Introduction
Mismatch negativity (MMN) is an event-related potential (ERP) component that has been studied extensively in schizophrenia (SZ). MMN is elicited in response to an unexpected, low probability deviant stimulus that is presented after a repeated train of high-probability standard stimuli (e.g. an oddball paradigm) (Näätänen et al., Reference Näätänen, Paavilainen, Rinne and Alho2007). The MMN is a negative deflection in the ERP that peaks approximately 100–150 ms after stimulus onset and is maximal at fronto-central scalp sites (Näätänen et al., Reference Näätänen, Paavilainen, Rinne and Alho2007; Garrido et al., Reference Garrido, Kilner, Stephan and Friston2009b; Todd et al., Reference Todd, Harms, Schall and Michie2013). MMN is traditionally measured in the deviant-standard ERP difference wave to facilitate distinguishing it from the earlier and partially overlapping N100 component evoked by both standards and deviants. Diminished MMN amplitude is a reliable finding in SZ, with meta-analytic reviews reporting large effect sizes (Umbricht and Krljes, Reference Umbricht and Krljes2005; Erickson et al., Reference Erickson, Ruffle and Gold2016). Notably, MMN amplitude is also related to functional outcomes in patients (Light and Braff, Reference Light and Braff2005; Kiang et al., Reference Kiang, Light, Prugh, Coulson, Braff and Kutas2007; Wynn et al., Reference Wynn, Sugar, Horan, Kern and Green2010; Friedman et al., Reference Friedman, Sehatpour, Dias, Perrin and Javitt2012; Light et al., Reference Light, Swerdlow, Thomas, Calkins, Green, Greenwood, Gur, Gur, Lazzeroni, Nuechterlein, Pela, Radant, Seidman, Sharp, Siever, Silverman, Sprock, Stone, Sugar, Tsuang, Tsuang, Braff and Turetsky2015; Hamilton et al., Reference Hamilton, Perez, Ford, Roach, Jaeger and Mathalon2017; Thomas et al., Reference Thomas, Green, Hellemann, Sugar, Tarasenko, Calkins, Greenwood, Gur, Gur, Lazzeroni, Nuechterlein, Radant, Seidman, Shiluk, Siever, Silverman, Sprock, Stone, Swerdlow, Tsuang, Tsuang, Turetsky, Braff and Light2017).
MMN has been viewed within several related conceptual frameworks, including echoic memory (Winkler et al., Reference Winkler, Reinikainen and Näätänen1993), short-term neuroplasticity (Stephan et al., Reference Stephan, Baldeweg and Friston2006; Näätänen, Reference Näätänen2008; Garrido et al., Reference Garrido, Kilner, Kiebel, Stephan, Baldeweg and Friston2009a; Breen et al., Reference Breen, Perez, Olmstead, Eisenberger and Irwin2014; Perez et al., Reference Perez, Tarasenko, Miyakoshi, Pianka, Makeig, Braff, Swerdlow and Light2017), and predictive coding (Baldeweg, Reference Baldeweg2007; Garrido et al., Reference Garrido, Kilner, Stephan and Friston2009b; Wacongne et al., Reference Wacongne, Changeux and Dehaene2012; Winkler and Czigler, Reference Winkler and Czigler2012; Wacongne, Reference Wacongne2016). Predictive coding may be an especially useful framework to understand important features of SZ, including auditory hallucinations and delusions (Fletcher and Frith, Reference Fletcher and Frith2009; Adams et al., Reference Adams, Stephan, Brown, Frith and Friston2013; Horga et al., Reference Horga, Schatz, Abi-Dargham and Peterson2014; Schmack et al., Reference Schmack, Schnack, Priller and Sterzer2015), and some aspects of impaired cognition and reward processing (Stephan et al., Reference Stephan, Friston and Frith2009; Lalanne et al., Reference Lalanne, Van Assche and Giersch2012; Friston et al., Reference Friston, Stephan, Montague and Dolan2014). Predictive coding is a hierarchical information processing model that posits interactions between lower order perceptual signals and higher order cognitive processes in a dynamic, iterative fashion to generate predictions about the environment and compare incoming stimuli with these predictions (Fletcher and Frith, Reference Fletcher and Frith2009; Nazimek et al., Reference Nazimek, Hunter and Woodruff2012). According to this model, neural responses to stimuli that match predictions are suppressed, whereas stimuli that are unexpected, violating these predictions, trigger a mismatch ‘prediction error’ signal (Schultz and Dickinson, Reference Schultz and Dickinson2000; Friston, Reference Friston2005; Garrido et al., Reference Garrido, Kilner, Kiebel, Stephan, Baldeweg and Friston2009a). The prediction error signals that updating of expectations is required to accommodate the discrepant stimuli.
