Introduction
Serotonin (5-HT) is a known neurotransmitter that is present and synthesized in the central nervous system, but has also been found in many peripheral tissues, e.g. the gastrointestinal tract (Bertrand et al., Reference Bertrand, Bertrand, Camello and Pozo2010), bone marrow cells (Weitzman et al., Reference Weitzman, Galli, Dvorak and Hammel1985; Ziegler et al., Reference Ziegler, Hultner, Egger, Kempkes, Mailhammer, Gillis and Rodl1993), blood platelets (Jonnakuty & Gragnoli, Reference Jonnakuty and Gragnoli2008) and human follicular fluid (Bodis et al., Reference Bodis, Bognar, Hartmann, Torok and Csaba1992).
The presence of serotonin has also been reported in avian embryonic tissues and oocytes, in the egg yolk and in early chick embryos (Emanuelsson et al., Reference Emanuelsson, Carlberg and Lowkvist1988; Moudgal et al., Reference Moudgal, Panda and Mohan1992), in oocytes, cumulus cells and early embryos of mice (Il'kova et al., Reference Il'kova, Rehak, Vesela, Cikos, Fabian, Czikkova and Koppel2004; Amireault & Dube, Reference Amireault and Dube2005b; Basu et al., Reference Basu, Desai, Balaji, Chaerkady, Sriram, Maiti and Panicker2008). Localized synthesis of 5-HT has been reported during chick morphogenesis (Wallace, Reference Wallace1982) and in sea urchin embryos (Buznikov et al., Reference Buznikov, Lambert and Lauder2001).
At present, there are 14 known and cloned serotonin receptors for mammals (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT5B, 5 -HT6, 5-HT7A), nine of which (5-HT1A, 5-HT1F, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT6 and 5-HT7A) are also known in birds (chick, turkey).
Thirteen receptors belong to the large family of transmembrane G protein-coupled receptors classified into seven families; the only receptor that does not belong to this family is 5-HT3, a ligand-gated K+/Na+ ion channel protein (Barnes & Sharp, Reference Barnes and Sharp1999; Bijak, Reference Bijak, Nowak and Zawilska2004).
In addition to brain and some somatic tissues, 5-HT receptors have been observed in mammalian oocytes and early embryos; the 5-HT1D receptor transcript (but not the 5-HT1B and 5-HT2C receptor transcripts) has been found in mouse oocytes and preimplantation embryos (Vesela et al., Reference Vesela, Rehak, Mihalik, Czikkova, Pokorny and Koppel2003; Il'kova et al., Reference Il'kova, Rehak, Vesela, Cikos, Fabian, Czikkova and Koppel2004). Wu et al. (Reference Wu, Dias, Kumar, Lauder and Singh1999) reported variable levels of 5-HT2A, 5-HT2B and 5-HT2C receptor transcripts in rat embryos from 9–21 days of development. The transient peak of 5-HT2B receptor mRNA expression has been reported for the mouse embryos at 8.5 day post coitus, whereas no expression of 5-HT2C and only weak expression of 5-HT2A were observed at that time (Choi et al., Reference Choi, Ward, Messaddeq, Launay and Maroteau1997; Nebigil et al., Reference Nebigil, Etienne, Schaerlinger, Hickel, Launay and Maroteaux2001). The same authors pointed also to dose-dependent abnormalities that arose during cardiac and neural development in the presence of ritanserin, the high-affinity antagonist of the 5-HT2B receptor, that indicated the participation of the receptor in the tissue differentiation. In sea urchin embryos, structures sensitive to the 5-HT3 receptor ligand (quipazine) localized in the inter-blastomeric cleft of Paracentrotus lividus (Shmukler et al., Reference Shmukler, Silvestre and Tosti2008). Over 20 years ago, reports described the presence of unidentified 5-HT receptors on hamster and rabbit spermatozoa (Meizel & Turner, Reference Meizel and Turner1983; Young & Laing, Reference Young and Laing1990), and, more recently, Jimenez-Trejo et al. (Reference Jimenez-Trejo, Tapia-Rodriguez, Queiroz, Padilla, Avellar, Rovas Manzano, Manjarrez-Gutierrez and Gutierrez-Ospina2007) have confirmed the immunoreactivity of 5-HT2A and 5-HT3 receptors in the flagella of the rat spermatozoa.
At present, it is generally accepted that serotonin plays a regulatory role in vertebrate and echinodermata development (Lauder et al., Reference Lauder, Wallace and Krebs1981, Reference Lauder, Wilkie, Wu and Singh2000; Lauder, Reference Lauder1993; Azmitia, Reference Azmitia2001; Buznikov et al., Reference Buznikov, Lambert and Lauder2001; Gaspar et al., Reference Gaspar, Cases and Maroteaux2003) and in mammalian follicles and oocytes (Amireault & Dube, Reference Amireault and Dube2005a, Reference Amireault and Dube2005b; Dube & Amireault, Reference Dube and Amireault2007; Basu et al., Reference Basu, Desai, Balaji, Chaerkady, Sriram, Maiti and Panicker2008). These authors stressed the role of serotonin in regulation of cAMP and Ca2+ levels by means of its receptors: 5-HT1 receptors that inhibited and 5-HT4, 5-HT6 and 5-HT7 receptors that stimulated cAMP formation, and 5-HT2 receptors that elevated Ca2+ levels.
Serotonin is a molecule that is related to melatonin, as it is the substrate for melatonin synthesis by two consecutive reactions: acetylation by arylalkylamine N-acetyltransferase (AA-NAT) and hydroxylation by hydroxyindole-O-methyl-transferase (HIOMT); both substances are present in the avian yolk. In contrast with serotonin, the activation of melatonin receptors usually results in the downregulation of cAMP (Slanar et al., Reference Slanar, Pelisek and Vanecek2000; Sampaio, Reference Sampaio2008; Dauchy et al., Reference Dauchy, Blask, Dauchy, Davidson, Tirell, Greene, Tirell, Hill and Sauer2009; Hardeland, Reference Hardeland2009). Both cAMP and Ca2+ play a regulatory role in oocyte activation and maturation, so both molecules influence the process of oocyte development. In fish oocytes, serotonin inhibited maturation-inducing hormone (MIH)- and melatonin-induced maturation. Germinal vesicle breakdown (GVBD) of carp oocytes (Chattoraj et al., Reference Chattoraj, Seth and Maitra2008), and the stimulatory effect of melatonin and inhibitory effect of serotonin were repressed in the presence of luzindole and metoclopramide, their respective specific blockers, in the incubation medium.
