Introduction
There are 16 mosquito species (Diptera: Culicidae) in New Zealand, of which 13 are endemic and three introduced (Belkin, Reference Belkin1968; Cane & Courtney, Reference Cane and Courtney2009). The endemic species feed primarily on avian hosts and most have minimal contact with human populations. Several species are found only in remote bush or swamp habitats and little is known of their behaviour and biology. Two of the three introduced species, Culex quinquefasciatus and Aedes notoscriptus, breed primarily in artificial containers and are usually found near sites of human habitation (Belkin, Reference Belkin1962, Reference Belkin1968; Laird, Reference Laird1995; Leisnham et al., Reference Leisnham, Lester, Slaney and Weinstein2006), while the third, Aedes australis, utilizes coastal rock pool habitats (Belkin, Reference Belkin1962, Reference Belkin1968).
The size and distribution of mosquito populations, particularly those species whose larvae develop in natural water bodies, can be strongly affected by environmental factors, such as rainfall and temperature (Ahumada et al., Reference Ahumada, Lapointe and Samuel2004; Carver et al., Reference Carver, Goater, Allen, Rowbottom, Fearnley and Weinstein2011; Beck-Johnson et al., Reference Beck-Johnson, Nelson, Paaijmans, Read, Thomas and Bjornstad2013). Mosquito populations require water bodies that are sufficiently stable to allow the immature stages to develop from egg to adult; rainfall and evaporation rates influence the availability of such water bodies (Chase & Knight, Reference Chase and Knight2003). Temperature can affect how quickly mosquito development occurs, with some species able to complete their life cycle in only a couple of weeks during warmer weather, but taking several months at other times of the year (Service, Reference Service, Lane and Crosskey1993b; Alto & Juliano, Reference Alto and Juliano2001).
Following the detection of the Australian Southern Saltmarsh mosquito Aedes camptorhynchus, an aggressive day-biting mosquito and a known vector of a number of mosquito-borne diseases, in several locations around New Zealand, programmes were established to eradicate this exotic pest (Kay & Russell, Reference Kay and Russell2013). The Kaipara Harbour (fig. 1), the largest harbour in the southern hemisphere, was one area infested by this pest mosquito species. In 2002, a programme was initiated by the Ministry of Health with the goal of eradicating Ae. camptorhynchus from the harbour. Monthly treatments of the juvenile growth hormone S-methoprene targeting the immature populations of Ae. camptorhynchus were carried out over the inundated portions (typically 40–50%) of 2700 ha of harbour saltmarsh habitat during the treatment phase of the eradication programme, September 2002 to June 2004. A small area (160 ha) of follow-up treatment continued from December 2005 to June 2006 at the southern end of the harbour until the eradication was deemed successful (Kay & Russell, Reference Kay and Russell2013). One component of the programme was an extensive network of light traps deployed throughout the harbour over this period. Sentinel traps were placed within and beyond the known limits of the pest mosquito population (Kay & Russell, Reference Kay and Russell2013). Mosquito specimens caught in the traps were identified to species (or occasionally genus for Coquillettidia spp.), and a large quantity of data on the occurrence, abundance and seasonality of several native and introduced mosquito species within the harbour were collected.

Fig. 1. Map of the Kaipara Harbour located in the north of the North Island of New Zealand. Climate data were recorded at meteorological stations in Dargaville and Warkworth. Land cover information derived from the New Zealand Land Cover Database v4.1, Landcare Research NZ Ltd (LRIS portal, 2017).
This paper examines the spatial and temporal trends of six New Zealand mosquito taxa obtained from 42 months of sampling in the Kaipara Harbour area. Collections of this intensity and extent, both spatially and temporally, are rarely analysed. We use these data to investigate patterns of regional and seasonal abundance, community structure and to test the relationship between population dynamics and environmental influences of temperature and precipitation.
Methods
Field collections
All mosquitoes were collected in carbon dioxide- and octenol-baited light traps. Light traps were supplied by Southern Monitoring Service – New Zealand Biosecure (SMS-NZB), made in-house following a design similar to light traps produced by the Centre for Disease Control and Prevention (CDC), USA. Carbon dioxide was provided from a cylinder at a flow rate of 350 ml min−1 and then mixed with octenol (1-octen-3-ol) from a sealed tube with a small wick slowly releasing the chemical. These traps target host-seeking adult females by mimicking hosts (Service, Reference Service1993a). The carbon dioxide and octenol simulates the breath of mammals, attracting those female mosquitoes in search of a blood meal. Aedes camptorhynchus is an aggressive day biter of predominantly mammals, prompting the use of octenol, which has been shown to be a successful attractant of host-seeking females, both day and night (Miller et al., Reference Miller, Wing, Cope, Davey and Kline2005).
