INTRODUCTION
Echinococcoses are serious parasitic zoonoses caused by the members of genus Echinococcus Rudolphi, 1801 (Cestoda: Taeniidae). The genus consists of 9 species: Echinococcus multilocularis, E. oligarthrus, E. vogeli, E. granulosus sensu stricto (s.s.), E. equinus, E. ortleppi, E. canadensis (including the genotypes G6, G7, G8 and G10) and E. felidis (Lymbery Reference Lymbery, Thompson and Lymbery1995; Eckert et al. Reference Eckert, Gemmell, Meslin and Pawlowski2001; Lavikainen et al. Reference Lavikainen, Lehtinen, Meri, Hirvelä-Koski and Meri2003; Xiao et al. Reference Xiao, Qiu, Nakao, Li, Yang, Chen, Schantz, Craig and Ito2005; Hüttner et al. Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008; Nakao et al. Reference Nakao, Yanagida, Okamoto, Knapp, Nkouawa, Sako and Ito2010b; Knapp et al. Reference Knapp, Nakao, Yanagida, Okamoto, Saarma, Lavikainen and Ito2011). The members of the E. granulosus sensu lato (s.l.) species complex morphologically resemble each other and are only distinguishable by molecular analysis. The causative agents of cystic echinococcosis (CE) referred to as E. granulosus s.l. utilize ungulates (sheep, goat, cattle, horse, etc.) as intermediate hosts, and dogs and wolves as definitive hosts (Abuladze, Reference Abuladze and Skrjabin1964; Foreyt et al. Reference Foreyt, Drew, Atkinson and McCauley2009). On the other hand, E. multilocularis causes alveolar echinococcosis (AE), and exploits rodents as intermediate hosts and foxes, dogs and wolves as definitive hosts (Abuladze, Reference Abuladze and Skrjabin1964; Ito et al. Reference Ito, Chuluunbaatar, Yanagida, Davaasuren, Sumiya, Asakawa, Ki, Nakaya, Davaajav, Dorjsuren, Nakao and Sako2013). Since the dissolution of the Soviet Union in 1991, human infections with Echinococcus spp. in Russia increased rapidly. According to the official website of Rospotrebnadzor (www.rospotrebnadzor.ru), only 190 cases were reported in 1992 but the recorded cases reached 553 in 2001. Among these 553 cases, only 30 cases were registered as AE. Based on serological studies, the Rospotrebnadzor also pointed out that the number of cases was underestimated and it could be as much as three times greater. Therefore, an extensive epidemiological survey is needed to understand the current situation of echinococcoses in Russia. For the epidemiology of echinococcoses, a reliable molecular method to identify the aetiological agents is necessary because of the morphological similarity among Echinococcus species. However, molecular identification of Echinococcus species and genotypes was rarely made in Russia until recently.
In all Russian territories, E. granulosus s.l. is widespread and biological strains using different host animals have been described (Yastreb, Reference Yastreb2010). Molecular identification studies revealed the distribution of E. granulosus s.s., E. ortleppi and E. canadensis (G6 and G7). The identification of species and genotypes has been conducted mainly by restriction fragment length polymorphism (Nikulina et al. Reference Nikulina, Garaev, Odoevskaya, Benediktov and Uspenskiy2003; Lukmanova et al. Reference Lukmanova, Tuigunov, Nartailakov, Bilalov and Gumerov2008) and the nucleotide sequence data of mitochondrial DNA (mtDNA) are available only for some cases (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a,Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob; Nakao et al. Reference Nakao, Yanagida, Konyaev, Lavikainen, Odnokurtsev, Zaikov and Ito2013b). E. multilocularis is also widespread in most parts of Russia. Based on the incidence records of human AE cases, nine endemic areas were identified (Lukashenko, Reference Lukashenko1975), and the largest focus was situated in Yakutia (Alperovich, Reference Alperovich1972). Another part of endemic area is located in western Siberia and most of the cases were distributed in the southern part of the region – Altai Krai and the Altai Republic (Gaenko, Reference Gaenko1958). For more than 50 years, researchers have paid special attention to the significant differences in the incidences of inhabitants in different territories. Although E. multilocularis infection is common in intermediate and definitive hosts, human AE cases are very rare in Yakutia and Taimyr and Yamal Peninsulas (Gubanov, Reference Gubanov1964; Gubanov and Fedorov, Reference Gubanov, Fedorov and Cherepanov1970; Martynenko et al. Reference Martynenko, Zorihina, Shcherbakov, Starkov and Suvorin1984; Bessonov, Reference Bessonov, Craig and Pawlowski2002, Reference Bessonov2003). One hypothesis to explain the difference in the incidence rate is that there are pathogenic (red fox strain) and low-pathogenic or non-pathogenic (Arctic fox strain) strains (Shakhmatova, Reference Shakhmatova and Maksimov1981). The recent phylogeographic analyses defined the division of E. multilocularis into European, Asian, Mongolian and North American genotypes (Nakao et al. Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009; Ito et al. Reference Ito, Agvaandaram, Bat-Ochir, Chuluunbaatar, Gonchigsenghe, Yanagida, Sako, Myadagsuren, Dorjsuren, Nakaya, Nakao, Ishikawa, Davaajav and Dulmaa2010). Among them, only one human AE case has been confirmed as the North American genotype until now (Yamasaki et al. Reference Yamasaki, Nakao, Nakaya, Schantz and Ito2008). To evaluate the pathogenicity of the genotypes to humans, extensive sampling of E. multilocularis from both humans and animals in Russia is essential.