In our previous papers we have shown the presence of melatonin receptor transcripts in primordial germ cells (PGC), oocytes, sperm, and early embryos of the Japanese quail (Oblap & Olszańska, Reference Obłap and Olszańska2001; Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008). We have also shown the presence of melatonin and AA-NAT and HIOMT activities (the enzymes engaged in melatonin synthesis from serotonin) in the yolk of Japanese quail eggs (Olszańska et al., Reference Olszańska, Majewski, Lewczuk and Stępińska2007).
The possibility exists that interactions of serotonin and melatonin via their respective receptors may occur and act as a regulatory mechanism before the appearance of the hormonal and nervous systems. Agomelatine, a novel antidepressant derivative of melatonin that acts as a melatonin agonist for the melatonin receptors MT1 and MT2 and as a serotonin antagonist against the 5-HT2C receptor, is a good example of such a mechanism (Arendt & Rajaratnam, Reference Arendt and Rajaratnam2008; San & Arranz, Reference San and Arranz2008; Millan et al., Reference Millan, Marin, Kamal, Jockers, Chanrion, Labasque, Bockaer and Mannoury la Cour2011), however its action has not yet been studied in developing organisms.
The finding of the receptors for the neurohormones (melatonin and serotonin) at early developmental stages suggests that both substances play a role during these stages, and that this role might be different from their roles in the adult organism. Thus, because no data on the presence of serotonin receptors and their action have been published for the gametes or early embryos of Aves, the present study investigated, for the first time, the serotonin receptor transcripts in the germ cells and early embryos of Japanese quail (Coturnix japonica), i.e. the biological material already tested for the presence of melatonin receptor transcripts.
Materials and methods
As this work is a continuation of previous studies (Obłap & Olszańska, Reference Obłap and Olszańska2001; Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008), we have tried to use the same materials and methods as in previous experiments as well as a similar experimental scheme.
Materials
Japanese quail (Coturnix japonica) were randomly bred at the Institute farm under a 14-h light/10-h dark regimen, with unlimited access to food and water. The freshly laid eggs were collected for incubation and embryo isolation. The uterine embryos were obtained from the quail females by delicate squeezing and massage of abdomen, without sacrificing the bird (Stępińska & Olszańska, Reference Stępińska and Olszańska1983). Oocytes were staged under a stereomicroscope in accordance with Eyal-Giladi & Kochav tables (Reference Eyal-Giladi and Kochav1976) and are designated with roman numerals. The embryos from laid eggs were staged in accordance with Hamburger & Hamilton (Reference Hamburger and Hamilton1951) stages and are designated with arabic numerals.
The female quails for oocyte collection were kept in individual cages; for a week preceding the experiments their laying times were noted in order to sacrifice the bird at the proper time before or after ovulation. The oocytes at different stages of oogenesis (Fig. 1) were cut out of the ovaries and their blastodiscs were segregated under a stereomicroscope. The first in hierarchy, non-ovulated oocytes, with a blastodisc that contained a clearly delineated nucleus (in the form of dark vesicle in the middle, Fig. 1 a,b) were designated as immature oocytes (F1 oocytes) before the breakdown of the nuclear envelope (GVBD); the oocytes without a visible nucleus (Fig. 1 c,d) were designated as having entered the maturation process, after GVBD. Ovulated oocytes were oocytes cut out of the ovary after GVBD and ovulated in vitro as described earlier (Olszańska et al., Reference Olszańska, Malewska and Stępińska1996). For RNA isolation the germinal discs/blastoderms were cut out of the oocytes or eggs and used for analysis; the PGC and sperm samples were frozen at –80°C until use.
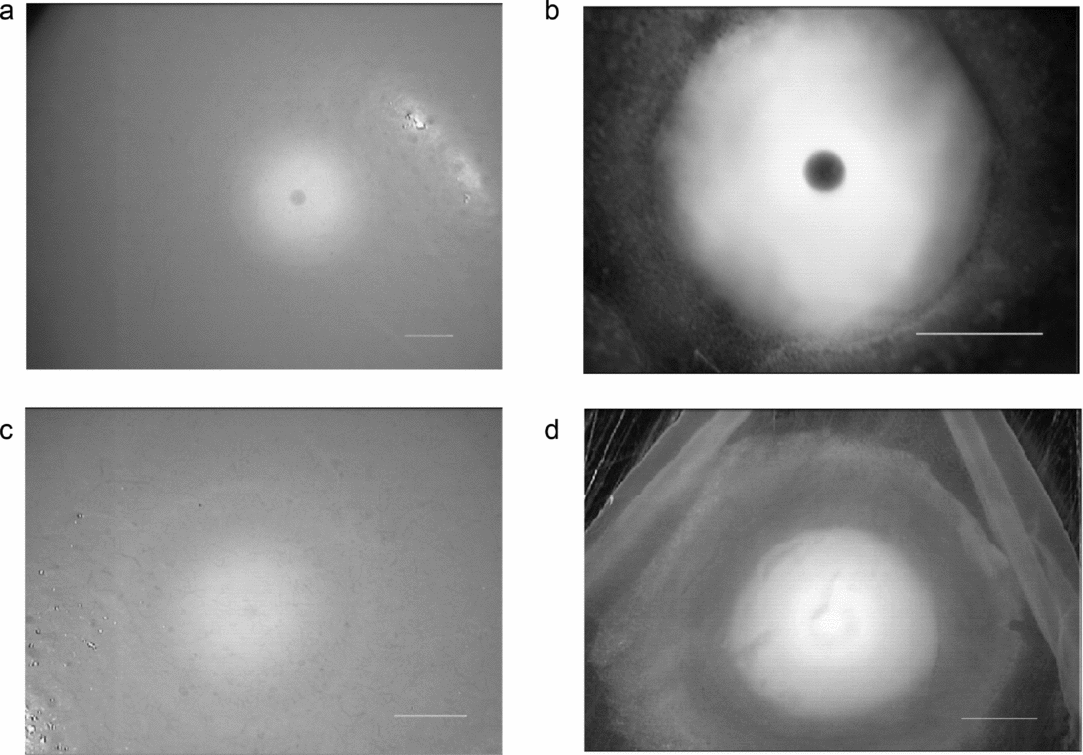
Figure 1 Germinal disc before maturation (a, b) and after maturation (c, d) of the oocyte, under a stereomicroscope, observed directly at the ovarian follicle surface (a, c) and at the excised germinal disc cleared of the yolk (b, d). Note the presence/absence of the oocyte nucleus. Scale bars represent 1 mm.
