INTRODUCTION
Schistosome eggs are responsible for both the progression of the life cycle of the parasite and the tissue pathology observed during infection. After being released into the mesenteric vasculature, immature eggs require approximately 5–6 days to achieve embryo differentiation and to begin secreting lytic and antigenic effectors through micropores in the egg shell (Andrade, Reference Andrade2009). At this time, mature eggs migrate across endothelial and mucosal barriers to the intestinal lumen, where they can be excreted into the external environment in the host's feces (Doenhoff et al. Reference Doenhoff, Hassounah, Murare, Bain and Lucas1986).
Animal studies indicate that the consumption of a high-fat diet modifies the morphology of the small intestine (Little et al. Reference Little, Feltrin, Horowitz, Meyer, Wishart, Chapman and Feinle-Bisset2008), gastrointestinal transit (de Wit et al. Reference de Wit, Bosch-Vermeulen, de Groot, Hooiveld, Bromhaar, Jansen, Müller and van der Meer2008), lipid metabolism, the cell cycle and the inflammatory and immune responses (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007). Previous studies have shown that mice fed a high-fat diet significantly increased both trapped eggs and faecal egg output during the acute (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007) and chronic phases of schistosomiasis (Alencar et al. Reference Alencar, Neves, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2009). Moreover, human studies have demonstrated a positive correlation between egg load and organ pathology (Cheever et al. Reference Cheever, Kamel, Elwi, Mosimann and Danner1977).
Despite these interesting observations, whether a schistosomiasis infection associated with a high-fat diet causes injury to the small intestinal morphology of mice has not been investigated. This study compares the histological features of the small intestine in mice fed a high-fat or a standard diet during the acute and chronic phases of schistosomiasis mansoni.
MATERIALS AND METHODS
Animals and dietary regimen
Three-week-old female Swiss Webster mice were obtained from the Laboratory Animal Breeding Center (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil). The mice were conventionally housed in polypropylene cages (40×33 cm) with screened covers made of stainless steel. The mice were maintained on a 12-h light-dark cycle with water provided ad libitum at a controlled temperature (21±1°C) and humidity (60±10%). All procedures were approved by the local Commission for Ethics in the Use of Animals (L-0036/07; CEUA-FIOCRUZ).
Animals were assigned to one of 2 nutritional groups for a 6-month period: (a) high-fat diet (47% carbohydrate, 24% protein, 29% lipid; 5·7 kcal/g body weight/day), or (b) standard laboratory diet for mice (Nuvilab CR-1-NUVITAL Nutrients Ltda., Colombo, Paraná, Brazil; 12% fat, 28% protein, 60% carbohydrate; 4·6 kcal/g body weight/day) (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2006). The lipid proportions were 354·0 g/kg saturated fatty acids, 500·0 g/kg monounsaturated fatty acids and 146 g/kg polyunsaturated fatty acids (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007). Body weight was recorded twice per week throughout the experiment. All mice were allowed to consume water and food pellets ad libitum.
Plasma cholesterol measurement
After the 6-month feeding period, blood was collected from food-deprived mice (overnight) by puncturing the retro-orbital sinus. Plasma was separated by low-speed centrifugation and stored at −20°C until use. The total serum cholesterol was determined using a previously described colourimetric enzymatic method (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2006). Lipid analysis was performed 1 day prior to experimental infection and again at 17 weeks post-infection.
Mouse infection procedures
Thirty mice were subcutaneously infected with approximately 50 Schistosoma mansoni (BH strain) cercariae (Martinez et al. Reference Martinez, Neves, de Oliveira, Machado-Silva and Rey2003) shed from laboratory-reared Biomphalaria glabrata snails provided by the Malacology Laboratory (Oswaldo Cruz Institute, Rio de Janeiro, Brazil) (Paraense et al. Reference Paraense and Corrêa1989).
The animals were divided into 6 groups of 5 mice as follows: SC (uninfected mice fed standard chow), HFC (uninfected mice fed high-fat chow), IHFCa (infected mice fed high-fat chow and euthanized during acute infection), IHFCc (infected mice fed high-fat chow and euthanized during chronic infection), ISCa (infected mice fed standard chow and euthanized during acute infection) and ISCc (infected mice fed standard chow and euthanized during chronic infection).