The present study aimed to identify the species and genotypes of Echinococcus spp. from humans and wild/domestic animals in Russia based on comparison of mtDNA sequences. For this purpose, results of our previous reports (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a, Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob; Nakao et al. Reference Nakao, Yanagida, Konyaev, Lavikainen, Odnokurtsev, Zaikov and Ito2013b) were combined with this study to overview the species and genotype compositions. Consequently we analysed 75 parasite samples from 14 host species include humans collected including human from a wide geographic range. The distribution, lifecycle and public health importance of each parasite species and genotype were discussed.
MATERIALS AND METHODS
From 2010 to 2012, both adult and larval stages of Echinococcus spp. were collected from various host species in Russia (Table 1). Adult worms were obtained from wolves (Canis lupus), red foxes (Vulpes vulpes) and arctic foxes (Vulpes lagopus). Metacestode larvae were collected from humans, a domestic cat, sheep (Ovis aries), elk (Alces alces), reindeer (Rangifer tarandus), a captive Senegal bushbaby (Galago senegalensis) and arvicoline rodents (Microtus gregalis, Microtus oeconomus, Myodes rufocanus, Alticola strelzowi and Alticola olchonensis). The detailed information of most of the human cases in the Altai region (Altai Krai and the Altai Republic) and the CE case of domestic cat in Saint Petersburg have been published previously (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a, Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob).
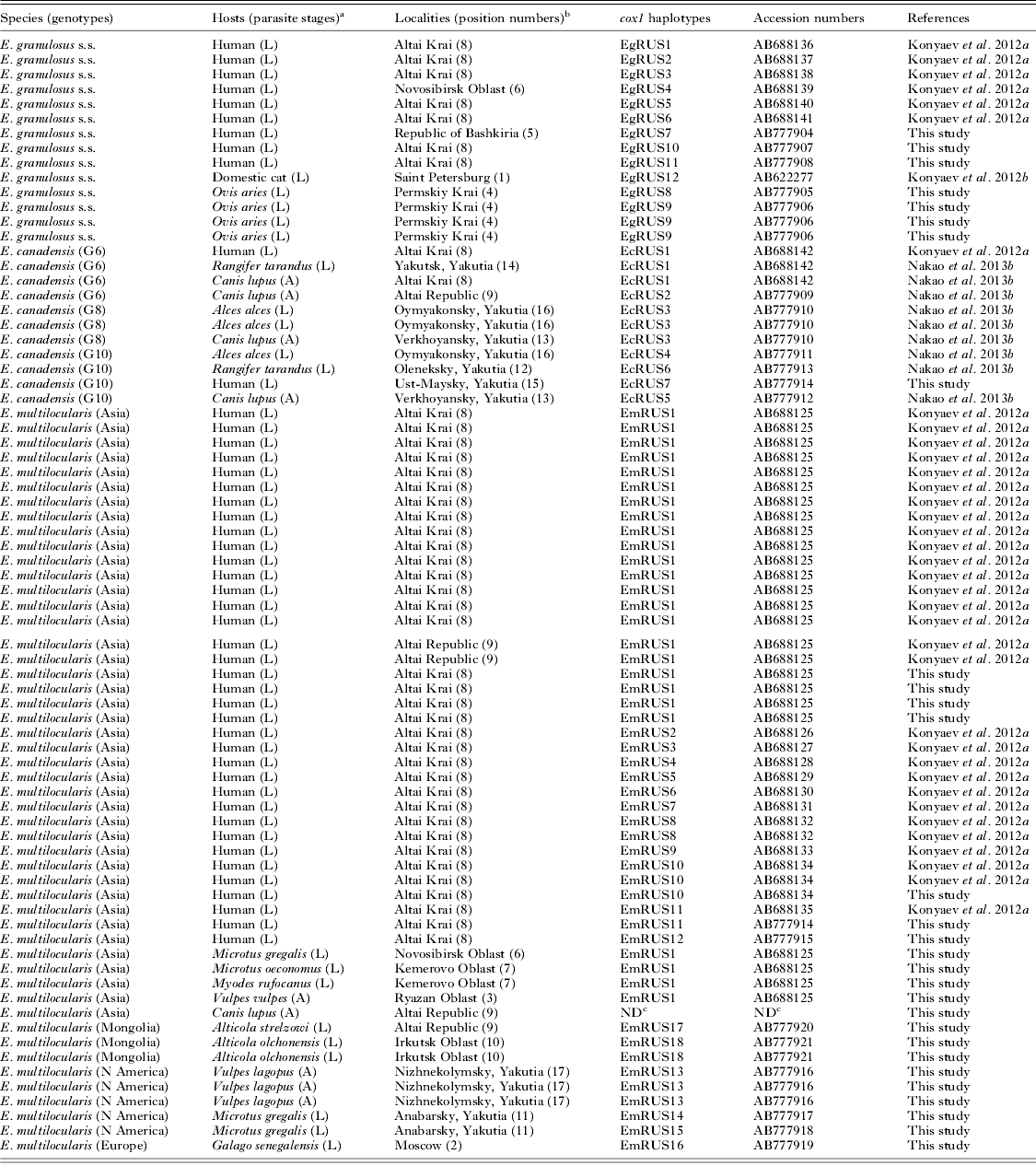
Table 1. Russian isolates of Echinococcus spp. used in this study.