The experimental material consisted of:
-
• manually isolated PGCs from the blood of 14–16 Hamburger-Hamilton (HH)-stage Japanese quail embryo, as described earlier (Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008)
-
• oocytes of 3 mm in diameter – at the beginning of vitellogenesis
-
• germinal discs of F1 oocytes (immature, before GVBD; see Fig. 1a,b)
-
• germinal discs of ovulated oocytes (mature, after GVBD; see Fig. 1 c,d)
-
• germinal discs of uterine embryos at Eyal-Giladi (E-G) stage II (see Fig. 2 a)
-
• germinal discs of uterine embryos at E-G stage IV (see Fig. 2 b)
-
• blastoderms of uterine embryos at E-G stage VII (area pellucida formation)
-
• blastoderms from the freshly laid eggs; HH embryo stage 1
-
• blastoderms from the eggs incubated ~18 h at 38°C, embryos at HH stage 4 (gastrulation)
-
• ejaculated sperm purified by gradient centrifugation as described in Kawashima et al. (Reference Kawashima, Stępińska, Kuwana and Olszańska2008) and examined for the absence of somatic cells under a microscope.
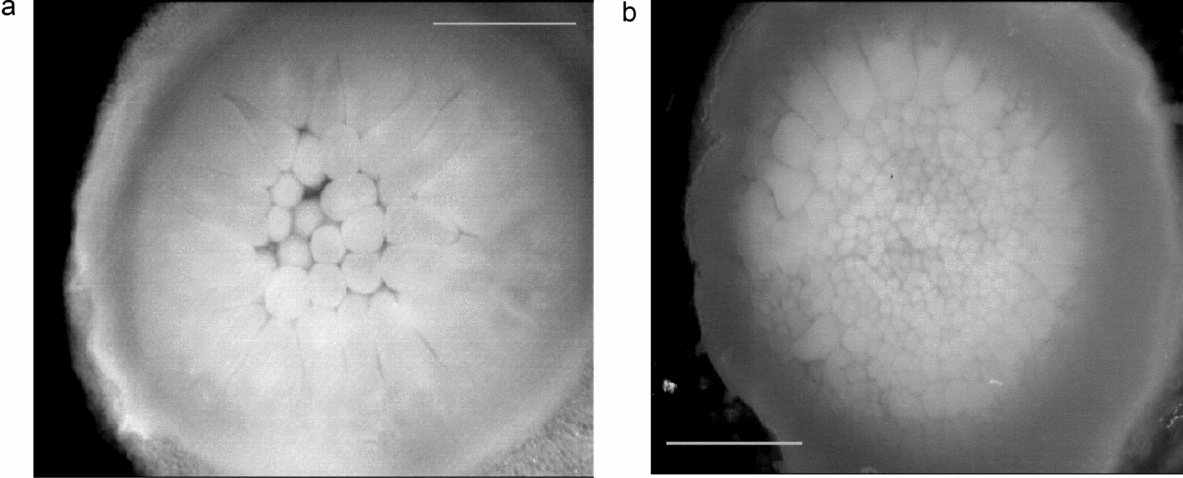
Figure 2 Uterine Japanese quail embryo (cleavage). (a) Stage II Eyal–Giladi tables (Reference Eyal-Giladi and Kochav1976). (b) Stage IV E-G tables. The scale bars represent 1 mm.
RNA isolation
Total RNA was extracted from one germinal disc or blastoderm, 2000 PGCs or 5 × 106 sperm cells per sample, using the InViSorb RNA kit II (InVitec GmbH, Berlin, Germany) according to the manufacturer's protocol. Briefly, the material was treated with a lysing solution to disrupt the cells, then DNA was removed with a DNA sorbent. Following phenol/chloroform and chloroform extraction of the aqueous phase, the RNA was precipitated with 1 volume of isopropanol and the pellet was washed twice with cold 70% ethanol.
RT-PCR
Total RNA isolated from an individual oocyte/embryo and brain as control tissue (~0.5 μg), from 2000 PGCs and ~5 × 106 sperm was used for RT-PCR analysis, as used in previous determinations of mel receptor transcripts (Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008). RT reactions were performed for 1 h at 42°C in a 40 μl reaction mixtures that contained 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3.0 mM MgCl2, 10 mM dithiothreitol (DTT), 0.3 mM deoxynucleotide triphosphate (dNTP) mix, 40 U of RNasin, 0.5 μg of oligo(dT)15 primer, and 200 U of M-MLV reverse transcriptase. All the reagents for the RT reactions were from Promega (Madison, WT, USA). PCR reactions were performed in a 25-μl volume that contained 2.5 μl of the mix from the RT reaction, 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.1 mM MgCl2, 0.01% gelatin, 0.2 mM dNTP mix, 10 pmol of each of the primers (0.4 μM), and 1.5 U of REDTaq® DNA polymerase (Sigma, St Louis, MO, USA). Each PCR reaction started with a 2 min denaturation step at 95°C, followed by 40 cycles of 30 s denaturation at 95°C, 30 s at the annealing temperature indicated in Table 1, and a 45 s extension at 72°C. A final extension of 5 min at 72°C concluded the PCR. The amplification conditions for each pair of primers were optimized in preliminary experiments and are listed in Table 1. RT and PCR reactions were performed using Peltier Thermal Cycler Tetrad 2 (BioRad Laboratories, Hercules, CA, USA). The RT-PCR products were separated by 2% agarose gel electrophoresis in Tris–borate–ethylenediaminetetraacetic acid (TBE) buffer, stained with ethidium bromide, and scanned with a Molecular Imager FX (BioRad Laboratories, Hercules, CA, USA). DNA size marker was plasmid UC (pUC) 19/HaeIII–Taq I (11 to 1444 bp), from InGen (Sieradz, Poland). The negative control consisted of 2.5 μl of H2O instead of the cDNA template. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sequence was used as a control for the RNA presence and integrity, as well as a control of purity of the individual RNA isolates: the appearance of the second band (656 bp) would be a signal of genomic DNA contamination and such preparations were discarded.
