Introduction
Over the past 40 years, assisted reproductive technology (ART) has evolved considerably from an ambitious and experimental procedure to mainstream medicine, and has resulted in the birth of more than 8 million children (Steptoe and Edwards, Reference Steptoe and Edwards1978; De Geyter et al., Reference De Geyter, Calhaz-Jorge, Kupka, Wyns, Mocanu, Motrenko, Scaravelli, Smeenk, Vidakovic and Goossens2018). The number of couples facing infertility issues has increased steadily, many of whom will ultimately need in vitro fertilization (IVF) treatments (Thoma et al., Reference Thoma, McLain, Louis, King, Trumble, Sundaram and Buck2013). Furthermore, in the last decades due to social and legal equality for same-sex couples, medically assisted reproduction (MAR) treatments are increasingly applied for these couples, as well as single women/men and transgender couples. Worldwide, approximately 2.5 million MAR cycles are performed annually, resulting in over 500,000 deliveries. Among the 39 countries in Europe offering ART treatments, in total in 2014, 56,516 egg-donation cycles were performed, with a sharp increment since 2013 (De Geyter et al., Reference De Geyter, Calhaz-Jorge, Kupka, Wyns, Mocanu, Motrenko, Scaravelli, Smeenk, Vidakovic and Goossens2018). The goal of reproduction treatment is a healthy live birth, but currently on average only one-third of all in vitro fertilization cycles results in pregnancy. Advances in embryo culture and cryopreservation over the past 15 years, have resulted in significant increases in embryo implantation rates (Rienzi et al., Reference Rienzi, Cimadomo, Maggiulli, Vaiarelli, Dusi, Buffo, Amendola, Colamaria, Giuliani, Bruno, Stoppa and Ubaldi2020). These advances allowed a reduction in the numbers of embryos being transferred, making the policy of elective single embryo transfer (eSET), a reality in many countries. Consequently, the number of multiple pregnancies and their related complications has decreased markedly. ART evolution has also facilitated the development of several strategies for oocyte cryopreservation. The first birth from a cryopreserved oocyte was obtained in Australia in 1986 (Chen, Reference Chen1986) using a slow-freezing protocol (van Uem et al., Reference van Uem, Siebzehnrübl, Schuh, Koch, Trotnow and Lang1987). However, this method did not yield optimal results for many years (Oktay et al., Reference Oktay, Cil and Bang2006). Moreover, there was a lack of progress in the field due to technical concerns and low success rates (Bernard and Fuller Reference Bernard and Fuller1996). Oocytes are challenging to cryopreserve, mainly due to their low surface area to volume ratio and high susceptibility to intracellular ice formation, which can induce irreversible damage to cells (Bianchi et al., Reference Bianchi, Macchiarelli, Borini, Lappi, Cecconi, Miglietta, Familiari and Nottola2014; Paynter et al., Reference Paynter, Cooper, Gregory, Fuller and Shaw1999). Early studies have highlighted the difficulties in predicting human oocyte membrane permeability characteristics, along with other biophysical components (Fuller et al., Reference Fuller, Hunter, Bernard, McGrath, Curtis and Jackson1992; Hunter et al., Reference Hunter, Bernard, Fuller, McGrath and Shaw1992). Several studies also reported the adverse effects of cryopreservation on microtubule stability and on the spindle in mammalian oocytes (Pickering and Johnson, Reference Pickering and Johnson1987; Pickering et al., Reference Pickering, Braude and Johnson1990). Furthermore, zona pellucida (ZP) hardening after cryopreservation has been reported as an extra complication resulting from the cryopreservation process (Vincent et al., Reference Vincent, Pickering and Johnson1990), therefore at warming the survived oocytes need to be mandatorily inseminated using intracytoplasmic sperm injection (ICSI) rather than standard IVF (Porcu et al., Reference Porcu, Fabbri, Seracchioli, Ciotti, Magrini and Flamigni1997). Research into oocyte cryopreservation has increased due to legal