Tissue processing and histopathology
The entire small intestine was collected from each mouse euthanized at 9 (acute) or 17 (chronic) weeks post-infection by cervical dislocation. The intestine was divided into 3 segments (proximal, middle and distal) of equal length (1·0 cm long). The fragments were processed routinely for histological preparation, embedded in paraffin, sectioned at a thickness of 5 μm, and stained with haematoxylin (Proquimios Comércio e Indústria Ltda, Brazil) and eosin (Vetec Química Sima Ltda, Brazil).
The intestinal mucosal or submucosal distribution of eggs and gut wall lesions (duodenum and jejunum) were analysed by light microscopy. Representative images were captured with an Olympus BX50 microscope (Tokyo, Japan) with a Nikon Eclipse E200 camera Nikon DS-Fi1 and Image Pro Plus software (Media Cybernetics, US). Tissues were evaluated for egg responses according to the predominant components as follows: exudative (E), exudative/exudative-productive (E/E-P), exudative-productive (EP) and productive (P) and percentage of confluent granulomas, which was expressed as a percentage of the total number of granulomas counted for acutely and chronically infected mice. An exudative stage does not fit the fundamental requirement of the granuloma concept: they do not produce a chronic inflammatory reaction. In the intermediate, E/E-P stages, granulomas are rich in eosinophils and monocytes and are surrounded by a disorganized zone rich in fibroblasts. In EP and P granulomas, a random reticular scaffold or mesh intermingled with the cells begins to appear, defining clear zones within the granuloma as follows: an inner, internal or paucifibrillar zone, which consists of macrophages with or without epithelioid transformation and, occasionally, giant cells; the middle or paracentral zone, which is rich in fibroblasts with or without mast cells; and the outermost or external zone, which, in the more chronic phase of the infection, is rich in lymphocytes (T and B) and plasma cells (Li-Hsü et al. Reference Li Hsü, Hsü, Davis and Lust1972; Lenzi et al. Reference Lenzi, Kimmel, Schechtman, Pelajo-Machado, Romanha, Pacheco, Mariano and Lenzi1998).
Morphometric measurements of mucosal thickness, small intestinal villi length (distance between the top of the villus and the upper edge of the muscularis mucosae) and height (distance between the top of the villi and the opening of the glandular crypts) and the abundance of goblet cells and enterocytes on the villous surface were made on digitally captured images (Nikon Eclipse E200 camera Nikon DS-Fi1) using Image Pro Plus software (Media Cybernetics, US).
Statistical analysis
The data were statistically analysed with GraphPad InStat (version 3.01). Groups were compared using a Student's t-test, Kruskal-Wallis test and ANOVA (Tukey post-test). P-values ⩽0·05 were considered significant.
RESULTS
Weight gain
Body mass significantly increased (P=0·003) in all mice by week 36, irrespective of diet. Mice fed the high-fat diet exhibited a higher body mass (HFC (58±9 g) and IHFC (59±12 g)) than those fed standard chow (SC (43±2 g) and ISC (39±4 g)).
Plasma cholesterol
SW mice fed a high-fat diet had significantly elevated plasma cholesterol before (HFC: 1·6–0·3; SC: 1·0–0·2; P=0·008) and after infection (HFC: 1·2–0·1; IHFC: 1·2–0·3; SC: 0·7–0·2; ISC: 0·9–0·2; P=0·005) compared with mice fed the standard diet.
Histoquantitative analysis
Changes in the small intestine of uninfected mice fed a high-fat diet
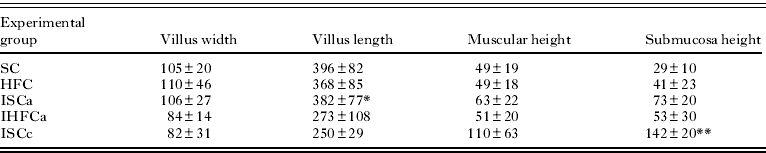
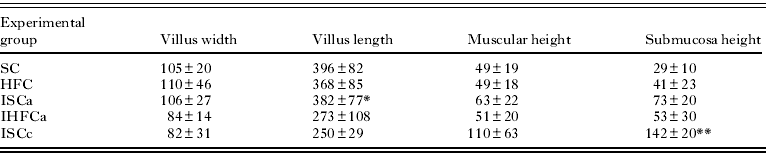
As shown in Table 1 and Fig. 1 (A,C), a high-fat diet substantially increased either the width of the duodenal villi (HFC, 110±46 μm; SC, 105±20 μm) or the height of the submucosa (HFC, 41±23 μm; SC, 29±10 μm). Villus length was reduced in mice fed a high-fat diet (HFC, 368±85 μm) compared with the control (Fig. 1 B,D) (SC, 396±82 μm). The muscular height was unchanged (HFC, 49±18 μm; SC, 49±19 μm).