Table 1 Serotonin (5-HT) receptor primers used in RT-PCR analysis
*Primers reported in Westbroek et al. (Reference Westbroek, Van der Plas, Rooij, Klein-Nulend and Nijweide2001).
Primers
The primer sequences for GAPDH and for 5-HT2B were taken from Westbroek et al. (Reference Westbroek, Van der Plas, Rooij, Klein-Nulend and Nijweide2001). The primer sequences for the remaining 5-HT receptors were designed using the Primer 3′ program (http://primer3.wi.mit.edu/), based on the predicted DNA sequences published in GenBank for the chick, as quail DNA sequences for serotonin receptors are not available. β-Actin mRNA (L08165) was chosen as the reference gene sequence for real-time RT-PCR, because of its high and stable expression in all tested materials. The primer sequences for β-actin mRNA are: GAGAAATTGTGCGTGACATCA (+) and CCTGAACCTCTCATTGCCA (–). All primers were synthesized at the Institute of Biochemistry and Biophysics of the Polish Academy of Sciences (Warsaw); the primer sequences, the position and product length are listed in Table 1.
Real-Time PCR
The levels of 5-HT3 and 5-HT4 mRNA were measured in relation to β-actin mRNA for the oocytes, blastoderms, stage 4 embryos and brain as control tissue. The RT mixture contained 100 ng of total RNA and the RT reaction was performed as described above. Real-time PCR mixture (25 μl) consisted of 1 μl of forward primer (10 μM), 1 μl of reverse primer (10 μM), 2 μl of cDNA from RT reaction, 12.5 μl of SYBR Green PCR Master Mix and 8.5 μl H2O. The analysis contained the negative control (the reaction mix without template), and dilution series of template cDNA (1, 1/4, 1/16, 1/64, 1/256) for generation of standard curve and calculation of reaction efficiency. Amplification was performed in 7500 ABI PRISM (Applied Biosystems, Foster City, USA), using the primers for the target genes as in Table 1, starting with 10 min of initial denaturation and then for 40 cycles with the cycle profile: 30 s denaturation at 95°C, 30 s annealing at 60°C and 45 s extension at 72°C. The results are shown as fold values of target mRNA in relation to β-actin mRNA (Pfaffl, Reference Pfaffl2001).
Results
The results showing the presence/absence of the investigated 5-HT receptor (5-HTR: 5-HT1A, 5-HT1F, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT6 and 5-HT7A) transcripts are summarized in Table 2. The number of analyses performed for each material from seven (for stage II embryos) to 15 (for PGCs) is shown in Table 2 and depended upon uniformity of the results. RNA was isolated individually from 7–12 oocytes/embryos or PGC (15 samples) and sperm (10 samples). The PCR reactions for the nine receptor sequences were performed for the cDNA from the same RT reaction. The RNA isolated from brain tissue was always included in the analysis as the positive control, in which all the receptor transcripts should be present. In PGCs only a single 5-HT4 transcript was constantly expressed (in 14 out of 15 samples); three transcripts were evidently absent (5-HT1A, 5-HT3 and 5-HT5A), while transcripts for the remaining five receptors (5-HT1F, 5-HT2B, 5-HT2C, 5-HT6 and 5-HT7A) were either absent or present in individual samples. In most sperm samples, the transcripts of 5-HT1A, 5-HT2C, 5-HT4 and 5-HT6 were present, the transcripts of 5-HT3 and 5-HT7A were absent, and others were sporadically present/absent.
Table 2 Summary of the results: serotonin receptors in germ cells and early embryos of Japanese quail
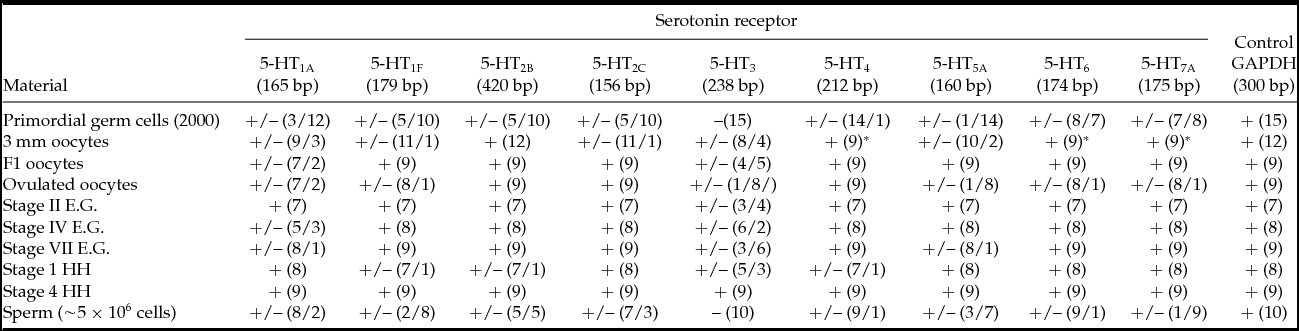
The designation +/– means the presence or absence of a receptor transcript in individual samples of the biological material.
The numbers in parentheses, e.g. +/– (2/9), means that for 11 individual samples the transcript was observed in two and absent in nine samples.
*For 3 mm oocytes the transcripts for receptors 5-HT4, 5-HT6 and 5-HT7A were determined only in nine samples.
E.G. Eyal–Giladi; HH, Hamburger–Hamilton.
In the 3-mm (beginning of vitellogenesis) and F1 oocytes (before maturation), practically all receptor sequences were found, with exception of 5-HT3 which was absent in about 30–50% of the samples. In the ovulated oocytes (after GVBD) the transcripts of 5-HT3 and 5-HT5A had to have undergone degradation, as they appeared only once per nine samples. In the developing embryos, from cleavage stage II Eyal–Giladi (EG) to stage 1 HH, all receptor transcripts were always present, again with exception of 5-HT3 which was not observed in ~50% of the samples. After the start of gastrulation (stage 4 HH), all nine transcripts were found in all the investigated samples without exception; this finding means that the presence/absence of a receptor transcript rather reflects real life situations in the studied material, and is not an accidental result.