restrictions on human embryo storage, especially in Italy, where embryo cryopreservation was not permitted for a specific time period (Benagiano and Gianaroli, Reference Benagiano and Gianaroli2004). A significant breakthrough was reported with the introduction of ‘vitrification’ in Japan and Australia (Kuleshova et al., Reference Kuleshova, Gianaroli, Magli, Ferraretti and Trounson1999; Kuwayama et al., Reference Kuwayama, Vajta, Kato and Leibo2005). Vitrification has been proposed as an alternative to the slow-freezing technique for human oocytes and is expected to give superior cryo-survival and pregnancy outcomes. The ability to cryopreserve human oocytes and embryos using vitrification has improved significantly over the last 20 years (Rienzi et al., Reference Rienzi, Gracia, Maggiulli, LaBarbera, Kaser, Ubaldi, Vanderpoel and Racowsky2017; Sciorio et al., Reference Sciorio, Thong and Pickering2018a). There is currently sufficient evidence to show that vitrification results are superior to those achieved using slow-freezing protocols (Cobo et al., Reference Cobo, Kuwayama, Pérez, Ruiz, Pellicer and Remohí2008; Loutradi et al., Reference Loutradi, Kolibianakis, Venetis, Papanikolaou, Pados, Bontis and Tarlatzis2008; Li et al., Reference Li, Wang, Ledger, Edgar and Sullivan2014). In the early 2000s, several studies reported a live-birth rate of 40% for vitrified–warmed oocytes and delivery rates similar to those for pregnancies from fresh oocytes (Cobo et al., Reference Cobo, Kuwayama, Pérez, Ruiz, Pellicer and Remohí2008; Cobo and Diaz, Reference Cobo and Diaz2011). The Human Fertilization and Embryology Authority (HFEA) has allowed the use of frozen oocytes for infertility treatment in the UK since 2000 (Wise, Reference Wise2000). The American Society for Reproductive Medicine (ASRM) in 2013 removed the experimental label applied to oocyte freezing (Practice Committees of ASRM, 2013) following randomized controlled studies (Cobo et al., Reference Cobo, Meseguer, Remohí and Pellicer2010; Rienzi et al., Reference Rienzi, Romano, Albricci, Maggiulli, Capalbo, Baroni, Colamaria, Sapienza and Ubaldi2010) that reported that IVF using vitrified–warmed oocytes could produce similar pregnancy outcomes to IVF with fresh oocytes. A systematic review of five studies, analyzing 4282 vitrified oocytes, reported that vitrification resulted in a higher oocyte survival rate, a higher fertilization rate, and a higher rate of top-quality embryos compared with slow freezing (Cobo and Diaz, Reference Cobo and Diaz2011). Another study compared the clinical outcomes between fresh donor oocytes to vitrified donor oocytes and reported similar clinical pregnancy rates (Cobo et al., Reference Cobo, Serra, Garrido, Olmo, Pellicer and Remohí2014). Concerning safety, several studies have established that there was no difference in birth weight (Chian et al., Reference Chian, Huang, Tan, Lucena, Saa, Rojas, Ruvalcaba Castellón, García Amador and Montoya Sarmiento2008) and congenital malformations (Noyes et al., Reference Noyes, Porcu and Borini2009) in infants born following oocyte vitrification compared with those born from natural conception or through conventional ART treatments. However, despite the increasing evidence demonstrating no differences between fresh and vitrified oocytes in egg-donation programmes, only restricted data have been published relating to egg-donation cycles achieved after egg banking (Domingues et al., Reference Domingues, Aquino, Barros, Mazetto, Nicolielo, Kimati, Devecchi, Bonetti, Serafini and Motta2017). Therefore, in this retrospective study, our main focus was to illustrate the establishment of an oocyte donation programme based on importing donated vitrified gametes from abroad and delineating the clinical and embryological workflow to increase IVF efficacy and reduce the risk of multiple pregnancies during egg-donation cycles. We also report our centre’s data on survival rates, fertilization, positive pregnancy rate, clinical pregnancy, and live-birth rates (LBR) of vitrified donor oocytes.