From a sensory echoic memory perspective, the elicitation of MMN by a ‘deviant’ stimulus depends on formation and maintenance of a memory trace for what has been ‘standard’ in the recent auditory stream. The predictive coding framework extended this perspective by demonstrating that there is a positive voltage deflection in the standard ERP that increases with successive repetitions of the standard stimulus. This positivity, referred to as the ‘repetition positivity’ (RP), is hypothesized to not only reflect the strength of the memory trace for the standard stimulus, but also the associated prediction that the standard stimulus will recur (Haenschel et al., Reference Haenschel, Vernon, Dwivedi, Gruzelier and Baldeweg2005; Baldeweg et al., Reference Baldeweg, Wong and Stephan2006; Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Baldeweg, Reference Baldeweg2007; Garrido et al., Reference Garrido, Kilner, Kiebel, Stephan, Baldeweg and Friston2009a, Reference Garrido, Kilner, Stephan and Friston2009b). When this prediction is violated by the appearance of a deviant stimulus, negativity is evident in the deviant ERP that is hypothesized to signal a prediction error. Importantly, the negativity evoked by the deviant stimulus increases as the number of standards preceding it increases, such that violations of stronger predictions evoke larger prediction error signals (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Haenschel et al., Reference Haenschel, Vernon, Dwivedi, Gruzelier and Baldeweg2005; Todd et al., Reference Todd, Harms, Schall and Michie2013; Baldeweg and Hirsch, Reference Baldeweg and Hirsch2015). Although this deviant-evoked negativity has traditionally been identified as the MMN, it has more recently been referred to as the ‘deviant negativity’ (DN) (Baldeweg et al., Reference Baldeweg, Wong and Stephan2006) to distinguish it from the MMN difference wave, which is now recognized to comprise two constituent parts: (1) the RP, reflecting the strength the memory trace for the standard stimulus and the associated prediction that it will recur, and (2) the DN, reflecting the strength of the prediction error signal (Garrido et al., Reference Garrido, Kilner, Stephan and Friston2009b; Heilbron and Chait, Reference Heilbron and Chait2017). Elucidation of predictive coding features of the MMN and its constituent RP and DN components can be optimized using ‘roving standard’ MMN paradigms (Cowan et al., Reference Cowan, Winkler, Teder and Naatanen1993; Winkler et al., Reference Winkler, Cowan, Csépe, Czigler and Näätänen1996; Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Haenschel et al., Reference Haenschel, Vernon, Dwivedi, Gruzelier and Baldeweg2005; Baldeweg, Reference Baldeweg2007).
In a roving standard MMN paradigm, a series of standards is presented, followed by a deviant, as in a typical oddball paradigm. However, after the first appearance of the deviant stimulus, it is repeated over successive trials, becoming the new standard. This new standard series is subsequently interrupted by a new deviant, and the process repeats. Relative to a traditional oddball sequence, the changing physical properties of each successive train of standard stimuli permits examination of the build-up of the memory trace for a new standard. By binning the standards according to their sequential position and generating standard ERPs for early (e.g. position 2), intermediate (e.g. position 6), and late (e.g. position 36) standards, a ‘memory trace effect’ is evident in the RP component of the standard ERPs, with late standards showing a more positive RP than early standards (e.g. Baldeweg, Reference Baldeweg2007). Similarly, a memory trace effect is evident in the DN, with deviants preceded by a longer train of repeating standards showing a more negative DN than those preceded by a shorter train (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Haenschel et al., Reference Haenschel, Vernon, Dwivedi, Gruzelier and Baldeweg2005; Todd et al., Reference Todd, Harms, Schall and Michie2013; Baldeweg and Hirsch, Reference Baldeweg and Hirsch2015). Finally, by calculating difference waves between the corresponding deviant and standard ERPs, a memory trace effect is also evident in the MMN (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Haenschel et al., Reference Haenschel, Vernon, Dwivedi, Gruzelier and Baldeweg2005; Todd et al., Reference Todd, Harms, Schall and Michie2013; Baldeweg and Hirsch, Reference Baldeweg and Hirsch2015), reflecting contributions from both RP and DN (Baldeweg, Reference Baldeweg2007).
