INTRODUCTION
Twenty out of ~10,000 Recent sponge species produce a massive basal calcium carbonate skeleton in addition to spicules. These ‘hypercalcified sponges’ belong to the classes Demospongiae and Calcispongiae.
In Recent seas, hypercalcified sponge species usually inhabit cryptic environments, such as submarine littoral caves or some deep fore reefs between 60 and 120 m (Lang et al., Reference Lang, Hartman and Land1975), with observations reported at extreme depths of 303 m and 530 m for Ceratoporella nicholsoni and Vaceletia crypta respectively (Vacelet, Reference Vacelet1988). Important skeletal fossil records revealed the large distribution of these reef builders through past seas. During the late Mesozoic, they were supplanted by more competitive reef builders, scleractinian corals, and survived only in cryptic environments.
The massive basal skeleton of hypercalcified sponges is composed of either calcite or aragonite according to the species. Only a thin veneer of soft tissues covers the apical part of this skeleton. It does not show annual density variations like coral skeleton, making age determinations difficult. Both direct staining (alizarin red and calcein) and indirect techniques (14C and 210Pb chronologies) have been used to determine the growth rate of hypercalcified sponges (Dustan & Sacco, Reference Dustan and Sacco1983; Willenz & Hartman, Reference Willenz, Hartman, Harmelin Vivien and Salvat1985, Reference Willenz and Hartman1999; Benavides & Druffel, Reference Benavides and Druffel1986). In situ staining of different Demospongiae species (Ceratoporella nicholsoni Hickson, Acanthochaetetes wellsi Hartman and Goreau, Astrosclera willeyana Lister) indicated very slow growth rates ranging from 100 to 300 µm/year (Willenz & Hartman, Reference Willenz, Hartman, Harmelin Vivien and Salvat1985, Reference Willenz and Hartman1999; Reitner & Gautret, Reference Reitner and Gautret1996; Wörheide, Reference Wörheide1998). A specimen of C. nicholsoni of 10 cm height, with a growth rate of 200 µm/year, may be 500 years old (Willenz & Hartman, Reference Willenz and Hartman1999). So far, the skeletal growth rate of hypercalcified Calcispongiae has not yet been determined.
With such long lifetimes and massive skeletal development, hypercalcified sponge skeletons have been highlighted as potential past temperature recorders (Swart et al., Reference Swart, Moore, Charles and Böhm1998). Rosenheim et al. (Reference Rosenheim, Swart, Thorrold, Willenz, Berry and Latkoczy2004) showed that the Sr/Ca ratio in the aragonitic skeleton of C. nicholsoni is directly related to temperature, with higher temperature sensitivity than in coral skeletons. Hypercalcified sponges provide palaeotemperature data complementary to those inferred from coral skeleton.
Although hypercalcified sponges occur usually in tropical and often deep habitats, one hypercalcified calcisponge, Petrobiona massiliana Vacelet and Lévi, is abundant in Mediterranean submarine littoral caves. This species has been recorded from the Gulf of Lion to Greece at shallow depths ranging from 0.5 to 25 m (Vacelet, Reference Vacelet1964). In addition to its calcareous spicules, P. massiliana produces a calcitic massive skeleton. Skeletal development in this species is still unclear, little information being available about its growth rate and patterns of growth. Reitner (Reference Reitner1989) described spicule incorporation in the growing massive skeleton, followed by a diagenetic process altering the spicule in a polycrystalline structure in the oldest part of the skeleton. However, Vacelet (Reference Vacelet, Reitner and Keupp1991) observed whole spicules at the basal part of the skeleton.
In order to improve the knowledge of the skeletal formation of hypercalcified Calcispongiae, we studied the morphology, growth patterns, and growth rates of the calcitic massive skeleton of Petrobiona massiliana, an easily accessible model. By comparing the skeletal chemistry of specimens collected in environmentally contrasted areas, the potential of this skeleton as a temperature recorder was evaluated.