Oocyte donation programme
In the last couple of decades, a critical decrease in women’s fertility has been reported, especially in women of advanced maternal age (>35 years) (van Noord-Zaadstra et al., Reference van Noord-Zaadstra, Looman, Alsbach, Habbema, te Velde and Karbaat1991; Bar-Hava et al., Reference Bar-Hava, Ferber, Ashkenazi, Orvieto, Kaplan, Bar, Peleg and Ben-Rafael1999; Perheentupa and Huhtaniemi, Reference Perheentupa and Huhtaniemi2009). Several conditions affect fertility potential, including premature ovarian failure, reduction in the ovarian follicular reserve, and a higher number of chromosomal abnormalities in the oocyte, which lead to a reduction in pregnancy rates (Munné et al., Reference Munné, Sandalinas, Escudero, Márquez and Cohen2002) and therefore women opting for oocyte donation (Sauer and Kavic, Reference Sauer and Kavic2006). This approach is now well established for age-related female infertility, where the oocyte quality is compromised. Therefore, embryo quality and viability might be optimized by donated oocytes from young women, resulting in high pregnancy rates and optimal obstetric outcomes observed in recipients (Budak et al., Reference Budak, Garrido, Soares, Melo, Meseguer, Pellicer and Remohí2007; Stoop et al., Reference Stoop, Baumgarten, Haentjens, Polyzos, De Vos, Verheyen, Camus and Devroey2012; Yadav et al., Reference Yadav, Bakolia, Malhotra, Mahey, Singh and Kriplani2018). The first practice of oocyte donation was described in Australia by Trounson et al. (Reference Trounson, Leeton, Besanko, Wood and Conti1983). Since then, the application of oocyte donation has become more common and is now considered a valid procedure by which to manage untreatable female infertility, repeated implantation failure, and recurrent miscarriages. Furthermore, oocyte donation has also been used in women when there is a high risk of transmitting a genetic disorder to the offspring, but when the preimplantation genetic screening option cannot be applied (Barri et al., Reference Barri, Coroleu, Martinez, Parera, Veiga, Calderon, Boada and Belil1992; Melnick and Rosenwaks, Reference Melnick and Rosenwaks2018). In Italy, gamete donation has historically been illegal. However, in 2014, the Constitutional Court (n.162/2014) modified the legislative scenario (Law 40/2004) (La Marca et al., Reference La Marca, Dal Canto, Buccheri, Valerio, Mignini Renzini, Rodriguez and Vassena2019), allowing gamete donation in MAR treatments for heterosexual couples, married or partners, and those who cannot rely on their own gametes. Since this change, in Italy more than 16,000 donor oocyte cycles have been performed (www.iss.it/pma; data from 2014 to 2017) (La Marca et al., Reference La Marca, Capuzzo, Bartolucci, Schirinzi, Dal Canto, Buratini, Mignini Renzini, Rodriguez and Vassena2020). Oocyte donation requires collecting oocytes from a donor, insemination with sperm from the recipient’s partner, fertilization, in vitro culture, and embryo transfer to the recipient’s uterine cavity. In Italy, it is challenging to carry out the donation of fresh oocytes due to the lack of donors. Therefore, the high accuracy of cryopreservation through the vitrification procedure has allowed the establishment of donor egg banks and the use of vitrified–warmed donor oocytes. This approach has overcome the limitations associated with the donor–recipient programme, including the need to synchronize the donor and the recipient, or potential cycle cancellation due to a poor response to ovarian stimulation. The oocytes need to be vitrified after retrieval and carefully transported to another IVF unit, provided that strict measures are applied to maintain oocyte viability and competence during shipping (Alikani and Parmegiani, Reference Alikani and Parmegiani2018). Over the last few years, Italian ART centres have established several collaborations with oocyte banks located abroad to manage the demand for oocyte donation cycles. Two strategies have been mainly adopted, the first involves the shipment of frozen sperm to the oocyte donor clinic, where the sperm will be thawed and used to inseminate fresh donor oocytes; the resulting embryos are then frozen and transported back to the referring IVF centre. Another strategy, applied in the current study, comprises the importation of donated vitrified oocytes, which are then warmed, and fertilized using ICSI and fresh sperm from the male recipient’s partner, followed by embryo transfer and the cryopreservation of viable supernumerary embryos (Figure 1). The Italian IVF registry, has recently reported that the number of couples who obtained IVF treatments involving donated gametes increased from 133 in 2014 to 2838 in 2017. In 2015, 1137 cycles were performed using vitrified donor oocytes with a biochemical pregnancy rate of 30.8% (www.iss.it/pma).