The traps were located around the perimeter of the Kaipara Harbour within or close to saltmarsh habitats or where Ae. camptorhynchus had been found. Figure 1 shows the extent of saline vegetation and saltmarsh habitat (which includes mangroves) fringing the harbour. Pasture and plantation forestry were the predominant land uses inland, while residential houses were few and far between. Traps were run continuously for 20–42 months (depending on location) and were serviced every 3–4 nights. These traps are typically lethal to mosquitoes; however, other insects often remain alive making sorting difficult; therefore, each collection was frozen overnight before processing the following day. The mosquito specimens were separated out, transferred to sample tubes and sent to the New Zealand BioSecure Entomology Laboratory (NZBEL) for identification. The specimens were identified to species using descriptions and taxonomic identification keys based on morphological characters (Belkin, Reference Belkin1962, Reference Belkin1968). The vast majority of specimens collected within the traps were females, as males seldom leave the breeding habitat; hence, no determination between the sexes was made for this analysis.
Data from 29 traps, all of which ran for a minimum period of 20 months, were selected for inclusion in this analysis. Sampling began at the end of the third week of January 2003, initially with 16 traps, which increased to 26 traps in February 2003. Over the period March 2003 to June 2005, between 26 and 29 traps were running at any one time. In June 2005, 12 traps situated in the northern half of the harbour were discontinued as Ae. camptorhynchus was pronounced eradicated from that area. The 14 remaining traps (all situated in the southern harbour) continued to run for an additional 12 months to the end of June 2006 and data from these are also included. In total, the dataset represents collections from more than 25,000 trap nights.
Data on Ae. camptorhynchus were not included in this analysis as the number of adults quickly dwindled to very low levels due to the targeted eradication treatments, which would have severely affected patterns of distribution and abundance.
Information on meteorological conditions for the Kaipara region (maximum and minimum daily temperature and daily precipitation) were obtained from the two nearest meteorological stations, Warkworth and Dargaville (fig. 1), downloaded from the NIWA National Climate Database (http://cliflo.niwa.co.nz/). Weekly summaries were calculated as follows: (1) mean temperature, as an average of the daily minima and maxima recorded across the two meteorological stations; and (2) rainfall values, accumulated for the week and averaged across the two stations. Data for Dargaville were not available for the period 1 January to 30 October 2003; hence, only Warkworth data were used for this initial period.
Data analyses
The number of specimens of each species was divided by the number of nights since the trap had last been serviced, to obtain a mean count per trap night for that 3–4 days period. Usually there were two collections per week, and these were averaged to obtain a mean count per trap night for the week. The weekly averages were further averaged across all active trap sites to provide a regional catch for the week. These data formed the basis of our 3½-year weekly time series of adult abundance, expressed in units of adults per trap night. A monthly average was also calculated across all trap catches made in each calendar month to show the typical annual trend in adult interception. Weekly averages for temperature and rainfall were calculated in the same manner described above.
Interspecific correlations in trap-level abundance were performed using Spearman's rank correlation. As the catches of traps close to each other may not be fully independent of each other, we did not calculate probability values for the test (Legendre, Reference Legendre1993). Cross-correlations between weekly mosquito catches and preceding meteorological conditions were investigated with time lags from 0 to 20 weeks. All analyses were performed in the R statistical and computing package R2.12.1; cross-correlations were performed with the ‘ccf’ function of the ‘stats’ base package (R Development Core Team, 2010).
Results
A total of 359,095 adult mosquitoes (excluding Ae. camptorhynchus) were trapped and identified over the 42-month period. There were seven species recorded; five native – Aedes antipodeus, Coquillettidia iracunda, Coquillettidia tenuipalpus, Culex pervigilans and Culiseta tonnoiri and two introduced: Ae. notoscriptus and Cx. quinquefasciatus. For the purposes of the eradication programme, the very rare Cq. tenuipalpus (<1% of the Coquillettidia adult specimens collected) was not routinely distinguished from the considerably more commonly occurring Cq. iracunda, so the data have been presented as Coquillettidia spp. and the trends interpreted as applying to Cq. iracunda.