a L, larva; A, adult.
b Position numbers are shown in Fig. 1.
c Cox1 haplotype was not determined because of the incomplete sequence, and the sequence was not deposited to the GenBank database.
The genomic DNA of each parasite isolate was extracted from the ethanol-fixed adult worms and metacestodes by DNeasy blood and tissue kit (Qiagen), and subsequently used as templates for polymerase chain reaction (PCR). Only one adult worm or an isolated metacestode from each host animal was used for the analysis. For a mtDNA genetic marker, the entire cytochrome c oxidase subunit I (cox1) was amplified by PCR as reported previously (Hüttner et al. Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008). The PCR products obtained were treated with illustra ExoStar (GE Healthcare) to remove excess primers and dNTPs, and directly sequenced with a BigDye™ Terminator v3.1 and a 3500 DNA sequencer (Life Technologies). The cox1 sequences of Russian Echinococcus spp. published in our previous reports (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a, Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob; Nakao et al. Reference Nakao, Yanagida, Konyaev, Lavikainen, Odnokurtsev, Zaikov and Ito2013b), were also added to the following analysis.
By using ClustalW 2.0 (Larkin et al. Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007), sequences of each cox1 haplotype in Russian Echinococcus spp. were aligned with representative cox1 sequences of Echinococcus spp. available in the GenBank database. A phylogenetic tree was constructed by neighbour-joining (NJ) method with Kimura's two-parameter model (Kimura, Reference Kimura1980), using the integrated software PAUP 4.0b10 (Swofford, Reference Swofford2002). The robustness of the phylogenetic tree was tested by bootstrapping with 1000 replicates. In the tree construction, Versteria mustelae was used as an outgroup because the species is sister to Echinococcus (Knapp et al. Reference Knapp, Nakao, Yanagida, Okamoto, Saarma, Lavikainen and Ito2011; Nakao et al. Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013a). To evaluate the relationship among E. multilocularis genotypes, a haplotype network was drawn by TCS 1.2 software (Clement et al. Reference Clement, Posada and Crandall2000) using statistical parsimony (Templeton et al. Reference Templeton, Crandall and Sing1992).
RESULTS
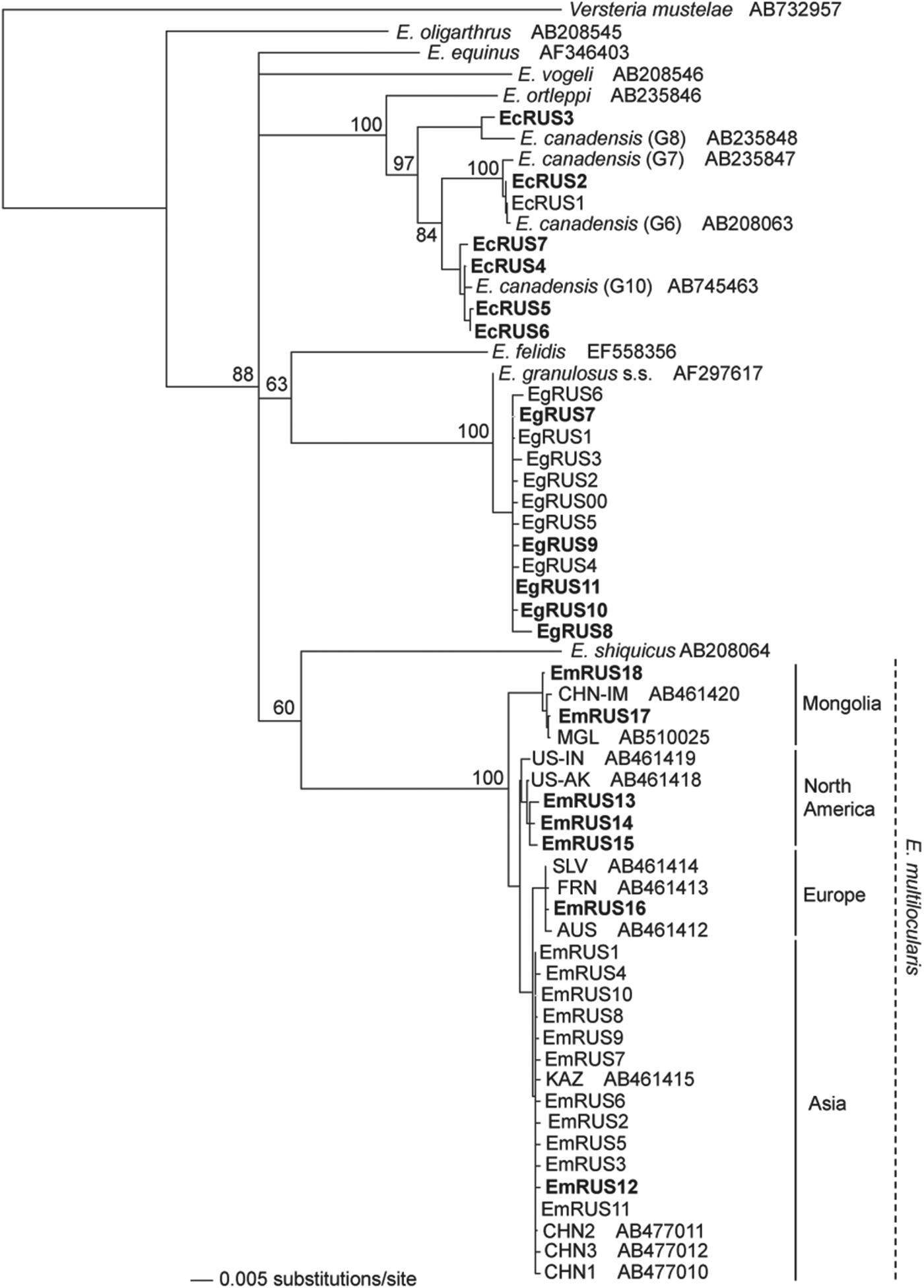