Figure 1. A schematic representation of the imported oocyte donation programme from a foreign country. eSET, elective single embryo transfer; ICSI, intracytoplasmic sperm injection; MII, metaphase II oocyte; OS, ovarian stimulation.
Materials and methods
This is a retrospective cohort study performed at the Donna Salus Women’s Health and Fertility Unit between September 2017 and December 2019. All consecutive oocyte donation cycles were included in the analysis. The oocytes were previously vitrified at an egg-donor bank (Ovobank, Marbella, Spain) from Caucasian women and shipped to our centre. After warming, the survived oocytes were injected using ICSI and fresh sperm obtained from the male partner. Following insemination, fertilization and embryo culture, single or double fresh embryo transfer was performed at the blastocyst stage on day 5. Alternatively, all blastocysts were vitrified and transferred in a subsequent frozen embryo transfer (FET) cycle. All patients, enrolled in the egg-donation programme, were evaluated for their general health status, including gynaecological examination, hormonal assessment, and infectious disease tests. The male partner was also subjected to a complete andrological evaluation, including semen analysis, infectious disease triage, and hormonal and genetic testing as appropriate. Psychological counselling was offered to all couples entering the programme.
Oocyte donor: vitrification and transport
Before starting the stimulation programme, all donors were screened for infectious and genetic diseases as required by law. Donors must also fulfil Italian and European regulation criteria and match the infertile couple seeking oocyte donation. All oocyte donors (age 20–35 years) had normal ovaries at a transvaginal ultrasound, adequate ovarian reserve as evidenced by an antral follicular measurement, and displayed an adequate response to ovarian stimulation. Ovulation was triggered when three or more follicles ≥18 mm diameter were present on both ovaries. Oocyte pick up (OPU) was performed 36 h after triggering with chorionic gonadotropin (hCG) administration, under sedation and transvaginal ultrasonography guidance. At 1 or 2 h after OPU, oocytes were denudated, and those at the metaphase II (MII) stage were cryopreserved using the vitrification method. The vitrification protocol adopted was the protocol originally proposed by Kuwayama et al. (Reference Kuwayama, Vajta, Kato and Leibo2005), using a combination of 15% dimethyl sulfoxide (DMSO), 15% ethylene glycol, and 0.5 M sucrose as the cryoprotectant, and the Cryotop device for oocyte storage (Kitazato, Japan). Two or three oocytes were loaded onto each cryo-device. The oocytes were then stored in liquid nitrogen for a variable period. An IVF courier using a vapour-phase nitrogen shipper as carry-on baggage transported the gametes from Spain to Italy. The shipper was equipped with an electronic detector to ensure that temperature was continuously monitored over the entire duration of the trip.