Jejunal measurements are reported in Table 2. With respect to the jejunal segment, a high-fat diet induced a higher villus width (HFC, 84±18 μm; SC, 77±13 μm) and height of the submucosa (HFC, 35±6 μm; SC, 26±7 μm). Villus length was reduced in mice fed a high-fat diet (180±38 μm) compared with those fed control chow (SC, 275±60 μm). The muscular height was unchanged (HFC, 49±17 μm; SC, 49±19 μm). No statistical difference (P>0·05) was observed between diet groups.
Histopathology
By week 9, mice fed a high-fat diet exhibited E, E/EP and EP granulomas, whereas in the control diet group, only E granulomas were found (Table 3). After week 17 of infection, mice fed a high-fat diet and control mice exhibited E, E/EP and EP ganulomas (Table 3; Fig. 3). Compared with acutely infected mice, chronically infected mice demonstrated a greater thickness of the mucosa and submucosa and shortened villi. The histological alterations were due to the presence of eggs and granulomas in the muscular, mucosal and submucosal layers (Fig. 2B, F). The muscular layer was primarily uniform in appearance among all groups, whereas focal thickening was observed only in control diet-fed mice (Fig. 2B). Chronically infected mice fed a high-fat diet exhibited higher granuloma and egg numbers than the acutely infected group. The jejunal transverse histological section was within normal limits of muscular thickness and was only increased by periovular granuloma formation (Fig. 2D).

Fig. 1. Photomicrographs of normal intestines from mice fed a high-fat (A and C) or standard diet (B and D). (A) Transverse section of the duodenum demonstrating the reduction and widening of the villi; increase in muscular thickness caused by the increase in muscular fibres. The mucosa exhibits the increased size of goblet cells (*). (B) General features of normal jejunal villi. (C) Intestinal villi and hyperplasia of goblet cells (*). (D) Intestinal villi exhibiting normal appearance. Haematoxylin-eosin staining. (A and B=X10; C and D=X40).

Fig. 2. Haematoxylin-eosin-stained sections of intestinal granulomas from acutely Schistosoma mansoni-infected mice fed a high-fat (A, C, E) or standard diet (B, D, F). (A) Transverse ileum section exhibits an increased muscle layer thickness caused by eggs within the mucosa and submucosa, polymorphonuclear infiltrates and hyperplasia of goblet cells (→). (B) Transverse duodenum section with thickening of the submucosal layer caused by schistosome eggs and mononuclear infiltrates. (C) Transverse section of jejune showing increased thickness of muscle layers, submucosa and mucosa due to the presence of lymphocytic and polymorphonuclear infiltrates; granulomas are present in the mucosa. (D) The histology of the transverse jejunal section was within normal limits of muscular thickness and only increased with periovular granuloma formation. (E) Detail of a granuloma of an egg in the mucosa with infiltrated polymorphonuclear cells. (F) Detail of an egg in the intestinal layer exhibiting infiltrated mononuclear and polymorphonuclear cells. (A, B, C, D=X10; E and F=X40).

Fig. 3. Haematoxylin-eosin-stained sections of intestinal granulomas of chronically Schistosoma mansoni-infected mice fed a high-fat (A, C, E) or standard diet (B, D, F). Exudative granuloma located in the duodenal (A) and jejunal (B) mucosa; exudative/exudative-productive granuloma in the duodenal mucosa (C) and in the mucosa (D); exudative-productive in duodenum (E) and jejunum (F) mucosa (X40).
Table 1. Morphometric parameters in the duodena of mice fed a high-fat or standard diet (mean±s.d.) (μm)
Letters indicate significant differences between values in each column (P<0·05). *Significant difference between ISCa and IHFCa; **significant difference between ISCc and IHFCc.
Table 2. Morphometric parameters in the jejune of mice fed a high-fat or standard diet (mean±s.d.) (μm)

Letters indicate significant differences between values in each column (P<0·01). *Significant difference between ISCc and IHFCc.