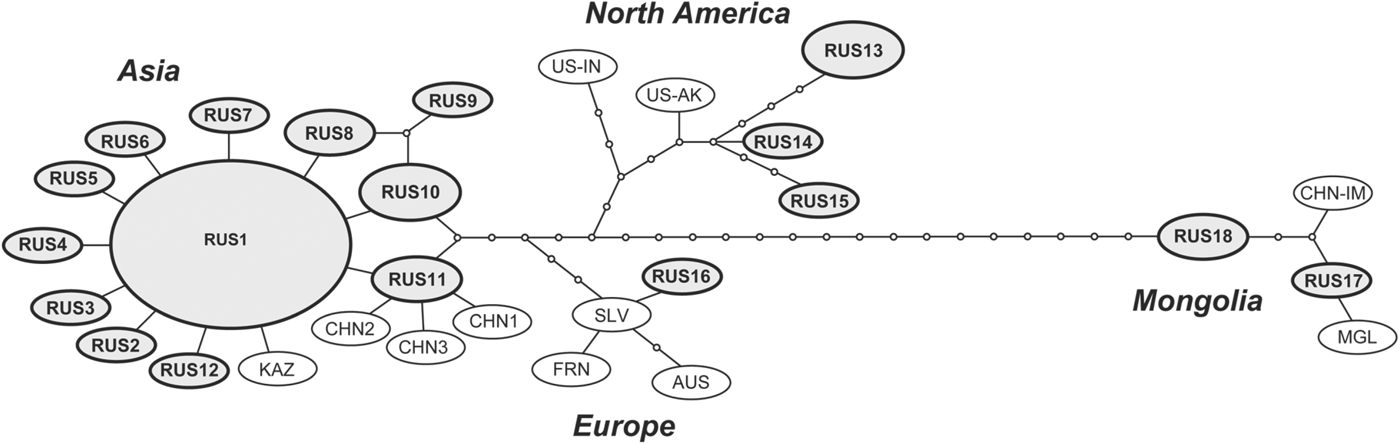
In the present study, 29 isolates of Echinococcus spp. were collected from various host species. The information on the hosts and localities of each isolate is shown in the Table 1 and Fig. 1. Nucleotide sequences of mitochondrial cox1 gene (1608–1609 bp) were determined for 4 adult worms and 24 metacestodes, and consequently 12 haplotypes were obtained. Only a partial (1053 bp) sequence was determined for an adult worm from a wolf in the Altai Republic. Together with the previous studies (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a, Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob; Nakao et al. Reference Nakao, Yanagida, Konyaev, Lavikainen, Odnokurtsev, Zaikov and Ito2013b), a total of 75 isolates of Echinococcus spp. including 37 cox1 haplotypes could be analysed. Three species of E. granulosus s.s., E. canadensis (G6, G8 and G10) and E. multilocularis were found in these isolates (Fig. 2), and the Russian isolates of E. multilocularis were further separated into four genotypes (Figs. 2 and 3).

Fig. 1. Spatial distribution of Echinococcus spp. in Russia. Localities are shown in serial numbers as follows: 1, Saint Petersburg; 2, Moscow; 3, Ryazan Oblast; 4, Permskiy Krai; 5, Republic of Bashkiria; 6, Novosibirsk Oblast; 7, Kemerovo Oblast; 8, Altai Krai; 9, Altai Republic; 10, Irkutsk Oblast; 11, Anabarsky District; Yakutia, 12; Oleneksky District, Yakutia; 13, Verkhoyansky District, Yakutia; 14, Yakutsk, Yakutia; 15, Ust-Maysky District, Yakutia; 16, Oymyakonsky District, Yakutia; 17, Nizhnekolymsky District, Yakutia.

Fig. 2. A neighbour-joining tree of Echinococcus spp. constructed from the nucleotide sequences of mitochondrial cox1 gene. Numbers on the nodes are bootstrap values. The names of the haplotypes obtained in the present study are shown in bold. CHN-IM, Chinese Inner Mongolia; MGL, Mongolia; US-AK, Alaska (St Lawrence Island); US-IN, Indiana; US-SD, South Dakota; SLV, Slovakia; AUS, Austria; FRN, France; KAZ, Kazakhstan; CHN, China (Sichuan).

Fig. 3. The parsimonious network of cox1 haplotypes of E. multilocularis. The haplotypes obtained in Russia are shown in circles with names in bold. CHN-IM, Chinese Inner Mongolia; MGL, Mongolia; US-AK, Alaska (St Lawrence Island); US-IN, Indiana; US-SD, South Dakota; SLV, Slovakia; AUS, Austria; FRN, France; KAZ, Kazakhstan; CHN, China (Sichuan).