Oocyte warming, insemination and embryo culture
Donor oocyte warming was performed according to the Kitazato protocol, as previously described (Kuwayama et al., Reference Kuwayama, Vajta, Kato and Leibo2005; Cobo et al., Reference Cobo, Serra, Garrido, Olmo, Pellicer and Remohí2014, Reference Cobo, García-Velasco, Domingo, Pellicer and Remohí2018). Briefly, at warming, each Cryotop was quickly plunged into 1 ml of 37°C prewarmed thawing solution (TS) containing 1.0 M sucrose for 1 min to remove the oocytes from the cryo-device. Subsequently, the oocytes were transferred to a dilution solution (DS) containing 0.5 M sucrose at room temperature for 3 min. Afterwards, two consecutive steps were performed in a washing solution (WS), for 5 min each. Lastly, the oocytes were transferred into equilibrated continuous single-step medium (CSCC, Fujifilm, Irvine Scientific, USA) at 37°C and 6% CO2, 5% O2, and nitrogen balance in a K-System incubator (K-System G210, CooperSurgical, USA) for about 1.5–2 h. Subsequently, ICSI insemination was performed with sperm obtained from the male partner. A single spermatozoon with normal morphology and progressive motility was selected under an inverted microscope (Olympus IX73, Olympus Corporation) and micro-injected with the use of electrohydraulic injectors (TransferMan®, Eppendorf AG, Hamburg, Germany). Sperm used for the ICSI procedure was collected by masturbation and processed using a standard method described by Bourne and colleagues (Reference Bourne, Edgar, Baker, Gardner, Weissman, Howles and Shoham2004). Fertilization was identified by the presence of two pronuclei at approximately 16–18 h after ICSI. At this stage, normally fertilized oocytes were cultured individually in 20 μl drops (CSCM, Irvine Scientific) up to the blastocyst stage (days 5 and 6) in a controlled atmosphere in a K-System incubator (K-System G210, CooperSurgical, USA). Morphological embryo assessment was performed according to the number of blastomeres, symmetry, percentage of fragmentation, as previously described by Sciorio et al. (Reference Sciorio, Thong and Pickering2018b). Blastocyst were classified using Gardner’s score according to blastocyst expansion, the morphology of the inner cell mass (ICM), and trophectoderm (TE). Single or double embryo transfer was carried out at the blastocyst stage on day 5 after insemination, as previously described (Sciorio et al., Reference Sciorio, Herrer Saura, Thong, Esbert Algam, Pickering and Meseguer2020). To obtain an optimal endometrium preparation, in total, 69 women had all blastocysts vitrified with subsequent embryo replacement after the warming procedure. Embryo replacement was performed under transabdominal ultrasound guidance using a soft transfer catheter (Wallace® Classic, CooperSurgical, USA). Endometrium preparation involved oestrogen (Progynova 2 mg, three times a day; Bayer Schering Pharma AG, Germany) and subcutaneous progesterone (Pleyris, 25 mg twice a day IBSA Farmaceutici Srl, Italy), and continued until the 12th gestation week. Biochemical pregnancy was defined as serum beta-hCG levels ≥5 IU/l, which was required to show an increase by 2 or 3 days later. Clinical pregnancy was defined as the presence of a gestational sac with a fetal heartbeat. A clinical pregnancy that resulted in at least one live birth was defined as a ‘live birth delivery’. Positive pregnancy tests, and the live-birth delivery rates were calculated using the number of transfers performed and the number of patient treated.
Results
In total, 100 patients (mean maternal age: 41 years) underwent an IVF cycle with imported donated vitrified oocytes. The main patient characteristics are reported in Table 1. The study included patients treated over 2 years (2017–2019). Of the 100 patients who underwent IVF using donor oocytes, 96 had at least one viable blastocyst to transfer. In total, 96 patients had 148 embryo transfers performed. Forty-four live births were obtained, 42 of which were singletons. Table 2 summarizes the embryological data, including all embryo transfers (fresh and warmed). In total, 681 oocytes were warmed with a survival rate of 79.1% (n = 539/681). The survived oocytes were injected by ICSI, resulting in a fertilization rate of 90.2% (n = 486/539). Blastocyst formation was 47.9% (n = 233/486). Overall, the ongoing clinical pregnancy rate per patient was 47.9% (n = 46/96), and 31.1% per transfer (n = 46/148). Live-birth and multiple pregnancy rates per transfer were respectively 28.4% (n = 42/148) and 0.7% (n = 1/148). Table 3 reports the characteristics of the patients who received fresh embryo transfer, whereas Table 4 summarizes data of patients who had all embryos frozen and subsequently transferred.