Table 3. Distribution (%) of schistosomal periovular and confluent granulomas in Schistosoma mansoni-infected SW mice fed a high-fat or standard diet during the acute and chronic phases of infection

A comparison of the granuloma stage with diet revealed that they were more common in IHFCa and IHFCc than in ISCa and ISCc. Productive granulomas were not found (Table 3).
The percentage of granulomas in the duodenum was higher in the IHFC group compared with the ISC group, which had a greater abundance of granulomas in the jejune. The percentage of confluent granulomas was higher in the IHFC group, independent of the time of infection (Table 3). Eosinophils, macrophages and fibroblasts behave differently according to the evolutionary stage of the granuloma. Exudative granulomas exhibited a diverse cellular composition, consisting of a preponderance of eosinophils, a reduced number of neutrophils, few macrophages, epithelioid cells and giant cells (Fig. 3A). As the infection progressed to the chronic phase, there was less cellular diversity, irrespective of the group assayed. Fibroblasts, lymphocytes and macrophages were the predominant cells in both E/EP and EP granulomas.
Changes in the small intestine of infected mice fed a high-fat diet
As demonstrated in Table 1, mice euthanized during acute infection exhibited a reduced duodenal villus width (IHFCa, 84±14 μm; ISCa, 106±27 μm; P<0·05), villus length (IHFCa, 273±108 μm; ISCa, 382±77 μm), muscular height (IHFCa, 51±20 μm; ISCa, 63±22 μm) and height of the submucosa (IHFCa, 53±30 μm; ISCa, 73±20 μm). The villus length was reduced in mice fed a high-fat diet (273±108 μm) compared with those that received control chow (382±77 μm).
As indicated in Table 2, the jejunal villus width was slightly reduced (IHFCa, 71±12 μm; ISCa, 78±12 μm), the villus length was increased (IHFCa, 334±42 μm; ISCa, 269±67 μm), the muscular height was reduced (IHFCa, 51±20 μm; ISCa, 63±22 μm) and the height of the submucosa was unchanged (IHFCa, 58±30 μm; ISCa, 59±16 μm).
During chronic infection, the duodenal villus width was slightly reduced (IHFCc, 76±9 μm; ISCc, 82±31 μm), the villus length was increased (IHFCc, 352±54 μm; ISCc, 250±29 μm), the muscular height was unaltered (IHFCc, 109±30 μm; ISCc, 110±63 μm) and the height of the submucosa was increased (IHFCc, 243±130 μm; ISCc, 142±20 μm; P<0·05) (Table 1).
Regarding the jejunum, the villus width was reduced (IHFCc, 52±14 μm; ISCa, 68±16 μm; P<0·05), the villus length was increased (IHFCc, 367±47 μm; ISCc, 265±38 μm), the muscular height was unaltered (IHFCc, 109±30 μm; ISCc, 110±63 μm) and the height of the submucosa was increased (IHFCc, 202±31 μm; ISCc, 122±22 μm; P<0·05) (Table 2).
DISCUSSION
The morbidity observed in gastrointestinal schistosomiasis is linked to the deposition of eggs in the small intestine and their subsequent passage to the intestinal lumen, which largely depends on the host and its cellular immune response (Doenhoff et al. Reference Doenhoff, Hassounah, Murare, Bain and Lucas1986; Andrade, Reference Andrade2009). This situation leads to a marked inflammation of the small intestine, which provokes structural and functional changes during both acute and chronic infection (Moreels et al. Reference Moreels, De Man, Bogers, De Winter, Vrolix, Herman, Van Marck and Pelckmans2001).