Attenuation of the MMN memory trace effect, reflected by a flatter slope of MMN amplitude change across deviants preceded by shorter v. longer trains of standards, has previously been reported in SZ compared with healthy controls (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Baldeweg and Hirsch, Reference Baldeweg and Hirsch2015). Moreover, greater attenuation of the MMN memory trace effect was associated with greater deficits in working memory and episodic memory (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Baldeweg and Hirsch, Reference Baldeweg and Hirsch2015). Beyond reporting an overall reduction in RP and DN amplitudes in SZ, one study (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004) found the memory trace effect to be attenuated for RP but normal for DN, suggesting that stimulus repetition failed to strengthen the predictive code but nonetheless modulated the strength of the prediction error signal.
The aim of this study was to develop and test a roving standard MMN paradigm to optimize evaluation of predictive coding in people with SZ. We hypothesized that SZ patients, relative to HC participants, would show: (1) overall MMN amplitude reduction when ERPs were derived by traditional averaging of all standards and deviants, (2) reduced amplitudes and attenuated memory trace effects for RP, DN, and MMN associated with short, intermediate, and long trains of standard stimulus repetitions and their immediately subsequent deviants. Additional study aims were to examine cognitive and clinical correlates of the roving standard MMN indices, and to assess the test-retest reliability of the indices over a 2-week interval.
Method
Participants
Clinical, cognitive, and EEG assessments were obtained from 43 SZ and 30 HC participants at baseline (Time 1). Of these participants, 43 SZ and 29 HC returned for a 2-week follow-up (Time 2). After excluding participants with unusable EEG data, 40 SZ and 30 HC had useable Time 1 data, 38 SZ and 27 HC had usable Time 2 data. In total, 43 SZ and 30 HC contributed usable data to at least one timepoint, and 35 SZ and 27 HC contributed usable data to both timepoints. SZ patients were recruited from local clinics and residences, and HC participants were recruited via Internet advertisements. Inclusion criteria for patients were: (1) DSM-IV (American Psychiatric Association, 1994) diagnosis of SZ based on SCID-I/P interview (First and Gibbon, Reference First, Gibbon, Hilsenroth and Segal2004), (2) age 18–60 years, and (3) stable outpatient status and no antipsychotic medication changes in the month prior to testing. For HC participants, inclusion criteria were: (1) no history of a DSM-IV diagnosis of recurrent major depressive disorder, bipolar disorder, or SZ-spectrum disorder (including schizoid, schizotypal, paranoid, and avoidant personality disorder) based on SCID-I/P and SCID-II interview (Benjamin, Reference Benjamin1994; First et al., Reference First, Williams, Karg and Spitzer2014), (2) no history of a SZ-spectrum disorder among first-degree relatives, and (3) age 18–60 years. Exclusion criteria for all participants were: (1) history of a neurological disorder or head injury resulting in loss of consciousness, and (2) alcohol or substance abuse or dependence in the 3 months prior to testing, and (3) benzodiazepine or sedative use in the 12 h before testing. Urine toxicology screenings were performed at each study visit. Sample characteristics are presented in Table 1.
Table 1. Clinical and demographic information by group

SZ, schizophrenia group; HC, healthy control group; MCCB, MATRICS Consensus Cognitive Battery; RFS, Role Functioning Scale; BPRS, Brief Psychiatric Rating Scale; CAINS, Clinical Assessment Interview for Negative Symptoms
Symptom severity and community functioning were rated by trained raters using the 24-item Brief Psychiatric Rating Scale (BPRS) (Lukoff et al., Reference Lukoff, Nuechterlein and Ventura1986) and the Clinical Assessment Interview for Negative Symptoms (CAINS) (Kring et al., Reference Kring, Gur, Blanchard, Horan and Reise2013), and the Role Functioning Scale (RFS) (Goodman et al., Reference Goodman, Sewell, Cooley and Leavitt1993). Each rater achieved a median intraclass correlation coefficient (ICC) of 0.80 or higher across all BPRS, CAINS, and RFS. For the SCID, clinical raters demonstrated an overall κ coefficient, κ sensitivity, and κ specificity >0.75, and a diagnostic accuracy κ > 0.85. The MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein and Green, Reference Nuechterlein and Green2006) was administered at Time 1 to assess cognition using age- and gender-corrected domain and overall composite scores.