As shown in Table 1, metacestodes of E. granulosus s.s. were confirmed from three human CE patients in Altai Krai and the Republic of Bashkiria and from four sheep in Permskiy Krai. Five cox1 haplotypes (EgRUS7-11) were confirmed among these seven isolates. A basic local alignment search tool (BLAST) revealed that the sequence of the haplotype EgRUS9 was the same as those of isolates from sheep in Iran (the database accession nos. JQ219962 and JQ250811), but the other haplotypes had no identical sequences in the GenBank database. Together with the previous studies (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a, Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob), a total of 12 cox1 haplotypes have been confirmed from 14 isolates of E. granulosus s.s. in Russia.
The distributional records of Russian isolates of E. canadensis have already been summarized (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a; Nakao et al. Reference Nakao, Yanagida, Konyaev, Lavikainen, Odnokurtsev, Zaikov and Ito2013b). The detailed information was as follows. The adult worms of E. canadensis G6 were collected from two wolves in Altai Krai and the Altai Republic. These G6 isolates were divided into two cox1 haplotypes (EcRUS1 and EcRUS2). The sympatric distribution of E. canadensis genotypes (G6, G8 and G10) was confirmed, especially in areas (Yakutsk and the Districts of Verkhoyansky, Ust-Maysky and Oymyakonsky) of Yakutia. The metacestode of G6 identified as the haplotype EcRUS1 was collected from a domestic reindeer in Yakutsk. Both adults and metacestodes of G8 were obtained from a wolf in Verkhoyansky District and two elk in Oymyakonsky District, respectively. Only one haplotype (EcRUS3) was found from the G8 isolates. The adult worms of G10 were obtained from a wolf in Verkhoyansky District, and the metacestodes were isolated from the lung and liver of an elk in Oymyakonsky District and a wild reindeer in Oleneksky District. G10 was also obtained from a CE patient in Oleneksky District. The cysts were removed from the lung of a young Yakutian boy. Four haplotypes (EcRUS4-7) were detected from these G10 isolates.
The adult worms of E. multilocularis Asian genotype were obtained from a red fox in Ryazan Oblast, southeast of Moscow. The partial (1053 bp) cox1 gene sequence of the adult worm from a wolf collected in the Altai republic was 100% identical to the Asian genotype (AB688125), but slightly different from the other genotypes. The metacestodes of the Asian genotype were found from seven human AE cases in Altai Krai, a narrow-headed vole (M. gregalis) in Novosibirsk Oblast and a root vole (M. oeconomus), and a grey red-backed vole (M. rufocanus) in Kemerovo Oblast. Although a total of 40 isolates of E. multilocularis Asian genotype have been obtained in Russia, 62·5% (25/40) of them had the same haplotype designated as EmRUS1 (Fig. 3). A BLAST search revealed this haplotype to be 100% identical to those from Japan (AB018440, AB385610 and AB461416). We detected E. multilocularis Mongolian genotype from a flat-headed vole (A. strelzowi) in Kosh-Agachsky District of Altai Republic and from two Lake Baikal mountain voles (A. olchonensis) in the Olkhon Island of Lake Baikal, Irkutsk Oblast. The metacestode of E. multilocularis European genotype was obtained from a captive Senegal bushbaby, small African primate belonging to the family Galagidae, reared in Moscow Zoo. In 2012, ten Senegal bushbabies died over short period in the zoo and were diagnosed as AE by morphological observation of histopathological specimens. One of the tissue specimens was subjected to molecular diagnosis and then identified as the European genotype. The adult worms of E. multilocularis North American genotype were found from three Arctic foxes in the Lower Kolyma, (Nizhnekolymsky District), northeast Yakutia. The metacestodes of the North American genotype were also found from narrow-headed voles around Yurung-Haya village in Anabarsky District, northwest Yakutia.
DISCUSSION
E. granulosus s.s
Seven isolates of E. granulosus s.s. were collected and five cox1 haplotypes were obtained in the present study. Together with the previous reports (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a, Reference Konyaev, Yanagida, Ivanov, Ruppel, Sako, Nakao and Itob), a total of 12 haplotypes have been found from 14 isolates in Russia. These haplotypes were geographically derived from European and Asian Russia. Other previous studies also suggest that E. granulosus s.s. is mainly distributed in western and central parts of Russia (Nikulina et al. Reference Nikulina, Garaev, Odoevskaya, Benediktov and Uspenskiy2003; Lukmanova et al. Reference Lukmanova, Tuigunov, Nartailakov, Bilalov and Gumerov2008). E. granulosus s.s. was originally recognized as the ‘sheep strain’ within E. granulosus s.l. and sheep farming strongly affects the distribution, although the involvement of cattle, goats and camels as intermediate hosts has also been confirmed (Cardona and Carmena, Reference Cardona and Carmena2013). In Yakutia, the sheep farming is almost non-existent because the weather and landscape are unsuitable. On the other hand, the indigenous people have a close contact with dogs which are often fed or scavenge on discarded offal of hunted or domestic cervids. Therefore, it is likely that most of the human CE cases are not caused by E. granulosus s.s. but by the ‘cervid strain’ of E. canadensis in Yakutia.