Table 1. Main couple and cycles features (oocyte donor–vitrification programme, Donna Salus, 2017–2019)
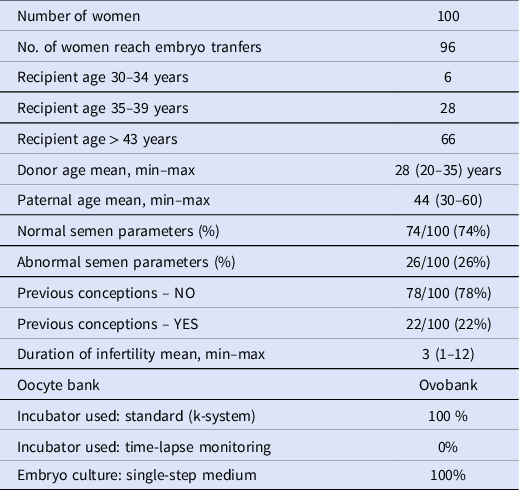
Table 2. Embryological and cycle data (oocyte donor–vitrification programme, Donna Salus, 2017–2019)

Table 3. Embryological and cycle data of patients who received fresh embryo transfer at blastocyst stage (oocyte donor–vitrification programme, Donna Salus, 2017–2019)

Table 4. Embryological and cycle data of patients who receive embryo transfer at blastocyst stage after vitrification and warming (oocyte donor–vitrification programme, Donna Salus, 2017–2019)

Discussion
This study reports the donor oocyte survival rates and pregnancy outcomes of an oocyte donation programme based on the shipment of vitrified gametes between countries. Of 100 women assigned to our egg-donor programme, 96 patients reached at least one embryo transfer event and, in total, 44 live births were obtained, mostly singletons. This system avoids the need to synchronize donor oocyte retrieval with embryo transfer to the recipients. Although egg-donor programmes are prohibited in many places, including Muslim countries and Germany (Audibert and Glass, Reference Audibert and Glass2015), it became legal in Italy in 2014. Oocyte cryopreservation has recently become a popular method with broad indications, including social freezing, fertility preservation in cancer patients and, in cases of severe diseases that may jeopardize future fertility (Cobo et al., Reference Cobo, García-Velasco, Domingo, Pellicer and Remohí2018; Sciorio and Anderson, Reference Sciorio and Anderson2020). However, donor recruitment in Italy is problematic, mainly due to the limited number of potential donors. Therefore, several reproductive units have imported vitrified oocytes from foreign countries. Over the few last years, oocyte cryopreservation methods have changed from slow freezing to vitrification. At this time, vitrification is the method of choice due to its safety and efficacy. In the last report of the Italian IVF registry, pregnancy data using vitrified donor oocytes for the year 2015 indicate a biochemical pregnancy rate of 30.8%, although the live-birth data are not provided (www.iss.it/pma). The efficacy of human oocyte vitrification made it possible to create oocyte banks that provide these gametes to clinics in which donor recruitment is problematic or not desired. Our study shows that the implementation of an egg-donation programme using imported vitrified oocytes is feasible. However, we had a learning curve on how to handle the imported oocytes. We found that the most important prerequisite for a successful banking programme is to have in place optimized and efficient freezing and warming procedures. During the vitrification process, a critical and challenging factor is to maintain the plasma and membrane integrity by preventing ice crystal formation, which damages the oocyte. Various permeating and non-permeating cryoprotectants have been used to prevent ice crystal formation. Because these compounds are toxic at high concentrations, a rigorous and well executed procedure is required to achieve successful survival rates, embryo development, and implantation. (Cousineau and Domar, Reference Cousineau and Domar2007; Cobo et al., Reference Cobo, García-Velasco, Domingo, Pellicer and Remohí2018; Colaco and Sakkas Reference Colaco and Sakkas2018). We stress the importance of the correct oocyte number that must be assigned to every couple to maximize outcomes. Our data indicated that a range between 6 to 8 warmed oocytes is associated with an increased probability of having at least one viable blastocyst for transfer in each couple. This finding is in agreement with a study published by Cobo and colleagues, who analyzed over 6000 vitrified–warmed cycles. The authors reported a cumulative live-birth rate of 15.8% with five warmed oocytes and 32.0% with eight warmed oocytes. For younger patients (<35 years old), 10 and 15 warmed oocytes provided success rates of 42.8% and 69.8%, respectively. The highest cumulative live birth was achieved in younger women when the number of oocytes vitrified was 24 (Cobo and Diaz, Reference Cobo and Diaz2011). An elective single embryo transfer (eSET) policy is also important to reduce the incidence of multiple pregnancies, which increases the risk of adverse outcomes for both mothers and babies (Korb et al., Reference Korb, Schmitz, Seco, Goffinet and Deneux-Tharaux2020). As