The majority of individuals from all species trapped (~80% of the catch) were Cq. iracunda, while Ae. notoscriptus at 0.6% of the catch was the rarest species in our sample (table 1).
Table 1. Total number of individual mosquitoes trapped (in order of decreasing abundance), number of positive trap locations and characteristic larval habitat for each species (after Belkin, Reference Belkin1968).

* = non-indigenous species.
1 Cane & Disbury, unpublished data, 2007.
2 NZ BioSecure, unpublished data, 2002.
Most of the species were widely distributed across the Kaipara Harbour (table 1); however, their relative abundance between sites was highly variable (fig. 2). The majority of the large numbers of Coquillettidia spp. were obtained from only four sites, although it was present across all locations. Aedes notoscriptus and Cx. quinquefasciatus were collected in relatively low numbers but were nonetheless detected at all (or all but one, respectively) of the trap sites. Culiseta tonnoiri was found at low numbers from only one-third of the locations. Aedes antipodeus, Coquillettidia spp. and Cs. tonnoiri appear to have similar spatial patterns of distribution and abundance, as do Cx. quinquefasciatus and Cx. pervigilans, as indicated by correlation coefficients >0.4 (fig. 2 and table 2).
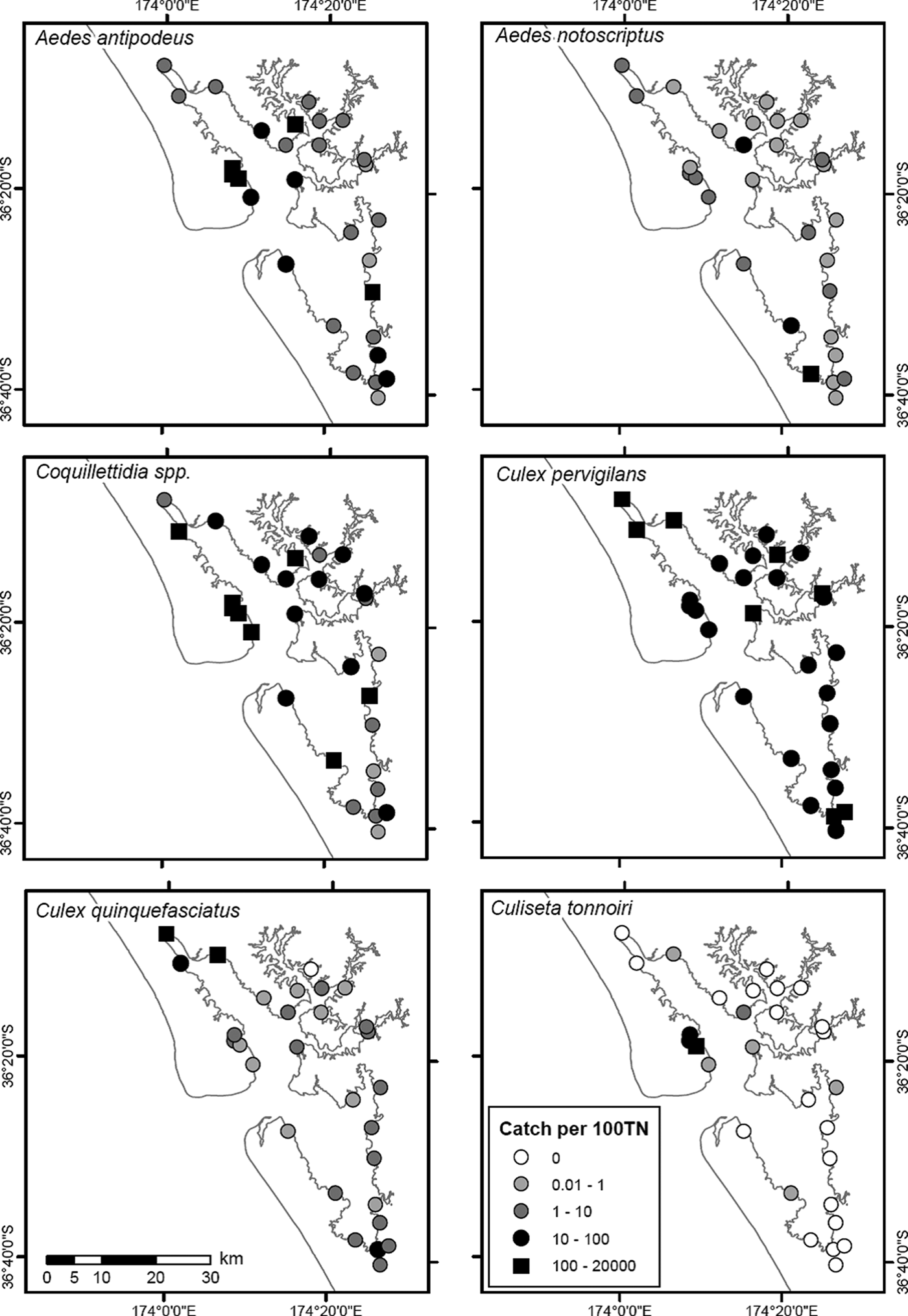
Fig. 2. Distribution of light traps and catches of the six species. Catches expressed as number of adults per 100 trap nights (TN), averaged over the 3½-year study period.