Diet-induced models have proven beneficial for evaluating the physiological changes that take place during the pathogenesis of diseases (Neves et al. Reference Neves, Machado-Silva, Pelajo-Machado, Oliveira, Coutinho, Lenzi and Gomes2001). Most experimental studies on schistosomiasis have demonstrated that the host-parasite relationship may be modified by the host's nutritional status (Kanuft and Warren, Reference Kanuft and Warren1969; Coutinho et al. Reference Coutinho, Ferreira, Assunção, Carvalho, Oliveira and Francelino2002). This work aimed to evaluate whether a high-fat diet could affect the architecture of the intestine in S. mansoni-infected mice. The present findings confirm that the high-fat diet caused greater body weight and higher plasma cholesterol levels compared with mice fed a standard diet (Galloway et al. Reference Galloway, Pallebage-Gamarallage, Takechi, Jian, Johnsen, Dhaliwal and Mamo2008; Stanley et al. Reference Stanley, Jackson, Griffiths and Doenhoff2009). Interestingly, S. mansoni-infected mice experienced a reduction in circulating cholesterol levels. Previous studies indicate that the anti-atherogenic effect of S. mansoni infection in mice was associated with the parasite-induced lowering of total blood cholesterol (Doenhoff et al. Reference Doenhoff, Stanley, Griffiths and Jackson2002). Studies in animal models provide evidence that schistosomes are closely associated with the modulation of lipid metabolism (Tallima and El-Ridi, Reference Tallima and El Ridi2005; Alencar et al. Reference Alencar, Neves, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2009), in which cholesterol from the host's bloodstream is used for biological functions (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2006; Stanley et al. Reference Stanley, Jackson, Griffiths and Doenhoff2009).
The consumption of a high-fat diet modifies small intestinal morphology (Little et al. Reference Little, Horowitz and Feinle-Bisset2007, Reference Little, Feltrin, Horowitz, Meyer, Wishart, Chapman and Feinle-Bisset2008), gastrointestinal transit, inflammation and the immune response (de Wit et al. Reference de Wit, Bosch-Vermeulen, de Groot, Hooiveld, Bromhaar, Jansen, Müller and van der Meer2008). In addition, the duodenum and proximal jejunum are the main sites for cholesterol absorption from the intestinal lumen (Balint et al. Reference Balint, Fried and Imai1980). In this study, uninfected mice fed a high-fat diet exhibited similar changes in the duodenal and jejunal segments, where measurements of villus width and submucosa height were higher than in the control group; however, the villus length was reduced compared with the control. These alterations likely resulted from an inflammatory reaction (Xu et al. Reference Xu, Barnes, Yang, Tan, Yang, Chou, Sole, Nichols, Ross, Tartaglia and Chen2003; Weisberg et al. Reference Weisberg, McCann, Desai, Rosenbaum, Leibel and Ferrante2003), in which the accumulation of T-lymphocytes (Kintscher et al. Reference Kintscher, Hartge, Hess, Foryst-Ludwig, Clemenz, Wabitsch, Fischer-Posovszky, Barth, Dragun, Skurk, Hauner, Blüher, Unger, Wolf, Knippschild, Hombach and Marx2008) could play an important role in the recruitment of macrophages (Nishimura et al. Reference Nishimura, Manabe, Nagasaki, Eto, Yamashita, Ohsugi, Otsu, Hara, Ueki, Sugiura, Yoshimura, Kadowaki and Nagai2009) and in the regulation of the inflammatory response to bacterial antigen (Feuerer et al. Reference Feuerer, Herrero, Cipolletta, Naaz, Wong, Nayer, Lee, Goldfine, Benoist, Shoelson and Mathis2009; Winer et al. Reference Winer, Chan, Paltser, Truong, Tsui, Bahrami, Dorfman, Wang, Zielenski, Mastronardi, Maezawa, Drucker, Engleman, Winer and Dosch2009). The gut microbiota is implicated in a variety of host functions involving intestinal development and function, including digestion and absorption of carbohydrates, proteins and fats, immune modulation and gastrointestinal motility. Gut bacteria suppress the expression and release of fasting-induced adipose factor (Fiaf) from the small intestine, resulting in the increased activity of lipoprotein lipase (Backhed et al. Reference Backhed, Ding, Wang, Hooper, Koh, Nagy, Semenkovich and Gordon2004, Reference Backhed, Manchester, Semenkovich and Gordon2007). It has been hypothesized that bacterial lipopolysaccharide (LPS) derived from gram-negative bacteria residing in the gut acts as a triggering factor linking inflammation to high-fat diet-induced metabolic syndrome. These results suggest that a high-fat diet increases endotoxaemia and affects the composition of the intestinal microbiota (Cani et al. Reference Cani, Amar and Iglesias2007). In addition, bacterial interactions promote pro-inflammatory signalling in multiple cell types in the intestine (Ding et al. Reference Ding, Chi, Scull, Rigby, Schwerbrock, Magness, Jobin and Lund2010). Studies in conventionally raised mice fed a high-fat diet indicate that changes in the intestinal microbiota and intestinal permeability may contribute to intestinal inflammation (Cani et al. Reference Cani, Amar and Iglesias2007). In accordance with this, our results demonstrate an intestinal inflammatory response. It will be interesting to determine the composition of the gut microbiota in our diet-induced model in further studies.