Recent phylogeographic studies have demonstrated the existence of a cosmopolitan cox1 haplotype, which is dominant in Europe, the Middle East (Iran and Jordan), China and Peru (Nakao et al. Reference Nakao, Li, Han, Ma, Xiao, Qiu, Wang, Yanagida, Mamuti, Wen, Moro, Giraudoux, Craig and Ito2010a; Casulli et al. Reference Casulli, Interisano, Sreter, Chitimia, Kirkova, La Rosa and Pozio2012; Yanagida et al. Reference Yanagida, Mohammadzadeh, Kamhawi, Nakao, Sadjjadi, Hijjawi, Abdel-Hafez, Sako, Okamoto and Ito2012), suggesting that E. granulosus s.s. has rapidly dispersed worldwide through the anthropogenic movement of domestic animals. The cosmopolitan haplotype corresponded to EgRUS1 in Russia from a human CE case in Altai Krai (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a). Russia has a large territory with the distribution of E. granulosus s.s., and is thus an important place to clarify the dispersal history of this cosmopolitan parasite. Further extensive epidemiological studies in the different part of Russia are needed.
E. canadensis
In Altai Krai, E. canadensis G6 has been found in a human CE patient (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a). The present study confirmed the natural distribution of G6 from a wolf in the Altai region. However, the larval stage was not yet confirmed from any domestic or wild animals in the region. Therefore, the infection cycle of E. canadensis G6 in the Altai region is still unknown. Although the G6 genotype has traditionally been considered as ‘camel strain’, less than 500 individuals of semi-free Bactrian camels (Camelus bactrianus) are distributed in the Altai region. Thus, it is unlikely that the G6 genotype is maintained solely by a wild wolf-camel life-cycle. Besides, the CE patient and the wolf infected with G6 were found in a region in which camels are absent (Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012a). Therefore, it is reasonable to assume the existence of the infection cycle not involving camels. Indeed, sheep, cattle and goats are also known to be susceptible to the G6 genotype (Omer et al. Reference Omer, Dinkel, Romig, Mackenstedt, Elnahas, Aradaib, Ahmed, Elmalik and Adam2010; Soriano et al. Reference Soriano, Pierangeli, Pianciola, Mazzeo, Lazzarini, Saiz, Kossman, Bergagna, Chartier and Basualdo2010; Hailemariam et al. Reference Hailemariam, Nakao, Menkir, Lavikainen, Yanagida, Okamoto and Ito2012). In addition, this genotype was found from a domestic reindeer in Yakutia. This is the first report of G6 infection in cervids. The present study indicates that the transmission cycle of E. canadensis G6 in Russia can be maintained by both domestic and wild animals.
The G8 and G10 genotypes of E. canadensis are considered as a ‘cervid strain’ (Bowles et al. Reference Bowles, Blair and McManus1994) and a ‘Fennoscandian cervid strain’ (Lavikainen et al. Reference Lavikainen, Lehtinen, Meri, Hirvelä-Koski and Meri2003), respectively. The sympatric distribution of these genotypes has been demonstrated in Estonia and North America (Thompson et al. Reference Thompson, Boxell, Ralston, Constantine, Hobbs, Shury and Olson2006; Moks et al. Reference Moks, Jogisalu, Valdmann and Saarma2008; Bryan et al. Reference Bryan, Darimont, Hill, Paquet, Thompson, Wagner and Smits2012; Schurer et al. Reference Schurer, Shury, Leighton and Jenkins2013). In this study, both G8 and G10 genotypes were collected from wolves and cervids in Yakutia. These results indicate that the two genotypes are sympatrically distributed through northern Eurasia and North America. Interestingly, E. canadensis G6 was also confirmed in Yakutia as mentioned above. The genotypes of E. canadensis have originally been recognized by the differences in their life-cycles and/or geographical distributions. However, sympatric distribution of different genotypes with the utilization of same definitive host species has been demonstrated. Furthermore, the recent phylogenetic studies have revealed that they are genetically closely related (Nakao et al. Reference Nakao, Yanagida, Konyaev, Lavikainen, Odnokurtsev, Zaikov and Ito2013b). Therefore, it is reasonable to assume that these genotypes can mate and produce hybrid offspring. A population genetic study in Yakutia where the three genotypes (G6, G8 and G10) are sympatrically distributed will shed light on the species status of E. canadensis. Instead of a maternally inherited mtDNA marker, the use of nuclear DNA markers is necessary to examine gene flow among the genotypes.