Table 2. Spearman rank correlations between species’ site-level abundances (mean adult catch per 100 trap nights, n = 29 sites).

Table structure and values in bold (r > 0.4) emphasize the three-way similarity in distribution between Ae. antipodeus, Coquillettidia spp. and Cs. tonnoiri, and the positive correlation between Cx. quinquefasciatus and Cx. pervigilans.
Five of the six species manifest strong annual cycles in abundance. Aedes antipodeus appears to have two peaks in abundance each year, occurring during spring and autumn, although the autumn peak was absent in 2005 (fig. 3). Coquillettidia spp., Ae. notoscriptus and Cx. quinquefasciatus show sizeable peaks in abundance during warmer months, from early to late summer, respectively, but are largely absent during winter months. Culex pervigilans tends to be present throughout the year, but is more abundant from spring to autumn (fig. 3).

Fig. 3. Seasonal trends in catches averaged across all light traps. Left = time series of weekly averages for the 3½-year period of sampling. Right = monthly averages. Catches are expressed as number of adults per trap night (TN) plotted on a logarithmic scale (left) and a linear scale (right). Note the change in limits of the y-axes between species (rows).
As may be anticipated from inspection of the seasonal trends (fig. 3 and online Supplementary material S1), there were strong positive correlations between (preceding) temperature and abundance of four species: Ae. notoscriptus, Cq. iracunda, Cx. pervigilans and Cx. quinquefasciatus, and negative associations between (preceding) temperature and abundance of the other two species: Ae. antipodeus and Cs. tonnoiri. The strongest relationships were between abundance of Ae. notoscriptus and mean temperature 4 weeks earlier and between Cq. iracunda and mean temperature 2 weeks earlier (table 3 and fig. 4). Relationships between abundance and rainfall were much weaker; the strongest result being a positive association between Cx. quinquefasciatus, and rainfall 5 weeks prior (table 3 and fig. 4). Significance values for these relationships must be interpreted with caution, since the temporal autocorrelation in both variables violates the assumption of independent sample units (Lennon, Reference Lennon2000).
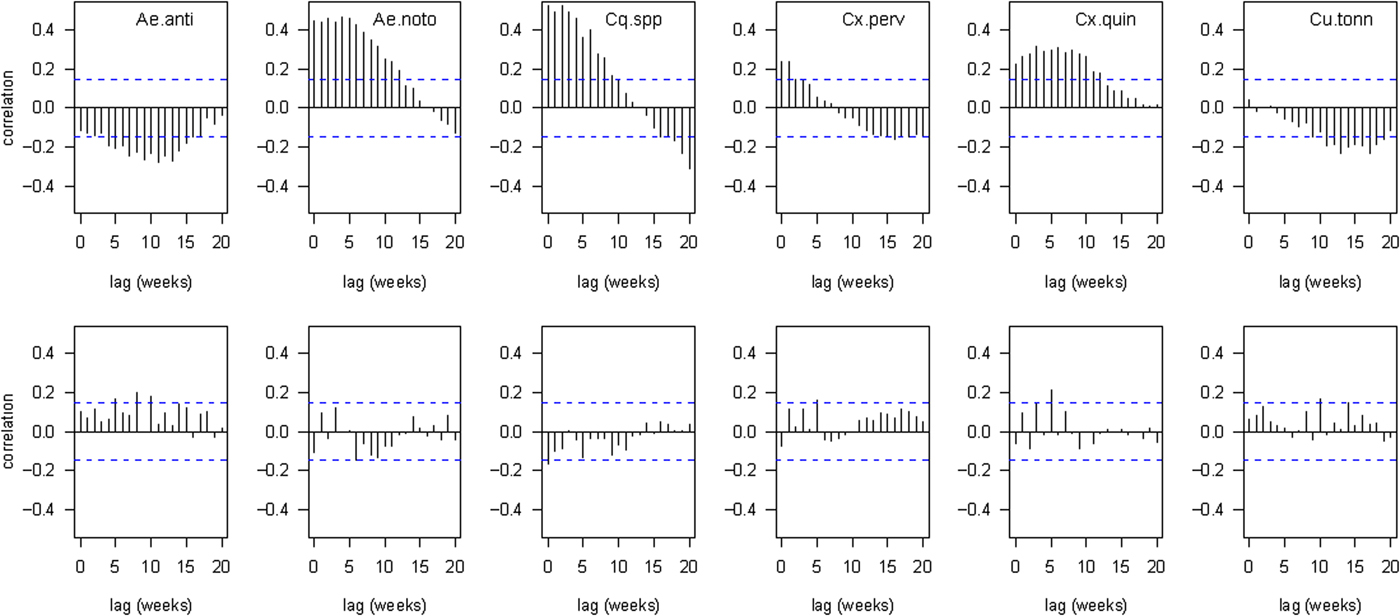
Fig. 4. Cross-correlations between adult abundance at traps and environmental conditions recorded k weeks earlier (x-axis indicates the value of k). Top = correlations with preceding temperature. Bottom = correlations with preceding rainfall. Dotted line indicates a significant correlation (P < 0.05) assuming no autocorrelation in the variables. Aedes notoscriptus, Coquillettidia spp. Culex pervigilans and Culex quinquefasciatus all show positive correlations between abundance and temperature during the preceding few weeks, whereas Aedes antipodeus and Culiseta tonnoiri exhibit a peak in abundance approximately 10 weeks after low temperatures. Correlations with rainfall were generally weak and inconsistent.
Table 3. Maximum correlations (r) between adult abundance and climatic variables recorded k weeks previously, where the value of k is the time lag.