An immunohistochemical analysis could help in characterizing the cellular composition and localization of the inflammatory response. Assessment of intestinal inflammation could be determined by immunofluorescence microscopy using antibodies against collagen I, collagen III, collagen IV, fibronectin, laminin and desmin (Silva et al. Reference Silva, Fernandes, Barbosa, Oliveira and Andrade2000). Alternatively, the infiltration of neutrophils (7/4), T lymphocytes (CD-3) and macrophages (F4/80) could be visualized by immunohistochemistry, and the mRNA levels of markers of inflammation, including intercellular adhesion molecule-1 (ICAM-1), monocyte chemo-attractive protein-1 (MCP-1) and macrophage inflammatory protein-2 (MIP-2), could be measured. In addition, the levels of TNF-α, IL-6, IL-17A, and keratinocyte-derived cytokine (KC) could be assessed (Park et al. Reference Park, Chen, Kim, Brown, Kolls, D'Agati and Lee2011).
A concomitant pathology could also compound the intestinal injury. Based on parasitological parameters, it has been previously demonstrated that the viability, maturity, and fecal excretion of eggs are much higher in mice fed a high-fat diet than those fed a standard diet during both acute (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007) and chronic infections (Alencar et al. Reference Alencar, Neves, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2009).
Based on previous reports (Little et al. Reference Little, Horowitz and Feinle-Bisset2007; Kvietys et al. Reference Kvietys, Specian, Grisham and Tso1991), we expected that mice fed a high-fat diet could develop greater gut pathology than the control group. Although the role of granulomas in damaging tissue has been extensively studied (Domingo and Warren, Reference Domingo and Warren1969; Moreels et al. Reference Moreels, De Man, Bogers, De Winter, Vrolix, Herman, Van Marck and Pelckmans2001; Stravitsky, Reference Stavitsky2004), researchers have also explored to some extent the tissue-protective potential of this inflammatory response. This observation supports the notion that a granulomatous response of diminished intensity is favourable to the host. Eggs deposited in tissues elicit an exudative response that can be characterized by a disorganized aggregation of cells as well as exudative-productive, productive, advanced or fibrotic-productive granulomas, which are morphologically organized (Lenzi et al. Reference Lenzi, Kimmel, Schechtman, Pelajo-Machado, Romanha, Pacheco, Mariano and Lenzi1998).
Among the parameters examined, a difference in granuloma morphology was demonstrated according to each diet assayed and the time of infection. Mice fed a high-fat diet possessed granulomas at different stages of maturation, whereas the control group exhibited only exudative granulomas during the acute phase. Although modifications in the appearance of intestinal granulomas with time have not been previously reported (Silva et al. Reference Silva, Fernandes, Barbosa, Oliveira and Andrade2000), our results suggest differences that may be related to diet. Accordingly, previous results showed that eosinophils, macrophages and fibroblasts behave differently according to the evolutionary stage of liver granulomas in mice fed a high-fat or a standard diet (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007). It is possible that the response induced by a high-fat diet may increase granuloma cell recruitment or be due to a higher intestinal egg load (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007).
The current data show that egg and granuloma distribution within the gut wall confirm previous histological descriptions of acute (Borgers et al. Reference Borgers, Moreels, De Man, Vrolix, Jacobs, Pelckmans and Van Marck2000) and chronic infections (Holmes, Reference Holmes1990). In the acute phase, the jejunum segment exhibited more granulomas than the duodena of control mice, irrespective of the phase of infection. Evidence has been presented that adult worms have a realised niche (portion of the fundamental niche occupied within the host) for egg-laying (Holmes, Reference Holmes1990), which is the distal region of the small intestine in mice (de Lima and Katz, Reference de Lima e Costa and Katz1982; Martinez et al. Reference Martinez, Neves, de Oliveira, Machado-Silva and Rey2003; Freire et al. Reference Freire, Rodrigues-Silva, Machado-Silva and Rey2003) and the large intestine in primate hosts (Nyindo and Farah, Reference Nyindo and Farah1999). Previous studies hypothesize that the colonization of homoeotherms by vascular trematodes requires precision in egg laying near the conduit to facilitate the passage of the egg to the external environment (Platt and Brooks, Reference Platt and Brooks1997).