In this study, a human CE patient in Yakutia was demonstrated to be infected with E. canadensis G10. As far as we know, this is the second confirmed human case of the G10 infection after the first report from Mongolia (Jabbar et al. Reference Jabbar, Narankhajid, Nolan, Jex, Campbell and Gasser2011). In a Canadian indigenous community, 11% of people were serologically positive for E. granulosus s.l. infection and the eggs of E. canadensis G10 were detected in 6% of environmentally collected canine faeces (Himsworth et al. Reference Himsworth, Jenkins, Hill, Nsungu, Ndao, Thompson, Covacin, Ash, Wagner, McConnell, Leighton and Skinner2010). This result suggests that the human infection of G10 is not rare. As shown in the present study, the life-cycles of G8 and G10 are maintained in Yakutia by wild wolves and cervids (elk and reindeer), and the indigenous people having close contact with the wildlife have a risk of CE caused by E. canadensis. Another risk of CE is the raising of reindeer. Indigenous people often feed shepherd dogs with offal of slaughtered reindeer, and sleep with the dogs in the traditional tent house ‘chum’. In Nizhnekolymsky District, 2·3% of people had antibody against Echinococcus spp. (Martynenko et al. Reference Martynenko, Zorihina, Shcherbakov, Starkov and Suvorin1984). In Chukotka Autonomous Okrug located in the Russian Far East, 11·5% of hunters and 12·3% of herders were seropositive to Echinococcus spp. (Vol'fson, Reference Vol'fson1968). Wilson et al. (Reference Wilson, Diddams and Rausch1968) observed 101 CE cases of Alaskan indigenous people and reported the differences in pathogenicity between the European (‘pastoral’) and the Alaskan (‘sylvatic’) forms of E. granulosus s.l. While the liver CE was most frequent (48–78%) in Europe, Australia, South America and the Middle East, lung CE was most frequent (66%) in Alaska. Similarly, 40% of echinococcosis patients in the Chukotka Autonomous Okrug had hydatid cysts in the lungs (Boĭtsov et al. Reference Boĭtsov, Telushkin, Tumol'skaia and Iarotskiĭ1992). There was also a significant difference in the degree of symptoms; the ‘pastoral form’ was often (80–95%) symptomatic, while the ‘sylvatic form’ was rarely (6–8%) symptomatic (Wilson et al. Reference Wilson, Diddams and Rausch1968). In fact, most of the patients in Alaska were admitted to hospital for the examination or treatment of tuberculosis, and the parasite cysts were incidentally found by routine roentgenography. Although there is no direct evidence, it is likely that the ‘sylvatic form’ of E. granulosus s.l. in Alaska was E. canadensis G8 or G10. Based on this assumption, it is possible that the CE patients infected with E. canadensis are easily overlooked because of the lack of typical symptoms. Therefore, extensive epidemiological surveys using modern sensitive immunodiagnostic methods for the human infection with E. canadensis are necessary to better understand the current situation of CE in Russia, especially in Yakutia. Also, molecular identification of the causative agent of human CE is essential to evaluate the difference in pathogenicity among species and/or the genotypes.
E. multilocularis
In the present study, all four geographic genotypes of E. multilocularis were confirmed in Russia. The Asian genotype was obtained in the Altai region bordering with Asian countries. As the Asian genotype was also found in European Russia, this genotype is expected to be widely distributed in North Eurasia. A wolf collected in the Altai republic was infected with the Asian genotype. The infection of wolves with E. multilocularis Mongolian genotype has also been found in Mongolia (Ito et al. Reference Ito, Chuluunbaatar, Yanagida, Davaasuren, Sumiya, Asakawa, Ki, Nakaya, Davaajav, Dorjsuren, Nakao and Sako2013). These results imply the importance of wolves in the transmission ecology of E. multilocularis. Two Lake Baikal mountain voles infected with the Mongolian genotype, were captured in Olkhon Island in Lake Baikal. The vole is an endemic species inhabiting rocky steppes in Olkhon and several other islands in the lake. This is the first record of a helminth parasite from the Lake Baikal mountain vole whose biology is poorly understood. The infection cycle is assumed to be maintained with red foxes as the definitive host, because the traces of foxes were found near the colony of the voles and only red foxes inhabit in the islands of Lake Baikal. The foxes can migrate freely from the mainland to the islands and vice versa across the ice bridge in winter. Another isolate of the Mongolian genotype was found in the Altai Republic. The sympatric distribution of the Asian and Mongolian genotypes in the Altai Republic indicates possible gene flow between the two genotypes. The Mongolian genotype was once described as a new species of Echinococcus russicensis from corsac fox (Tang et al. Reference Tang, Cui, Qian, Kang, Wang, Peng, Lu and Chen2007) and later regarded as a synonym of E. multilocularis based on the comparative genetic studies (Nakao et al. Reference Nakao, Xiao, Okamoto, Yanagida, Sako and Ito2009, Reference Nakao, Yanagida, Okamoto, Knapp, Nkouawa, Sako and Ito2010b; Ito et al. Reference Ito, Agvaandaram, Bat-Ochir, Chuluunbaatar, Gonchigsenghe, Yanagida, Sako, Myadagsuren, Dorjsuren, Nakaya, Nakao, Ishikawa, Davaajav and Dulmaa2010). The human case caused by the Mongolian genotype has not been found yet in Russia, although its pathogenicity to humans was demonstrated in Mongolia (Ito et al. Reference Ito, Agvaandaram, Bat-Ochir, Chuluunbaatar, Gonchigsenghe, Yanagida, Sako, Myadagsuren, Dorjsuren, Nakaya, Nakao, Ishikawa, Davaajav and Dulmaa2010).