Length of the time series: n = 183 weeks, degrees of freedom = n-k-2, * = nominal P-value < 0.01.
In summary, the spatial distribution at a regional scale, local abundance and temporal variability of each species can be categorized according to a spatio-temporal analogue of Rabinowitz's (Reference Rabinowitz and Synge1981) seven forms of rarity, a 2 × 2 × 2 classification (table 4). The most abundant and widespread taxon, Coquillettidia spp. is the most highly seasonal, while the regionally restricted and locally rare Cs. tonnoiri is most consistent in its year-round occurrence. Both introduced species, Ae. notoscriptus and Cx. quinquefasciatus, can be categorized as regionally widespread, highly seasonal and of low numerical abundance in the area sampled.
Table 4. Summary of the spatial and temporal distribution patterns of the six mosquito taxa.

* = non-indigenous species.
Discussion
Mosquito communities can be highly variable in space and time, and dynamics in mosquito populations can be driven by both direct and indirect, biotic and abiotic influences (Chase & Knight, Reference Chase and Knight2003; Beck-Johnson et al., Reference Beck-Johnson, Nelson, Paaijmans, Read, Thomas and Bjornstad2013). Here, we investigated the spatial and temporal distribution patterns of six mosquito taxa resident around agricultural and salt marsh habitats of Kaipara Harbour, New Zealand. Our sampling was spatially extensive (across 29 sites), but in particular, was temporally intensive: involving bi-weekly collections sustained for 3½ years. As a consequence, the patterns observed in temporal variability are among the most robust of any reported thus far for any region in New Zealand.
Abundance and distribution
All species were widespread throughout the Kaipara Harbour area apart from Cs. tonnoiri, which was very restricted in range. This may be expected as this species usually occurs in native forest habitat (Pillai, Reference Pillai1968) and not within the saltmarsh environment that exists throughout much of the harbour environs. Some national surveys of New Zealand have reported Cx. pervigilans to be the most abundant species (Belkin, Reference Belkin1968; Laird, Reference Laird1990, Reference Laird1995); however, these surveys sampled larvae from a range of breeding habitats and it is possible that their results were more reflective of the range and frequency of micro-sites sampled, and/or octenol-baited light traps are not as sensitive for detecting this species, relative to other species. Another possibility is that the treatments with S-methoprene may have affected a proportion of the populations of Cx. pervigilans and Ae. antipodeus, as larvae of both species develop in, but are not limited to, saltmarsh habitats. The treatments were carried out monthly, and therefore while possibly reducing the overall abundance of these species at particular sites, the seasonal trends should remain largely unaffected. No effect is anticipated on the other species since their larvae develop outside of the saltmarsh habitat, for example, in native bush or container habitats.