MATERIALS AND METHODS
Sample collection, morphology and chemistry of the skeleton
Specimens of Petrobiona massiliana were collected by SCUBA diving in Mediterranean submarine caves located in La Vesse (Bouches-du-Rhône, France) at a depth of 11 m, in Capo Caccia (Sardinia, Italia), 18 m depth, and in Kalithea Bay (Rhodes, Greece), 2.5 m depth. After collection, specimens were dried in an oven at 50°C for 48 hours. Triplicate water samples were collected at the collection site in 50 ml Nalgene vials, filtered on a Millipore 0.22 µm filter with addition of HgCl2 and stored at 4°C until analysis.
Massive skeletons (devoid of substrate) were cleaned from associated organic tissues with a Proteinase K solution (P6556 SIGMA) in a 100 mmol/l Tris HCL buffer (pH = 7.5) at 37°C for 3 hours. Cleaned skeletons were then rinsed with a thin Milli Q water spray (Broxo Jet) and dried at 50°C for 48 hours.
Cleaned and uncleaned skeletons of P. massiliana collected in La Vesse (France) were fractured or sectioned with a low speed diamond saw (Labcut 1010). They were sputter-coated with gold-palladium and observed on a JEOL JSM-6100 or a Philips/FEI XL30 ESEM TMP scanning electron microscope (SEM).
For chemical analyses, 0.1 to 0.25 g cleaned skeletal samples were mineralized in a Milestone 1200 mega microwave oven in 2.5 ml HNO3 and 1 ml H2O2. The mineralized solution was filtered on a GF/A Whatman filter and brought to a final volume of 25 ml. Magnesium, strontium and calcium contents of the 10 times diluted solutions and of 20 times diluted seawater samples were analysed with an Iris Advantage (Thermo Jarrel Ash) inductively coupled plasma atomic emission epectroscope (ICP-AES). The calibration was achieved using artificial multi-elemental solutions made from certified mono-elemental solutions (Merck) and using certified reference materials for quality check: HPS CRM Seawater (High Purity Standard) for the seawater samples and JCp-1 (coral) and JCt-1 (giant clam) (Standard Geological Survey of Japan). Results for the certified reference materials were always within ±10% of the certified values.
In situ calcein labelling experimentation
Individual sponges, together with a small piece of their substrate, were delicately removed from the rocky wall with hammer and cold chisel and cemented underwater on polymethyl methacrylate plates with a 2 components epoxy resin (Spreadsub T260 Resipoly-Chrysor). Seven to ten individuals were glued on each plate (12×12 cm). Plates were firmly screwed on the original substrate, respecting the natural location of the samples. In order to mark the newly deposited calcite, specimens were incubated with calcein, a fluorochrome that binds permanently at the site of precipitation of calcium carbonate. Two staining experiments were conducted in two littoral caves near Marseille (France): in the first one, sponges were labelled immediately after being cemented on plates (Figuier cave (FC)); in the second one, they were marked after a two months recovery period following their transfer on plates (La Vesse cave (LVC)). Staining was performed in situ to reduce the sponge stress: plates were unscrewed from the substrate and gathered in a polymethyl methacrylate holder which was enclosed in a plastic bag and left on the bottom of the cave during the incubation. Calcein, dissolved in seawater, was injected into bags to reach a final concentration of 100 mg/l. Bags were removed after 24 hours in FC and 72 hours in LVC (Table 1). After incubation, plates were immediately replaced on the wall of the caves.
Table 1. Dates of transfer on plates, calcein staining, collection and total period of skeletal growth of specimens of Petrobiona massiliana in the 2 locations.

Plates were collected about one year after staining. Eight to ten specimens were sacrificed and fixed in ethanol 70°. After dehydration in absolute ethanol, they were embedded in Spurr's epoxy according to Spurr (Reference Spurr1969) and sectioned along their principal growth axis with a low speed diamond saw (Labcut 1010). Central sections along the main growth axis were ground on a series of diamond grinding discs to a thickness of about 10 µm. The linear extension from calcein stained line to the edge of the massive skeleton was measured under epifluorescence microscopy (Nikon Optishot-2 microscope) to calculate annual growth rate.
Data analysis
Mean annual temperature of the field locations were obtained from MEDAR/MEDATLAS regional datasets available on http://doga.ogs.trieste.it/medar/ (MEDAR Group, 2002).