We should take into account that the amount of RNA from the sperm and PGC samples taken for RT-PCR analysis, was lower than that from the oocyte or embryo, and that the RT-PCR method does not permit quantitative estimation of the individual sequence. Therefore, determinations were performed for 7–15 samples, and an individual result could be interpreted only in relation to the visual presence of other receptor sequences and to the reference sequence of GAPDH by gel electrophoresis. Previously, it had been determined that the amount of RNA from 2000 PGCs and 5 × 106 sperms was sufficient for visualization of rare mRNA sequences (Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008).
The example analyses of two PGC samples (10 and 11) and two sperm samples (7 and 8) are given in Figs. 3 and 4, respectively. For PGCs (Fig. 3), five receptor transcripts are absent (for 5-HT1A, 5-HT1F, 5-HT2B, 5-HT3, 5-HT5A and 5-HT7A) and three are present (for 5-HT2C, 5-HT4 and 5-HT6); for sperm (Fig. 4) – the transcripts for 5-HT5A, 5-HT2C and 5-HT4 are present and the other ones are absent.
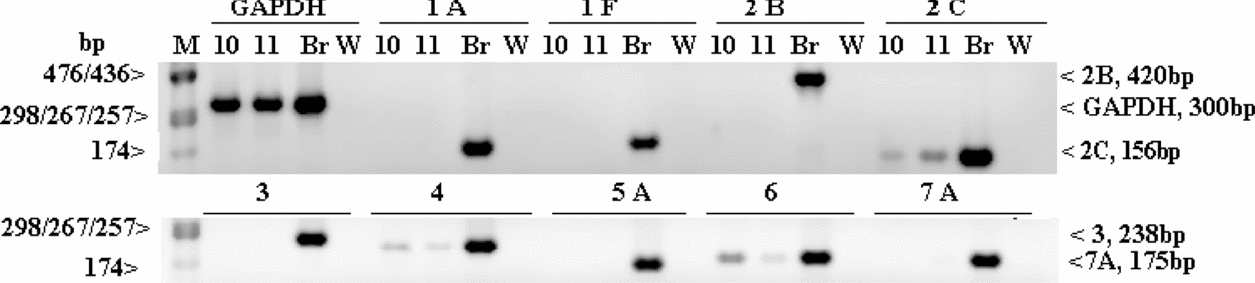
Figure 3 Gel electrophoresis of RT-PCR products showing the presence/absence of 5-HT receptor transcripts in two samples of PGCs (sample nos. 10 and 11). 1A, 1F, 2B, 2C, 3, 4, 5A, 6, and 7A, serotonin receptor transcripts; Br, the brain (control tissue); GAPDH, glyceraldehyde 3-phosphate dehydrogenase (as a control of the RNA presence and integrity); M, molecular weight marker; W, water (negative control).
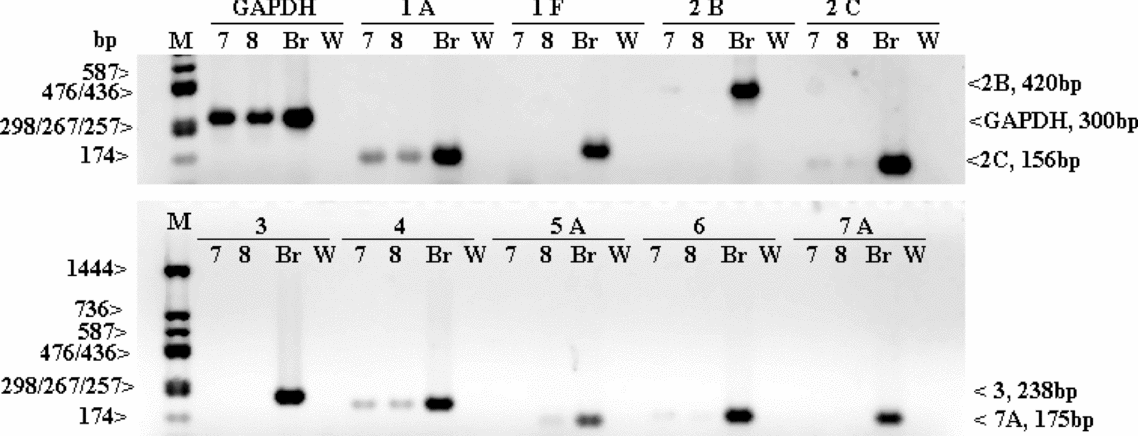
Figure 4 Gel electrophoresis of RT-PCR products for nine serotonin receptor transcripts (1A, 1F, 2B, 2C, 3, 4, 5A, 6, and 7A) present/absent in two sperm samples (sample nos. 7 and 8) and GAPDH (as a control of the RNA presence and integrity). Br, the brain (control tissue); M, molecular weight marker; W, water (negative control).
The results of real-time RT-PCR for 5-HT3 and 5-HT4 mRNAs in relation to β-actin as reference mRNA are shown in Fig. 5 a,b. The relative levels of the transcripts expressed as the percentage of their levels in brain tissue are shown in Table 3. In comparison with the brain, the expression of 5-HT3 in oocytes is practically non-existent (Fig. 5 a and Table 3). A slight accumulation of the transcript is detected in 3-mm oocytes but the transcript is destroyed at ovulation (after GVBD). Its detectable transcription can be seen in blastoderm (stage 1 embryo) from the laid eggs and in stage 4 embryos (gastrulation). The expression of the 5-HT4 receptor in all investigated samples, expressed as a percentage of the level in the brain, is clearly higher than the 5-HT3 expression (Fig. 5 b and Table 3). The results are consistent with the data in Table 2.
Table 3 Expression of 5-HT3 and 5-HT4 receptors in quail oocytes and gastrulating embryos in relation to their expression in brain tissue

*Relative values of the target mRNA versus β-actin mRNA, determined by real-time RT-PCR.
HH, Hamburger–Hamilton.