Coquillettidia iracunda adults were present in light trap collections throughout summer with an abundance far exceeding the other species trapped. Graham (Reference Graham1939) noted that adults of this species often occur in vast swarms. Belkin (Reference Belkin1968) suggested that this species may have a considerable flight range with adults found in offshore locations. This could explain why collections of this species were made throughout the Kaipara at all locations, despite the specific breeding habitat requirement for freshwater swamps.
It is interesting that the two introduced species present within the Kaipara Harbour occur in very low numbers compared with endemic species from within the same genera (Cx. quinquefasciatus vs. Cx. pervigilans ~1:14 and Ae. notoscriptus vs. Ae. antipodeus ~1:13). This may be readily explained by the reliance of these two introduced species on containers for larval development, as opposed to the ubiquitous natural habitats of the two endemic species (Laird, Reference Laird1995). It is reasonable to expect that there would not be a high proportion of containers (artificial or natural) within the saltmarsh environment, an area largely devoid of trees and not suitable for situating buildings; and any adults present had presumably flown in from neighbouring farms or towns where suitable habitat exists.
The presence of the two introduced species in nearly all trapping locations (Cx. quinquefasciatus absent from one), despite their low overall abundance, supports the suggestion that the adults of these two species have a high dispersal capacity, flying in from neighbouring areas outside of the saltmarsh where suitable breeding habitats exist (see also Schreiber et al., Reference Schreiber, Mulla, Chaney and Dhillon1998; Lapointe, Reference Lapointe2008). In recent decades, both species have increased their range southwards across New Zealand (Laird, Reference Laird1995).
Seasonal trends
As well as being the most abundant taxon in our sampling, Cq. iracunda also showed the most pronounced seasonality, with adult densities peaking in January, and being virtually absent from May to November. These observations are similar to data on the Australian species Coquillettidia linealis, which has also been observed to peak dramatically in abundance at certain times of year (Johnson, Reference Johnson2006). It appears that this may be a characteristic of the genus.
We did not routinely distinguish between the two Coquillettidia species (Cq. iracunda and Cq. tenuipalpus) in our samples as they were not the primary focus of the surveillance programme. Graham (Reference Graham1939) has reported two records of egg-laying females of Cq. tenuipalpus during January and May at two locations, suggesting that this species may have a similar seasonal prevalence as Cq. iracunda, although at significantly reduced abundance (NZ BioSecure, unpublished data 2006).
On a week-to-week basis, Coquillettidia sp. and Ae. notoscriptus showed the strongest positive correlations in adult abundance with temperature measured 2–4 weeks prior. Development of Ae. notoscriptus has been described as ongoing throughout the year in temperate environments with peaks in numbers during wetter and warmer months (Liehne, Reference Liehne1991; New Zealand BioSecure, unpublished data 2006). Graham (Reference Graham1939) reported this species to pass winter months in both adult and larval stages. Our survey did not record adults during winter months. One possible explanation for this inconsistency is the different types of environment examined: Graham (Reference Graham1939) included samples from forested areas, where this species is naturally more abundant, utilizing natural containers such as tree holes. As these sites dry up, it is possible that during summer months, adult females need to fly further to find breeding habitats containing water and are therefore more commonly encountered in trap catches.
Aedes antipodeus adults have been reported as being active year round (Marks & Nye, Reference Marks and Nye1963; Belkin, Reference Belkin1968), with breeding occurring mainly in the cooler months of March to October (Lee et al., Reference Lee, Hicks, Griffiths, Russell and Marks1984), hence the common name ‘winter mosquito’ given to this species. Our results, however, show the Ae. antipodeus adult population with two peaks per year in spring and autumn, apart from 2005 when there was no autumn peak. This can probably be explained by the dry spell that occurred from March to mid-May 2005, as the flooded surface pools in which the larvae of this species develop, may have dried out too quickly and not supported the autumn population through to emergence. This suggestion is supported by the correlation between this species’ abundance and high rainfall 6–10 weeks prior. This could also explain the apparent inhibition of this species to breed during the summer, as the ephemeral pools are less likely to be flooded at this time.