All statistical analyses were carried out using SIGMASTAT and SYSTAT software. Significance level was fixed at 0.05. All linear growth increments were normalized as annual growth rate prior to their analysis. Non-parametric Kruskal–Wallis one-way analysis of variance on ranks tests and Wilcoxon matched pairs signed ranks test were performed. The relationships between temperature and skeletal element to calcium ratios were investigated using linear regression. The comparison of element to calcium ratios of seawater was achieved using one-way ANOVA. Data normality and homoscedasticity were checked prior to the use of parametric tests.
RESULTS
The massive skeleton of the hypercalcified calcisponge Petrobiona massiliana appears as a solid mass of calcite with crests and depressions (Figure 1A). These crests are organized along multi-directional axes (Figure 1B, C) and support the thin living tissues, which cover the upper part of the skeleton and contain an abundance of spicules. Longitudinal sections of the massive skeleton presented homogeneous density. Narrow canalicules containing remains of living tissues (in uncleaned specimens) and numerous rugose diactines (length 30 to 40 µm) were observed along the entire thickness of the skeletons. Fractured specimens showed various types of spicules randomly entrapped inside the massive skeleton and imprints of some other spicules, ripped during fracture (Figure 1D, E).

Fig. 1. Petrobiona massiliana (SEM): (A) longitudinal section perpendicular to surface, along major growth axis (scale bar = 1 mm). Cr, skeletal crests; D, depression of the skeleton; LT, living tissues; S, spicules; Sk, massive skeleton; Ca, canalicules; (B) edge of massive skeleton showing multi-directional growth of crests (scale bar = 100 µm); (C) detail of crest edge of cleaned skeleton, showing cone-shaped protuberances (C) and multi-directional growth of a single crest around main growth direction (scale bar = 100 µm); (D) detail of fractured skeleton showing entrapped spicules (ES), and imprints of some other (SI) (scale bar = 10 µm); (E) diapason triactine entrapped during skeletal growth (scale bar = 10 µm); (F) skeletal pit shaped excavations (arrows), probably occasioned by a Cliona sp. (scale bar = 100 µm).
All specimens observed in this study presented smooth and circular excavation pits in their calcareous skeleton (Figure 1F). The surface of the skeleton presented skeletal cone-shaped protuberances, ranging from 50 to 125 µm in height, with a smooth tip emerging from the rough surface of the skeleton (Figure 2A). Several cone-shaped protuberances were stained during the calcein incubation, and were totally embedded within the skeleton one year later (Figure 2B). Some free spicules were also stained, as they were growing. One year later, these spicules were located in the superficial spicule coat. Ground sections of the skeleton of P. massiliana stained with calcein revealed a spatially discontinuous labelling under epifluorescence microscopy (Figure 3). Fluorescent marks observed were more intense in LVC specimens stained for 72 hours after a two month recovery period following their installation on plates. Out of a total of 18 observed specimens, only one specimen, from FC, did not survive the experimentation.

Fig. 2. Petrobiona massiliana: (A) SEM: skeletal surface of the massive skeleton. Detail of a cone shaped protuberance with smooth tip (ST) emerging from the skeletal rough surface (R) (scale bar = 10 µm); (B) epifluorescence microscopy: cone shaped protuberance, calcein labelled (scale bar = 50 µm).
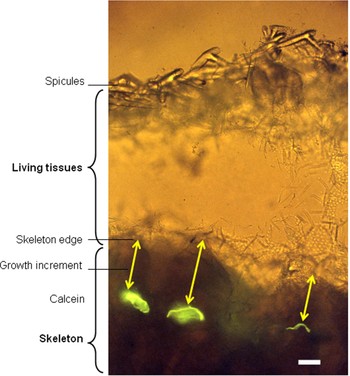
Fig. 3. Petrobiona massiliana (epifluorescence microscopy): ground section of a specimen collected one year after calcein staining (scale bar = 50 µm). Arrows indicate growth increment.
