI) at 160 ms following stimulus onset. Brain areas shown in red demonstrate regions of stronger activation during the transverse patterning (TP) task versus the Elemental task for the older children and adult; and show greater activation during Elemental than TP for the younger children. Areas shown in blue demonstrate greater activation during the Elemental task versus the TP task, for the younger children and adults; and demonstrate greater activation during TP versus Elemental for the younger children. (B) Group average of difference waveforms for TP minus elemental for the right hippocampal ROI from LV2 [IJKafni coordinates: (21, 17, 7)] for all three groups. Circles denote a bootstrap ratio ≥ 2.5, Xs denote a bootstrap ratio ≤ −2.5.
Brain-behavior correlations
We investigated the relations among laterality of hippocampal activation, age, accuracy, and reaction time. A hippocampal laterality coefficient was calculated for all groups using the average right and left source location coordinates of the hippocampal regions of interest found in LV1 (Figure 2), which showed robust task differences across all groups [Right Hippocampal IJKafni coordinates: (23, 20, 7); Left Hippocampal IJKafni coordinates: (10, 21, 6)]. The laterality coefficient was calculated as a ratio: (right source strength – left source strength)/(right source strength + left source strength), as in Hanlon et al. (Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005). Therefore, a positive number indicates stronger right hippocampal activation and a negative number indicates stronger left activation. This laterality coefficient was calculated at 20-ms intervals, between 200 and 700 ms following stimulus onset during performance of the TP and elemental tasks, and then all 25 values were averaged for each task to obtain one laterality coefficient per task which could be used to investigate brain-behavior correlations.
The TP task elicited greater right-lateralized hippocampal activation than the elemental task across all age groups, illustrated by a significant main effect of task (F(1,34) = 6.6; p = .02) in an ANOVA comparing the laterality coefficient during TP and elemental performance across the three age groups. There was also a trend for right hippocampal lateralization to increase with age, regardless of task (F(2,34) = 2.6; p = .09).
Overall, higher accuracy on TP was correlated with stronger right hippocampal lateralization (i.e., a more positive total laterality coefficient; r = 0.37; p = .02). There was no significant correlation between elemental task accuracy and total laterality (r = 0.19; p = .22). No significant correlations were detected between the total laterality coefficient and reaction time or age. A multiple regression analysis was used to test if laterality and age significantly predicted participants’ accuracy. The two factors explained 27.3% of the variance of TP accuracy (R2 = 0.23; F(2,36) = 6.372; p = .04). Age significantly predicted TP accuracy (β = 0.367; t = 2.496; p = .018), as did total laterality (β = 0.336; t = 2.284; p = .029). When looking at elemental accuracy, total laterality predicted accuracy (β = 0.399; t = 2.541; p = .016), but age did not (β = 0.065; t = 0.413; p = .682).
Correlations were also examined within each individual age group. For the younger children, stronger right hippocampal lateralization correlated with superior accuracy on TP (r = 0.64; p = .05), but not on the elemental task (r = −0.05; p = .88). In addition, increased right hippocampal lateralization was correlated with longer reaction times in younger children (r = 0.66; p = .04). No significant correlations between laterality of hippocampal activation and either accuracy or reaction time were found for the older children or adult groups separately.
Inspection of the laterality coefficient values (Figure 8) and t tests revealed significant differences between the younger children and older children, and between the adults and older children. The younger and older children showed significant differences between 460 and 500 ms, and 560–600 ms (p < .05). The older children and adults showed significant difference between 440 and 480 ms, and 540–640 ms (p < .05).

Fig. 8 Group averaged transverse patterning (TP) laterality coefficients. Positive numbers indicate more right in TP, and negative numbers indicate more left in TP. Red asterisks indicate significant differences between the adult and older children groups, and blue asterisks indicate significant differences between the younger children and older children groups.