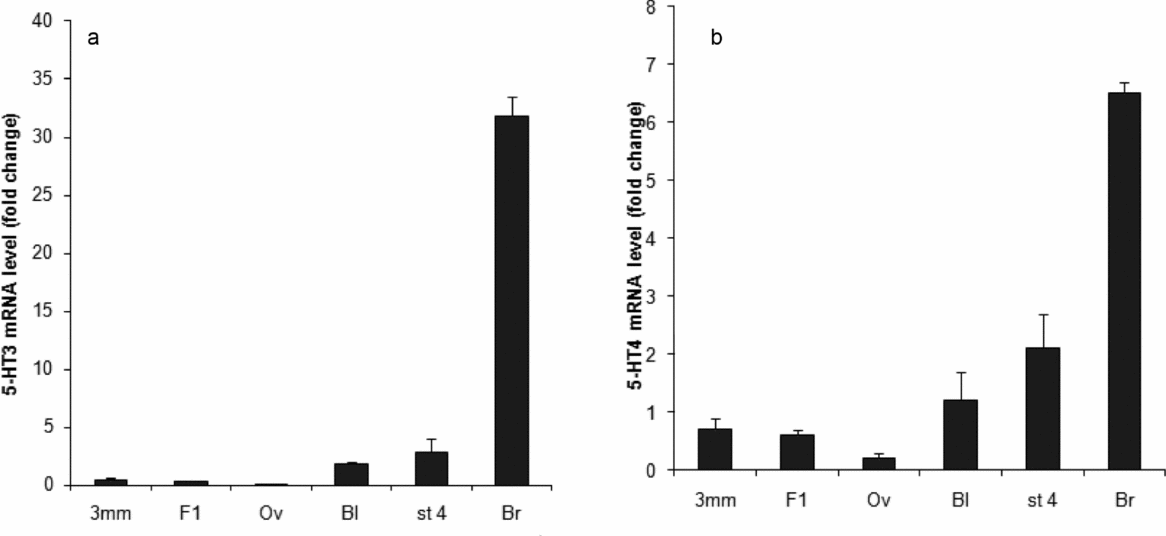
Figure 5 Relative expression of mRNA measured by real-time RT-PCR. (a) 5-HT3 mRNA. (b) 5-HT4 mRNA, at different stages of quail oocyte and embryo development. The mRNA levels are expressed in relation to the level of β-actin mRNA. 3 mm, oocyte of 3 mm in diameter; Bl, blastoderm from the laid eggs; Br, brain tissue (as control); F1, large immature oocyte; Ov, ovulated oocyte; St4, stage 4 blastoderm. Error bars are standard error of the mean (SEM) values for three separate measurements.
Discussion
The results obtained demonstrate the presence of 5-HT receptor transcripts at different stages of gamete formation and in early embryos in birds. To our knowledge this report is the first to describe the presence of 5-HT receptor transcripts in the avian germ line cells and shows their selective appearance at different stages of oogenesis, in PGCs, and in sperm cells. However, we should keep in mind that, although the presence of a coding sequence is a strong indication of the possible synthesis of the corresponding functional protein, it is not a definitive proof of this.
An avian egg contains relatively large amounts of serotonin in the yolk (Emanuelsson et al., Reference Emanuelsson, Carlberg and Lowkvist1988; Moudgal et al., Reference Moudgal, Panda and Mohan1992), which is probably transported from the outside during oogenesis and may be the substrate for melatonin synthesis. In the chick blastoderm from unincubated egg, Fukumoto et al. (Reference Fukumoto, Kema and Levin2005) found the accumulation of 0.38 pmol (66.96 pg) of serotonin in the cells around the periphery of the area opaca, i.e. in the cells that contained large amounts of yolk granules. During gastrulation these amounts increased to 1.3 pmol (229.08 pg) at the end of stage 3 H.H. and localized to the primitive streak. They also reported the presence of ~3 pmol (528.66 pg) serotonin per fertilized Xenopus oocyte (by HPLC analysis) that decreased gradually below 0.5 pmol/embryo at stage 31. This serotonin was localized radially in the vegetal part of the oocyte, and with progression of cleavage became restricted to the small subset of blastomeres originating from the right ventral blastomere of the 4-cell embryo. The authors (Fukumoto et al., Reference Fukumoto, Kema and Levin2005; Levin et al., Reference Levin, Buznikov and Lauder2006) concluded that serotonin may be implicated in the formation of the left–right axis of the embryos. However, the recent work by Beyer et al. (Reference Beyer, Danilchik, Thumberger, Vick, Tisler, Schneider, Bogush, Andre, Ulmer, Walentek, Niesler, Blum and Schweickert2012) reported no specific distribution of serotonin in Xenopus blastomeres between the 32-cell and blastula stages and attributed the discrepancy in serotonin localization to methodological artifacts. The authors proposed that serotonin is only indirectly engaged in symmetry formation.
In contrast with avian oocytes, mouse oocytes do not store serotonin in large amounts, hamster oocytes seem to contain much more, as estimated by visual observation of indirect fluorescence under confocal microscopy. Oocytes and early embryos do not contain the isozyme tryptophan hydroxylase (TPH)1, which is responsible for 5-HT synthesis in non-neural peripheral tissues (Amireault & Dube, Reference Amireault and Dube2005b; Basu et al., Reference Basu, Desai, Balaji, Chaerkady, Sriram, Maiti and Panicker2008). Another isozyme, TPH2, is expressed and is responsible for 5-HT synthesis, mostly in neural tissues. However, TPH2 transcripts were also found in mouse oocytes and in 2-cell embryos, but disappeared from 8-cell embryos and blastocysts (Basu et al., Reference Basu, Desai, Balaji, Chaerkady, Sriram, Maiti and Panicker2008). Thus, it seems that synthesis of 5-HT in oocytes may follow the same pattern as in neural tissues and use a different isozyme than in adult peripheral somatic cells. When incubated in a serotonin-containing medium, mouse and hamster oocytes had the ability to accumulate 5-HT to a great extent. The surrounding cumulus cells contained serotonin, the serotonin-synthesizing enzyme (TPH1) and serotonin transporter (SERT) transcripts and proteins. SERT was also present on the surface of mouse oocytes (Amireault & Dube., Reference Amireault and Dube2005b; Basu et al., Reference Basu, Desai, Balaji, Chaerkady, Sriram, Maiti and Panicker2008). However, even when there was a possibility of 5-HT synthesis in situ in the cumulus complex, the mouse embryo required a supply of maternal serotonin for its development, and embryos that originated from mutant tph1 − mothers showed considerable abnormalities in brain development, irrespective of their own genotype (Côté et al., Reference Côté, Fligny, Bayard, Launay, Gershon and Mallet2007). As the avian embryo is separated from the maternal circulation, all substances needed for its development, including serotonin and melatonin, must have accumulated in the egg during oogenesis, or have been synthesized by the embryo itself from the very beginning of its development. The above data indicate that serotonin is engaged in developmental processes from the very beginning; it would be useful to see what are the mechanisms and means of its action, and are they the same or different during the course of development/differentiation.