Calcein lines were observed at the tips of the skeletal crests, in flat surfaces or in skeletal depressions. The individual mean growth from measurements on skeletal tips was compared to those made in skeletal depressions: a Wilcoxon test revealed that both type of measurements did not differ significantly (P = 0.3).
Mean annual growth rates of the massive skeleton of P. massiliana did not differ between locations (Mann–Whitney, P = 0.646): 245 µm/year (±116, N = 50) in FC and 220 µm/year (±34, N = 41) in LVC. The global mean individual growth rate was 236 µm/year (±90). The annual growth rate of the 7 LVC individuals did not differ (Kruskal–Wallis one-way analysis of variance on ranks, P = 0.918). On the contrary, a significant variability between the 10 FC specimens was observed (Kruskal–Wallis one-way analysis of variance on ranks, P = 0.007).
Magnesium to calcium (Mg/Ca) and strontium to calcium (Sr/Ca) ratios of the massive skeleton differed according to the collection site (Table 2). The Mg/Ca ratio (mmol/mol) showed a positive linear relationship with temperature (°C) expressed as regression equation y = 5.2x+25.3 (R2 = 0.72, P = 7*10−5). Seawater Mg/Ca and Sr/Ca ratios did not differ between locations (ANOVA, P = 0.977 and P = 0.746 respectively).
Table 2. Skeletal Mg/Ca and Sr/Ca ratios in the massive skeleton of Petrobiona massiliana according to sampling sites (mean±standard deviation, N=5).

DISCUSSION
Up to now, growth rate of massive basal skeletons of Calcispongiae was unknown. In the present study, in situ calcein labelling experiments showed that the average skeletal growth rate of the calcisponge Petrobiona massiliana was 236 µm/year. This value is close to average growth rates (ranging from 100 to 300 µm/year) reported for massive basal skeletons of tropical species of Demospongiae (Ceratoporella nicholsoni, Astrosclera willeyana and Acanthochaetetes wellsi), despite the differences between tropical and Mediterranean field conditions (Willenz & Hartman, Reference Willenz, Hartman, Harmelin Vivien and Salvat1985, Reference Willenz and Hartman1999; Reitner & Gautret, Reference Reitner and Gautret1996; Wörheide, Reference Wörheide1998). A maximum height of 20 mm has been reported for hemispherical shaped P. massiliana living in caves protected from hydrodynamism (Vacelet, Reference Vacelet1964). In regards of growth rates measured in this study, and considering that growth is constant in time, lifespan of this species can be estimated to be approximately 85 years, a relatively short lifetime in comparison with other hypercalcified sponges, which can live several centuries (Swart et al., Reference Swart, Moore, Charles and Böhm1998).
Our SEM and epifluorescence microscopy observations highlighted some processes involved in the skeletal growth of P. massiliana. As previously described by Vacelet (Reference Vacelet, Reitner and Keupp1991), some calcareous spicules were entrapped within the calcitic mass during growth. These entrapped spicules (or their imprint) were also observed across the entire thickness of fractured skeletons, confirming that spicules do not dissolve in the mass of the skeleton (Vacelet, Reference Vacelet, Reitner and Keupp1991). Intense calcein fluorescence was observed on the conical protuberances at the skeleton surface, indicating that they were actively growing at the time of incubation.
The discontinuous calcein lines suggest that growth was spatially discontinuous. However, growth rates were similar at the apex of skeletal crests and in skeletal depressions or flat surfaces. Discontinuous growth could result from an inhomogeneous spatial distribution of the basopinacocytes (i.e. the skeleton forming cells), or from differences in their activity. Ultrastructural studies are needed to answer these questions. Furthermore, nothing is known about the seasonal variations of the calcification rate. Physiological process could also affect energy allocated to skeletal growth. In C. nicholsoni a slower growth rate was observed in small individuals in comparison to older specimens (Willenz & Hartman, Reference Willenz and Hartman1999).
All specimens observed in SEM presented pit shaped excavations in their basal skeleton. The smooth and regular micro-patterns of these excavations are typical of boring sponges (Calcinai et al., Reference Calcinai, Arillo, Cerrano and Bavestrello2003). Several boring sponge species, including Aka labyrinthica, Alectona millari and Cliona sp., have been previously described in the massive skeleton of P. massiliana (Vacelet, Reference Vacelet1964).