Discussion
This work examined the neural organization of relational memory in children compared to adults using the transverse patterning task. In particular, we compared predictions of the compensation hypothesis (bilateral activation in children would lead to better TP performance) and the maturation hypothesis (performance supported by right-lateralized pattern of hippocampal activation). The results support the maturation hypothesis: a more adult-like pattern of increased right hippocampal lateralization in children led to superior performance on the TP task. Additionally, dynamic changes of lateralization were observed throughout the time course for all age groups including adults.
Similarities between Age Groups
Overall, the transverse patterning task elicited robust hippocampal activation across all groups. Although similar task differences in hippocampal activation were found across groups, the effect was most strongly expressed in the younger children. All age groups had stronger right hippocampal activation for the TP versus the elemental task very early in the trial at 80 ms, as well as later in the trial at 505 ms. This finding supports previous work with adults implicating the right hippocampus in TP (Hanlon et al., Reference Hanlon, Weisend, Huang, Lee, Moses, Paulson and Cañive2003, Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005, Reference Hanlon, Houck, Lundy, Euler, Weisend, Thoma and Tesche2011; Moses et al., Reference Moses, Ryan, Bardouille, Kovacevis, Hanlon and McIntosh2009). However, all groups also showed stronger left hippocampal activation for the TP versus the elemental task between 110 and 500 ms. Examination of the waveforms showed that the younger children had the largest amplitude differences between tasks for both left and right hippocampal activity. Thus, TP elicits both right and left hippocampal activation. It is possible that the left hippocampal activation found here reflects verbalization of the TP task, despite the stimuli being pictorial in nature. In support of this idea, all subjects reported naming the stimuli, despite their novel abstract nature. While this set of results does not distinguish between the maturation and compensation hypotheses, it does demonstrate the importance of examining the entire activation time course rather than looking only at specific times, which may provide incomplete information.
Differences across Age Groups
Although the younger children showed similar task differences to the two other groups, group differences in hippocampal amplitude between the TP and elemental tasks were also observed. All groups had a higher amplitude of left hippocampal activation during the TP compared to the elemental task between 440 and 500 ms with the older children having the largest amplitude difference between the two tasks. Additionally for right hippocampal amplitude, the younger children showed greater activation for the TP versus the elemental task early in the trial, at 150–190 ms and 225–250 ms, whereas the older children and adults showed stronger activation for the elemental versus TP task at the same time period. Thus, the younger children were showing distinct patterns of activation in comparison with the other two groups.
Brain-Behavior Correlations
By examining correlations between a laterality coefficient of hippocampal activation and accuracy of behavioral performance, we contrasted the compensation hypothesis, which would predict that less lateralization in children would lead to better TP performance as it would provide supplemental resources to compensate for an immature right hippocampal network, with the maturation hypothesis, which would predict that more right lateralized hippocampal activation, indicative of a more mature brain, would lead to better performance. Our findings support the maturation hypothesis.
We found that TP accuracy was correlated with stronger right hippocampal activation across all groups, and that this relationship was the strongest in the younger children. No such relation was found for the elemental control task. We also found tendencies for right hippocampal laterality to increase with age, regardless of task, and for accuracy on the transverse patterning task to increase with age. Thus, the more a child shows an adult-like right lateralization pattern of hippocampal activation, the more accurate their relational memory performance as indicated by the TP task. This is in line with previous developmental research which shows that increased lateralization with age is associated with more accurate responses. For example, Tsuiji et al. (2009) found that children who had increased right hemisphere lateralization showed better navigation performance during a working memory task. Similarly, Everts et al. (Reference Everts, Lidzba, Wilke, Keifer, Mordansini, Schroth and Steinlin2009) found that lateralization was strengthened for both left and right hemisphere functions as age and performance increased, with children who had strong left lateralization showing strong verbal task performance and children who have strong right lateralization showing strong spatial task performance. In addition, Chou et al. (Reference Chou, Booth, Bitan, Burman, Bigio, Cone and Cao2006) found that activation amplitude in the left middle temporal gyrus and inferior parietal lobule was associated with increasing age in a semantic processing task suggesting a more elaborated semantic representational system similar to that of adults. These studies indicate that the development of working memory and verbal, visuospatial, and semantic functions may have similar trajectories to that of relational memory function; all move from a poorer performance mediated by a less lateralized organization to a superior performance mediated by a more lateralized organization.