much as possible, we applied eSET to our patient population. We found a trend for a better clinical outcome with the fresh transfer of a single blastocyst after oocyte warming, fertilization, and embryo culture, compared with culture and freezing of all the embryos at the blastocyst stage and replacement in a subsequent FET cycle. The live-birth rate was 34.1% in the fresh group and 26.2% in the FET group, but our numbers were too small to make firm conclusions (Tables 3 and 4). Embryo vitrification generated from vitrified oocytes has been, overall, successful (Farhat et al., Reference Farhat, Zentner, Lossos, Bdolah, Holtzer and Hurwitz2001; Smith et al., Reference Smith, Roots and Dorsett2005; Kumasako et al., Reference Kumasako, Otsu, Utsunomiya and Araki2009; Murakami et al., Reference Murakami, Egashira, Murakami, Araki and Kuramoto2011), but the experience is still very limited (Murakami et al., Reference Murakami, Egashira, Murakami, Araki and Kuramoto2011). As stated earlier, the survived oocytes relied on the mandatory use of the ICSI, rather than standard IVF insemination (Porcu et al., Reference Porcu, Fabbri, Seracchioli, Ciotti, Magrini and Flamigni1997). This choice was mainly due to ZP hardening after the vitrification–warmed procedures, which might be associated with increased risk of failed fertilization using the standard IVF insemination (Vincent et al., Reference Vincent, Pickering and Johnson1990). An alternative oocyte donation programme was based on the shipment of frozen sperm from the partner to the egg bank. In this scenario, fresh donor oocytes are used and the resulting embryos vitrified and shipped to the referring IVF centre. This method has been recently described by La Marca et al. (Reference La Marca, Dal Canto, Buccheri, Valerio, Mignini Renzini, Rodriguez and Vassena2019). The authors analyzed, in total, 2617 embryos from 630 patients and reported a survival rate after warming of 98.5% and a live-birth rate of 30.6%, which was similar to our results of 28.4% LBR. In another study, similar to ours, Rienzi and colleagues reported equivalent results with oocytes purchased from three different Spanish cryo-banks (Rienzi et al., Reference Rienzi, Cimadomo, Maggiulli, Vaiarelli, Dusi, Buffo, Amendola, Colamaria, Giuliani, Bruno, Stoppa and Ubaldi2020). In their longitudinal cohort study, including 273 couples, the survival rate after warming was 86%, and the live-birth rate was 35%. For sperm quality, our study included a broad range of phenotypes, including normozoospermia, moderate male factor, and severe oligoasthenoteratozoospermia. Despite the overall successful outcomes, our sample size was relatively small. As the paternal genome plays a crucial role in the fertilization and embryo development processes, future studies must determine the ideal number of oocytes needed to maximize the chances of achieving a healthy live birth when defective sperm are used (Verza and Esteves, Reference Verza and Esteves2008). Many couples travel abroad to undergo IVF treatments with donated gametes due to the lack of oocytes or prohibitive use of donor oocytes in their countries (Shenfield et al., Reference Shenfield, de Mouzon, Pennings, Ferraretti, Andersen, de Wert and Goossens2010). Travelling to foreign countries implies an increased financial burden associated with travel, housing, and work absenteeism. In addition, infertility and MAR treatments play an important role in patient psychosocial wellbeing; the need to travel to foreign countries to be treated increases the emotional burden to the already stressful IVF cycle (Pasch et al., Reference Pasch, Holley, Bleil, Shehab, Katz and Adler2016). Therefore, it might be advantageous to IVF centres located in countries with limited availability of donors to implement an egg-donation programme that relies on imported vitrified oocytes. For this, excellent process management between the units is paramount. Moreover, the shipment should be synchronized and performed by a third-party company familiar with the process to avoid risks associated with loss or damage of the gametes.
In conclusion, the importation of donated vitrified oocytes from a foreign country is a viable and safe approach to counteract the lack of egg donors. Our data indicate that adequate pregnancy can be obtained with this approach, with advantages for patients and clinics alike.
Conflict of interest
The authors declare to have no conflict of interest.