Hypercalcified sponge skeletons have been proposed as reliable temperature recorders. Rosenheim et al. (Reference Rosenheim, Swart, Thorrold, Willenz, Berry and Latkoczy2004) calibrated a strong negative relationship between Sr/Ca and temperature in the aragonitic skeleton of C. nicholsoni. Conversely, Rosenheim et al. (Reference Rosenheim, Swart and Thorrold2005) showed a negative correlation between Mg/Ca ratio and temperature, and assumed that few vital effects influence elemental incorporation in the aragonitic skeleton of C. nicholsoni. This is in agreement with the statements of Böhm et al. (Reference Böhm, Joachimsky, Dullo, Eisenhauer, Lehnert, Reitner and Wörheide2000) and Rosenheim et al. (Reference Rosenheim, Swart and Willenz2009), who showed that C. nicholsoni precipitates aragonite close to oxygen isotopic equilibrium. Chemical analyses revealed an important temperature dependence of bulk skeletal Mg/Ca ratio in P. massiliana. The fitted regression on temperature accounts for 72% of the Mg/Ca ratio variations in the skeleton of this species (R2 = 0.72). Such relationships between temperature and skeletal Mg content are well known in biogenic calcites (Chave, Reference Chave1954) and have successfully been used to reconstruct past temperatures, in combination with other skeletal temperature proxies such as δ18O (i.e. in foraminiferal calcites; Kristjándóttir et al., Reference Kristjánsdóttir, Lea, Jennings, Pak and Belanger2007). On a geological timescale, Mg/Ca ratios of biogenic carbonates are also influenced by seawater Mg/Ca ratios (Ries, Reference Ries2004). However, considering that seawater Mg/Ca did not differ between locations, this ratio was not involved in the trend observed here. In this study, we observed a linear increase in Mg/Ca of 5% per °C in the skeleton of P. massiliana. This value is closer to that reported for inorganic calcite precipitation (3.1%; Oomori et al., Reference Oomori, Kaneshima and Maezato1987) than to that observed in foraminiferal calcite (10%; Lea et al., Reference Lea, Mashiotta and Spero1999). Our results, which agree with those reported for C. nicholsoni (Rosenheim et al., Reference Rosenheim, Swart and Thorrold2005), highlight the importance of the thermodynamic control of magnesium incorporation in hypercalcified sponge skeleton. Even if skeleton deposition occurs in an organized chronological way, morphology or growth modalities can restrict the use of the skeleton of hypercalcified sponges as temperature recorder. For the massive aragonitic skeleton of A. willeyana, the use of Sr/Ca ratio as a temperature recorder is limited by a thickening of the skeletal material at the ‘living’ edge of the skeleton (Fallon et al., Reference Fallon, McCulloch and Guilderson2005).
The present study allows evaluating the potential use of the massive skeleton of P. massiliana as a reliable temperature recorder. In contrast with most hypercalcified sponges of the class Demospongiae, several factors such as multidirectional growth axis, spatially discontinuous growth and a relatively short lifetime complicate the use of this species in high resolution temperature reconstructions.
ACKNOWLEDGEMENTS
The authors thank R. Barbieri, A. Ereskovsky, S. Fally, F. Ledda, R. Manconi, C. Marshall, P. Paneels, P. Van de Steen, the members of ‘Au Delà Plongée’ (La Vesse) and ‘The Waterhoppers’ (Rhodes) diving centres for their SCUBA diving assistance. Laboratory technical support was provided by Ph. Pernet, L. Despontin, N. Dakhani and L. Berry. We thank H. Zibrowius who provided information to access samples in Rhodes. This work was supported by a ‘Plan Action 2’ grant (contract No. WI/36/F02) and the CALMARS II project (No. NR SD/CS/02A) from the Belgian Federal Science Policy, Brussels, Belgium. Ph. Dubois is a senior Research Associate of the National Fund for Scientific Research (FRS–FNRS Belgium).