Our findings with children contrast those of adults who have impaired neural function. Whereas TP performance was supported by a more right-lateralized pattern of hippocampal activation, Hanlon et al. (Reference Hanlon, Weisend, Huang, Lee, Moses, Paulson and Cañive2003) showed that performance in an adult patient with a damaged right hippocampus was associated with increased left hippocampal activation. Similarly, Hanlon et al. (Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005) showed that patients with schizophrenia who had increased left hippocampal activation had higher accuracy on TP. Therefore, the patterns of neural activation in children are not commensurate with adults with cognitive impairments, but rather show that higher performance in children is associated with healthy, adult-like patterns of neural activity, indicating a more mature and efficient brain.
Importantly, we examined whether hippocampal laterality is consistent during processing on trials or whether the dynamics vary across the trial. The plot of the temporal dynamics of the laterality coefficient revealed that hippocampal laterality is indeed variable across the trial. For example, at earlier time points (200–250 ms), adults showed right lateralized hippocampal activation, consistent with previous literature (Hanlon et al., Reference Hanlon, Weisend, Huang, Lee, Moses, Paulson and Cañive2003, Reference Hanlon, Weisend, Yeo, Huang, Lee, Thoma and Cañive2005, Reference Hanlon, Houck, Lundy, Euler, Weisend, Thoma and Tesche2011; Moses et al., Reference Moses, Ryan, Bardouille, Kovacevis, Hanlon and McIntosh2009), whereas the younger and older children showed left lateralized hippocampal activation. However, during later time points (380–450 ms) this laterality pattern flips: the younger and older children showed right lateralized hippocampal activation, whereas the adult group showed left lateralized hippocampal activation. Left-lateralized activation in the older children and adults was contrary to the predictions, and may be suggestive of verbal processing occurring in the trial (Opitz & Friederici, 2003). Nonetheless, the findings of changes in lateralization patterns throughout the trial point to the fact that we must be cautious when interpreting conclusions about lateralization of brain activation.
Future Directions and Implications
Stronger right hippocampal lateralization and increased age lead to higher accuracy on the relational memory task, demonstrating that the neural bases of relational memory function changes over childhood including adolescence. The current study used a cross-sectional design and future research should consider using a longitudinal design to better track the brain laterality changes with age. These preliminary findings may lead to considerations for the surgical excision of affected brain tissues in children with brain disorders such as childhood epilepsy in the future. Accuracy was high for all age groups and future research should examine the impact of task difficulty as this has been found to have an effect on the lateralization of adults (e.g., Reiterer et al., Reference Reiterer, Erb, Droll, Anders, Ethofer, Grodd and Wildgruber2005; Helton et al., Reference Helton, Warm, Tripp, Matthews, Parasuraman and Hancock2010). Future research could also focus on differences in laterality during the TP task in children with brain disorders as these populations may have increased hippocampal differences that are critical when considering treatment options.
However, because MEG allows for accurate temporal information of brain activation, this work further showed that hippocampal lateralization was variable over time throughout these tasks; suggesting not only that future researchers should be attentive when analyzing hippocampal lateralization points, but this variability of laterality over time may be an important factor in considering the outcomes of cognitive processing following surgical excision.
Acknowledgments
This work was funded by a Natural Science and Engineering Council Discovery grant awarded to SNM and by the Canada Research Chair Program for JDR. The authors would like to thank Gabrielle Singh Cadieux, Marc Lalancette, and Travis Mills for data collection, contribution to the analysis, and technical support, and Randy McIntosh for his support and advice. The authors declare no conflicts of interest. Grant sponsor: Natural Science and Engineering Council Discovery grant awarded to S.N.M.