Adult activity of Cx. quinquefasciatus in New Zealand has been reported to cease during the cooler months of July to September (Lee et al., Reference Lee, Hicks, Debenham, Griffiths, Marks, Bryan and Russell1989; Weinstein et al., Reference Weinstein, Laird and Browne1997). Our sampling did not collect any adults between July and October, and numbers were relatively low even during their peak in late summer (April). Temperature is most likely a limiting factor as this species has been found to overwinter as quiescing adults in underground habitats, such as storm drains (Eldridge, Reference Eldridge1968). In Hawaii, Cx. quinquefasciatus has been recorded as present year round at warmer, low-elevation sites (mean annual temperature of 23°C), while highly seasonal at high-elevation sites with a mean annual temperature of 13° (Ahumada et al., Reference Ahumada, Lapointe and Samuel2004). The population dynamics modelled for these high-elevation Hawaiian sites appear very similar to the empirically observed dynamics observed here around the Kaipara Harbour where mean annual temperatures were approximately 14°C. If mean annual temperatures in Kaipara were to rise a further 1° or 2°, the models of Ahumada et al. (Reference Ahumada, Lapointe and Samuel2004) suggest that Cx. quinquefasciatus could increase in abundance and become present throughout the year. As Cx. quinquefasciatus is a competent vector of avian malaria, such a scenario may have important consequences for local wildlife (Tompkins & Gleeson, Reference Tompkins and Gleeson2006).
The two species with the least pronounced seasonality were Cx. pervigilans and Cs. tonnoiri. Miller & Phillipps (Reference Miller and Phillipps1952) reported Cx. pervigilans adult activity levels at their peak in the spring to late summer months and larvae surviving over winter, but with no development taking place. This is consistent with our data, which showed adults of this species to be present year round, but with increased abundance during the spring-early autumn period. In a year-long study in Wellington, New Zealand, Lester & Pike (Reference Lester and Pike2003) recorded larval densities of Cx. pervigilans close to zero between the months of July and September. Culex pervigilans is another species implicated in the spread of avian malaria and therefore a greater understanding of the factors affecting its abundance is of considerable interest to wildlife managers (Niebuhr et al., Reference Niebuhr, Poulin and Tompkins2016).
Dumbleton (Reference Dumbleton1965) noted Cs. tonnoiri adult females were most abundant in January and February, breeding in the summer and appearing to overwinter as gravid females. Pillai (Reference Pillai1968) recorded small numbers of adults active and feeding throughout the year, including during winter. Our results showed adults to be present throughout the year, although most abundant in the warmer months, the highest peaks ranged from October to February and varied from year to year. These two species also displayed the weakest correlations with temperature and no relationship with preceding rainfall.
Conclusion
The data we have presented are the result of some of the most extensive and sustained sampling efforts of adult mosquitoes for any region in New Zealand. We recorded seven species of mosquito (in addition to the eradication target Ae. camptorhynchus) using carbon dioxide- and octenol-baited light traps. Catches at individual sampling sites varied from one individual of a species in over 1000 trap nights, to over 20,000 of the same species over 100 trap nights. Most species were widespread across the region. Introduced species were generally much less common than their native congeners. Four of the species were highly seasonal in their occurrence, with peaks in abundance between spring and late summer. These differences in spatial and temporal distribution, as well as differences in the type of breeding habitat employed, suggest a variety of niche differences between existing species, and the potential for further underutilized niches. New Zealand has a relatively depauperate mosquito fauna (16 as opposed to 33 in the UK), and it is plausible that a lack of biotic resistance may make the country vulnerable to future introductions (Juliano & Lounibos, Reference Juliano and Lounibos2005). Further work on the effects of climatic variables such as rainfall, temperature and tidal height (for saltmarsh mosquito species) would greatly improve our understanding of the biology and ecological requirements of New Zealand's endemic native fauna and the potential for competition with introduced species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485317000736.
Acknowledgements
The authors would like to thank the Ministry of Health who funded the Kaipara southern saltmarsh mosquito eradication programme from 2002 to 2006 and permitted us to use their data. The authors thank Shaun Maclaren for his part in managing the field operations and data provision, Mark Disbury for assistance with data management and the large number of SMS New Zealand BioSecure field workers and laboratory employees who worked on this programme. The authors also thank John Haywood, Catherine Duthie and David Saville for comments on an earlier version of the manuscript.