A Senegal bushbaby reared in Moscow Zoo was demonstrated to be infected with E. multilocularis European genotype. Until now, E. multilocularis infections have been reported in zoo's captive primates including the Old World monkeys, Macaca spp. (Brack et al. Reference Brack, Tackmann, Conraths and Rensing1997; Bacciarini et al. Reference Bacciarini, Gottstein, Pagan, Rehmann and Gröne2004; Sato et al. Reference Sato, Kawase, Yano, Nagano, Fujimoto, Kobayashi, Miyahara, Yamada, Sato and Kobayashi2005; Boufana et al. Reference Boufana, Stidworthy, Bell, Chantrey, Masters, Unwin, Wood, Lawrence, Potter, McGarry, Redrobe, Killick, Foster, Mitchell, Greenwood, Sako, Nakao, Ito, Wyatt, Lord and Craig2012), the gorilla, Gorilla gorilla (Kondo et al. Reference Kondo, Wada, Bando, Kosuge, Yagi and Oku1996; Rehmann et al. Reference Rehmann, Gröne, Lawrenz, Pagan, Gottstein and Bacciarini2003), the orangutan, Pongo pygmaeus (Taniyama et al. Reference Taniyama, Morimitsu, Fukumoto, Asakawa, Ohbayashi, Uchino and Sato1996) and the ring-tailed lemur, Lemur catta (Kondo et al. Reference Kondo, Wada, Bando, Kosuge, Yagi and Oku1996). In the present study, the infected bushbaby was reared in the zoo located in the centre of Moscow, and there were no stray dogs or wild foxes inside. All canids regularly had deworming twice in every year. Therefore, it is not likely that there were carrier animals as the infectious source of echinococcosis in the zoo. However, the infected bushbabies were born and bred in the zoo, and thus they must have become infected there. The most probable source of Echinococcus eggs was mulch used as ground cover in the cage. Mulch is made of different natural materials such as woods and straws that came from Yaroslavl Oblast and occasionally from Baltic countries (Latvia or Estonia). These Baltic countries are known to be endemic for E. multilocularis (Moks et al. Reference Moks, Saarma and Valdmann2005; Bagrade et al. Reference Bagrade, Šnábel, Romig, Ozoliņš, Hüttner, Miterpáková, Ševcová and Dubinský2009) and it is possible that the mulch was contaminated with eggs of the parasite.
In this study, the North American genotype was found in northeast and west Yakutia. The result indicates that this genotype has trans-Beringian or circumpolar distribution. Martynenko (Reference Martynenko1984) suggested that there were three different patterns of the transmission cycle of E. multilocularis in Yakutia; ‘Arctic fox-lemming’ cycle in tundra, ‘red fox-vole’ cycle in taiga and ‘dog-mouse/rat’ cycle in towns. Retrospective analyses of E. multilocularis infections in definitive hosts in Yakutia from 1961 to 2006 indicated that the prevalence could vary quite widely, but was always relatively high in Arctic foxes and low in the red fox (Gubanov, Reference Gubanov1964; Martynenko, Reference Martynenko1984; Odnokurtsev and Sedalishchev, Reference Odnokurtsev, Sedalishchev, Seryodkin and Miquelle2012). Although up to 100% of the foxes were infected in the Lower Kolyma region of Yakutia, only a single human AE case was recorded from 1972 to 1981 (Martynenko et al. Reference Martynenko, Zorihina, Shcherbakov, Starkov and Suvorin1984). Moreover, human AE has never been found in Taimyr and Yamal Peninsulas, where infected Arctic foxes were confirmed (Bessonov, Reference Bessonov2003). Interestingly, human AE cases are also very uncommon in North America (excepting Alaska), even though E. multilocularis is highly prevalent in rodents and canids (Rausch, Reference Rausch, Thompson and Lymbery1995; Storandt et al. Reference Storandt, Virchow, Dryden, Hygnstrom and Kazacos2002). One of the only two reported human AE cases in the USA was analyzed retrospectively and was demonstrated to be caused by the North American genotype of E. multilocularis (Yamasaki et al. Reference Yamasaki, Nakao, Nakaya, Schantz and Ito2008). To date, this is the only confirmed human AE case caused by this genotype. Based on these epidemiological observations, it is speculated that E. multilocularis North American genotype has a low infectivity to humans, and this might correspond to the non-pathogenic ‘Arctic fox strain’ postulated by Shakhmatova (Reference Shakhmatova and Maksimov1981). Further epidemiological surveys in Siberia and North America are needed to clarify the difference of pathogenicity among E. multilocularis genotypes.
CONCLUSIONS
The present molecular survey revealed the rich genetic diversity of Echinococcus spp. in Russia, using 75 isolates from 14 host species including humans. The species and genotype compositions were disclosed as follows: three species (E. granulosus s.s., E. canadensis and E. multilocularis), three genotypes of E. canadensis (G6, G8 and G10) and four genotypes of E. multilocularis. The nationwide survey also suggests that distributions of these taxa overlap in Europe, Asia and far eastern Russia, depending on their complicated ecosystems. The present distributional records of Echinococcus spp. and their genotypes will become the basis of public health strategy to control cystic and alveolar echinococcoses in Russia. This study demonstrated the importance of Russia in investigating the biological and clinical features of Echinococcus spp. Further extensive epidemiological surveys with molecular identification of the causative agents are needed to clarify the distribution, life cycle and pathogenicity of each species and genotype.
FINANCIAL SUPPORT
This study was supported by a RFBR research project (Nos.13-04-10140, 12-04-0018 and 12-04-31203) to S.K. and by Grants-in-Aid for Scientific Research (Nos. 21256003 and 24256002) from JSPS, JSPS-Asia/Africa Scientific Platform Fund (2006–2011) and the Special Coordination Fund for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT) (2010–2012) to A.I. Field surveys of rodents were conducted under the for Program fundamental researches of presidium RAN, Project 30.12.