In our studies the characteristic feature of germ cell differentiation was the absence of 5-HT3 and 5-HT5A receptor transcripts in PGCs, and of 5-HT3 and 5-HT7A in sperm. This absence was the main difference between PGCs/sperm and oocytes, in which all receptor transcripts were observed. We do not know exactly at what time in early oogenesis (prior to the 3-mm oocyte) that these transcripts appeared, however we should take into account that synthesis of RNA in the avian oocyte occurs on the lampbrush chromosomes, on the maternal template, and ends with their condensation, when the oocytes are about 1.5 mm in diameter (Callebaut, Reference Callebaut1973; Malewska & Olszańska, Reference Malewska and Olszańska1999). Thus, we propose that the transcripts present in 3-mm oocytes are either transcribed before lampbrush chromosome condensation or, on the basis of data for mouse (Amireault & Dube, Reference Amireault and Dube2005b; Basu et al., Reference Basu, Desai, Balaji, Chaerkady, Sriram, Maiti and Panicker2008), rather are transported from granulose cells into the oocytes at the beginning of vitellogenesis, as for most other substances. At ovulation, two receptor transcripts (for 5-HT3 and 5-HT5A) underwent degradation, as observed, but in one ovulated oocyte per nine (Table 2). This situation may be explained by the previously reported degradation of total RNA and mRNA in the mouse and quail oocytes at ovulation (Bachvarova et al., Reference Bachvarova, De Leon, Johnson, Kaplan and Paynton1985; Malewska & Olszańska, Reference Malewska and Olszańska1999). In earlier experiments, we have also seen a similar disappearance of melatonin mel1a and mel1b receptor transcripts at ovulation and their re-transcription in the early quail embryos (Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008).
The five isoforms of 5-HT3 receptors have been found in human central nervous system (CNS) and gastrointestinal tract and some of them in rodents (Niesler et al., Reference Niesler, Kapeller, Hammer and Rappold2008; Barnes et al., Reference Barnes, Hales, Lummis and Peters2009), but to our knowledge there are no reports on the 5-HT3 receptor transcripts or proteins in PGCs, oocytes or spermatozoa of any species. However, there is a report based on the appearance of a membrane current after the addition of 5-HT3 agonists, indicating the presence of the 5-HT3 receptor in the inter-blastomeric cleft at the first cleavage of sea urchin embryo (Shmukler et al., Reference Shmukler, Silvestre and Tosti2008).
The absence of 5-HT3 receptor transcripts in PGCs and spermatozoa suggests that these ion channel proteins do not function in these cells, thus they are not indispensable for the cell functioning. The transcripts started to accumulate in the 3-mm oocytes, i.e. at the beginning of vitellogenesis but were degraded at ovulation, and became stably expressed at stage 4 H.H., when the primitive streak structure is already well developed. This finding is consistent with the studies of Fukumoto et al. (Reference Fukumoto, Kema and Levin2005) who did not observe (by immunochemistry) 5-HT3 protein in unincubated chick blastoderm but found it in the primitive streak of embryonic stages 2–5 H.H, i.e. at the beginning and end of gastrulation.
We did not detect 5-HT5A receptor transcripts in most PGCs and in the majority of the sperm samples, but these transcripts were present in most of the remaining material (oocytes, embryos), again with the exception of the ovulated oocytes where it was degraded (Table 2). The 5-HT5A receptor transcripts and proteins were found in various parts of mammalian brain but the transcripts were absent in peripheral tissues such as heart, liver, kidney, spleen or small intestine; the presence of proteins was not tested (for a review see Barnes & Sharp, Reference Barnes and Sharp1999; Thomas, Reference Thomas2006). We have found no reports on the transcripts/proteins in avian tissues. By analogy with mammals, their presence in the quail brain is not surprising, however the accumulation of the transcripts in the oocytes and their presence in the cleaving and gastrulating embryos, as opposed to adult peripheral tissues, is probably significant, even when we cannot, at present, guess their function.
As to the 5-HT1A receptor, the transcripts were present in most of the quail samples, at each of the oocyte and embryo stages and in the sperm, but not in PGCs – where it was mostly absent. As in 5-HT5A, the presence and function of the 5-HT1A receptor was investigated in brain tissue of mammals but not in the avian tissues. Receptors 5-HT4 and 5-HT6 seem to play a most important role for gametes and early embryos, as they were stably expressed in all samples of the investigated materials. They were found in every quail oocyte and embryo stages, as well as in the PGCs and sperm samples. This finding would mean that their presence is indispensable for function and/or differentiation of the PGCs, sperm and cleaving embryos. The presence of coding RNA sequences in sperm is not surprising as it has already been reported earlier that they may provide additional information for early embryonic development (Miller & Ostermeier, Reference Miller and Ostermeier2006). Previously, we have also found two melatonin receptor transcripts (mel1a and mel1c) in the quail sperm (Kawashima et al., Reference Kawashima, Stępińska, Kuwana and Olszańska2008). Lately, two reports have appeared that showed the enhanced hyperactivation of the hamster and human sperm in the presence of 5-HT in the medium and pointing to the presence of 5-HT receptors – 5-HT2 and 5-HT4 in hamster (Fujinoki, Reference Fujinoki2011) and 5-HT1B, 5-HT2A and 5-HT3 in human (Jimenez-Trejo et al., Reference Jimenez-Trejo, Tapia-Rodriguez, Cerbon, Kuhn, Manjarrez-Gutierrez, Mendoza-Rodrigues and Picazo2012) spermatozoa. The discrepancy between the presence of 5-HT3 receptor protein in human sperm and the lack the receptor transcript in quail sperm (our data) may be attributed to differences between species. Another explanation might be that the receptor protein is inserted into the sperm membrane during the process of sperm maturation in epididymis, without transcription of its gene.