As the infection progressed to the chronic phase, reduced cellular diversity was observed, irrespective of the group assayed, which corresponds to the process of cellular organization (Lenzi et al. Reference Lenzi, Kimmel, Schechtman, Pelajo-Machado, Romanha, Pacheco, Mariano and Lenzi1998). The data presented here show that mice fed a high-fat diet had higher granuloma and egg numbers than the acutely infected group. These findings raise some questions. First, animal (Rocha et al. Reference Rocha, Rocha, Pedroso, Colosimo and Coelho1995) and in vitro studies (Barth et al. Reference Barth, Fernandes and Rodrigues1996) have demonstrated that fecal egg excretion progressively declines during chronic infection. Although the mechanisms due to which the egg output declines are not well understood, some studies have correlated this decline with structural and functional changes in the host intestine (Moreels et al. Reference Moreels, De Man, Bogers, De Winter, Vrolix, Herman, Van Marck and Pelckmans2001). In contrast, animal experiments have shown that older male and female schistosomes demonstrate age-induced morphological alterations in the reproductive system (Cheever et al. Reference Cheever, Mosimann, Deb, Cheever and Duvall1994; Machado-Silva et al. Reference Machado-Silva, Neves and Rodrigues-Silva2010). Second, previous studies have demonstrated that there is no tendency for egg output to decline among chronically infected mice fed a high-fat diet (Alencar et al. Reference Alencar, Neves, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2009). The most straightforward interpretation of this finding is that cholesterol may induce higher egg production among adult females, consistent with our previous report (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007). Cholesterol represents a key node in cellular physiology because it is involved in membrane fluidity, the regulation of membrane traffic, signalling pathways and reproduction. Caenorhabditis elegans is a widely used model to study cholesterol distribution in living worms. The accumulation of lipids in oocytes and spermatozoa has been observed in this system, which suggests a possible role for cholesterol in sperm development (Matyash et al. Reference Matyash, Geier, Henske, Mukherjee, Hirsh, Thiele, Grant, Maxfield and Kurzchalia2001). Given that schistosomes do not synthesize cholesterol de novo, male and female worms could be maintained in medium containing radio-isotope labelled cholesterol (Rumjanek and Simpson, Reference Rumjanek and Simpson1980). We suggest that an imaging assay based on fluorescence microscopy could be used to address the location of the labelled cholesterol (Matyash et al. Reference Matyash, Geier, Henske, Mukherjee, Hirsh, Thiele, Grant, Maxfield and Kurzchalia2001).
Considering the paucity of morphometric data on the mucosa of the small intestine of schistosomiasis-infected mice fed a high-fat diet, our histoquantitative results showed that villus, muscle and submucosa measurements were significantly modified. Concurrent infection with S. mansoni significantly altered the aforementioned anatomical sites mainly during chronic infection, irrespective of diet and the intestinal segment. Our results suggest that morphological alterations begin by week 9 and are aggravated by week 17, consistent with other studies of intestinal pathology (Borgers et al. Reference Borgers, Moreels, De Man, Vrolix, Jacobs, Pelckmans and Van Marck2000; Couto et al. Reference Couto, Ferreira, Rocha, Duarte, Assunção and Coutinho2002; Siqueira et al. Reference Siqueira, Ferraz, Campos, De Lima Filho, Albuquerque, de Lima Aires, Ribeiro, Cavalcanti, De Lima, Cavalcanti and Ferraz2010). In this study, the damage to the intestinal tissue could be the result of inflammation (Trobojević-Stanković et al. Reference Trobojević-Stanković, Milićević, Milosević, Despotović, Davidović, Erceg, Bojić, Bojić, Svorcan, Protić, Dapcević, Miljković and Milićević2010) due to the higher number of eggs trapped in the intestine in mice fed a high-fat diet (Neves et al. Reference Neves, Alencar, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2007, Alencar et al. Reference Alencar, Neves, Aguila, Mandarim-de-Lacerda, Corrêa and Machado-Silva2009). In conclusion, a high-fat diet and schistosomiasis infection had a significant impact on the architecture of the small intestine.
FINANCIAL SUPPORT
This work was supported by grants from FAPERJ (E-26/101965/2009 and E-26/111538/2010) and Capes.