Seven receptors (5-HT1A, 5-HT1F, 5-HT2B, 5-HT2C, 5-HT4, 5-HT6 and 5-HT7A) accumulated in 3-mm oocytes and were not degraded in the ovulated oocytes (Table 2). This finding suggests that new transcription on the embryonic template would not be enough to support cleavage, or that new transcription of their sequences begins later in development. This situation is different from the behaviour of 5-HT3 and 5-HT5A, which, after initial accumulation in 3-mm oocytes, were degraded at ovulation and started to be re-transcribed at the beginning of cleavage. The results of the real-time RT-PCR analysis for two representative transcripts (for 5-HT3 and 5-HT4 receptors) confirmed the qualitative results shown in Table 2, i.e. the maternal transcripts that accumulated in oocytes degraded partly (5-HT4) or completely (5-HT3) at ovulation and then appeared again after fertilization. If we accept the relative levels of the target transcripts in the brain as 100%, we can also see that the relative levels of the target mRNAs change during development. For example, the transcripts of the 5-HT3 receptor (absent in sperm and PGCs) in 3-mm and F1 oocytes consist of ~1% of its level in the brain, and this percentage increases to ~6 and ~9% in stage 1 and stage 4 embryos, respectively. The transcripts of the 5-HT4 receptor, which are permanently observed in PGCs, sperm, oocytes and embryos, accumulated in 3-mm oocytes to a level of 11% compared with the transcript in the brain and were degraded partly at ovulation. Its embryonic transcription increased significantly to ~32% that of the brain. These results do not agree with the data published recently for Xenopus oocytes (Nikishin et al., Reference Nikishin, Kremnyov, Konduktorova and Shmukler2012), in which 5-HT3 and 5-HT4 transcripts were found neither in the oocytes nor in the embryos before neurulation. This discrepancy is difficult to explain because the total amounts of maternal RNA are similar in Xenopus and hen and quail oocytes (Malewska & Olszańska, Reference Malewska and Olszańska1999), however mature Xenopus oocytes were used for RT-PCR analysis, therefore some mRNA could be destroyed at GVBD. The results reported in Tables 2 and 3, for the presence/absence of the transcripts in an oocyte, led us to conclude that this situation might vary depending on the oocytes taken for analysis, prior or after GVBD, or maybe even on the advancement of their maturation process, thus this factor should be taken into account when interpreting the data of different authors.
In general, there are not much published data on the presence of serotonin receptors in oocytes and early embryos; the available data are concerned mostly with mouse embryos with respect to the development of differentiated tissues (Choi et al., Reference Choi, Ward, Messaddeq, Launay and Maroteau1997; Lauder et al., Reference Lauder, Wilkie, Wu and Singh2000; Nebigil et al., Reference Nebigil, Etienne, Schaerlinger, Hickel, Launay and Maroteaux2001). The presence of 5-HT1D receptor transcripts in mouse unfertilized oocytes and in embryos up to the blastocyst stage (Vesela et al., Reference Vesela, Rehak, Mihalik, Czikkova, Pokorny and Koppel2003; Il'kova et al., Reference Il'kova, Rehak, Vesela, Cikos, Fabian, Czikkova and Koppel2004) was found by RT-PCR analysis, but not for 5-HT1B and 5-HT2C. We cannot confirm the 5-HT1B and 5-HT1D results, as they were not included in our experiments but 5-HT2C receptor transcripts were present in all oocyte stages, in most sperm and in some PGC samples in quail (see Table 2). Also, the three isoforms of the 5-HT7 family (5-HT7A, 5-HT7B and 5-HT7C) transcripts have been found previously in mouse oocytes and embryos up to the 4-cell stage (Amireault & Dube, Reference Amireault and Dube2005a), but disappeared at the 8-cell stage and later. This finding is contrary to our data, which showed the presence of 5-HT7 transcripts in quail oocytes, cleaving and gastrulating embryos, and the data of Nikishin et al. (Reference Nikishin, Kremnyov, Konduktorova and Shmukler2012), who found 5-HT7 receptor transcripts in Xenopus oocytes and embryos from the 2-cell to tadpole stages. This discrepancy may be explained by the fact that mouse oocytes contain much less maternal RNA than those of Xenopus and quail (~0.45 ng and 5–6 μg, respectively; Malewska & Olszańska, Reference Malewska and Olszańska1999), thus it could be destroyed after GVBD and before embryonic transcription. This explanation would also be consistent with the absence of 5-HT2C, 5-HT4 and 5-HT6 transcripts observed by Amireault & Dube (Reference Amireault and Dube2005a) in ovulated mouse oocytes, i.e. after GVBD. Our results suggested that the 5-HT7A transcript was present in half of the PGC samples, in all oocytes but not in the sperm. However, we cannot actually say whether this proportion in PGCs is meaningful or just coincidental. Sheng et al. (Reference Sheng, Wang, Liu, Montplaisir, Tiberi, Baltz and Liu2005) suggested the possible role of the 5-HT7 receptor in maintaining oocyte maturation in frog and mouse.
In conclusion, we can state that serotonin receptor mRNAs appear in avian germ cells and in very early embryos much earlier before specialized tissues start to differentiate. This finding means that serotonin might act at such early developmental periods not as a neurotransmitter but as a signaling molecule. Previously, such a role has already been ascribed to this molecule by several authors (Lauder, Reference Lauder1993; Buznikov et al., Reference Buznikov, Lambert and Lauder2001; Amireault & Dube, Reference Amireault and Dube2005a; Dube & Amireault, Reference Dube and Amireault2007; Shmukler et al., Reference Shmukler, Silvestre and Tosti2008). We have extended the study to Aves and to cells not investigated previously, such as PGCs and sperm, and have come to the conclusion that the 5-HT receptors, as for those of melatonin, are rather selectively expressed in germ cells, and thus might be involved in the earliest events of differentiation.
Acknowledgements
We are deeply grateful to Anne-Lise Haenni from the Institute Jacques Monod (Paris) for reading and correcting the English in the manuscript. The study was supported by the grant from National Science Center (no. N N311179 637).