Procedures
Roving standard mismatch negativity paradigm
Prior work has shown that MMNs elicited by different types of auditory deviance (e.g. pitch v. duration) are subserved by different neural generators (Giard et al., Reference Giard, Perrin, Pernier and Bouchet1990; Paavilainen et al., Reference Paavilainen, Alho, Reinikainen, Sams and Näätänen1991; Alho, Reference Alho1995; Csépe, Reference Csépe1995; Deouell et al., Reference Deouell, Bentin and Giard1998; Molholm et al., Reference Molholm, Martinez, Ritter, Javitt and Foxe2004). Further, there is variability in relative sensitivity of pitch v. duration MMN to SZ across the illness course (Michie et al., Reference Michie, Budd, Todd, Rock, Wichmann, Box and Jablensky2000; Umbricht and Krljes, Reference Umbricht and Krljes2005; Todd et al., Reference Todd, Michie, Schall, Karayanidis, Yabe and Näätänen2008; Näätänen and Kähkönen, Reference Näätänen and Kähkönen2009; Bodatsch et al., Reference Bodatsch, Ruhrmann, Wagner, Müller, Schultze-Lutter, Frommann, Brinkmeyer, Gaebel, Maier, Klosterkötter and Brockhaus-Dumke2011; Nagai et al., Reference Nagai, Tada, Kirihara, Yahata, Hashimoto, Araki and Kasai2013; Perez et al., Reference Perez, Woods, Roach, Ford, McGlashan, Srihari and Mathalon2014; Erickson et al., Reference Erickson, Ruffle and Gold2016; Avissar et al., Reference Avissar, Xie, Vail, Lopez-Calderon, Wang and Javitt2017; Haigh et al., Reference Haigh, Coffman and Salisbury2017) in that paradigms that produce larger MMNs tend to show larger MMN deficits in SZ (Javitt et al., Reference Javitt, Grochowski, Shelley and Ritter1998; Avissar et al., Reference Avissar, Xie, Vail, Lopez-Calderon, Wang and Javitt2017). Also, MMN amplitude is enhanced when multiple types of deviance are combined in a single stimulus (Levanen et al., Reference Levanen, Hari, McEvoy and Sams1993; Schroger, Reference Schroger1995; Takegata et al., Reference Takegata, Paavilainen, Naatanen, Winkler, Näätänen and Winkler1999; Paavilainen et al., Reference Paavilainen, Valppu, Naatanen and Näätänen2001; Wolff and Schröger, Reference Wolff and Schröger2001). Hence, the paradigm tested here used pitch + duration ‘double deviants’, similar to our prior studies (Perez et al., Reference Perez, Woods, Roach, Ford, McGlashan, Srihari and Mathalon2014; Hay et al., Reference Hay, Roach, Srihari, Woods, Ford and Mathalon2015).
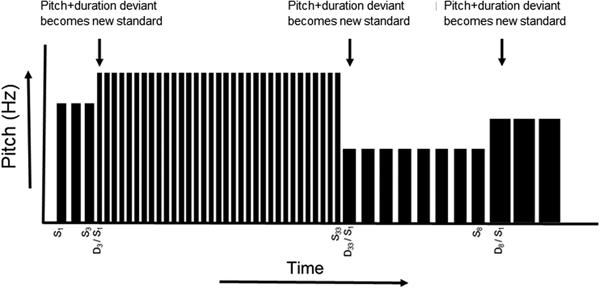
Participants completed the roving standard MMN at Time 1 and Time 2. The paradigm comprised five blocks (block duration: 317 s), each separated by a 30 s break, during which tones of 50 or 100 ms duration, varying in pitch between 700 and 1250 Hz, were presented binaurally (80 dB, 5 ms rise/fall time, 400 ms SOA) through insert earphones (ER-2, Etymotic Research, Inc., Elk Grove Village, IL, USA). The stimulus sequence comprised short (3), intermediate (8), and long (33) trains of identical standard tones followed by a deviant tone differing from the standard in both pitch (minimum change 100 Hz, maximum change 300 Hz) and duration (±50 ms). The deviant tone then repeated, effectively becoming the new standard, with a train of 3, 8, or 33 standard repetitions preceding the next deviant (see Fig. 1). A randomly permuted sequence of short, intermediate, and long trains of identical standard repetitions was generated for each block, with all five blocks comprising 90 occurrences of each train length. Participants were instructed to ignore the tones while they performed a tactile oddball distractor task and maintained visual fixation on a central cross on a computer screen. In the tactile oddball task, participants held a game controller with both hands that transmitted a pseudorandom sequence of three distinct vibration patterns: a frequent (p = 0.8) standard (right side), an infrequent (p = 0.10) target (right side), and an infrequent (p = 0.10) foil (left side). Participants responded with a button press to targets only.

Fig. 1. Roving standard MMN task design. For the roving standard MMN paradigm, an identical standard tone is repeated three, eight, or 33 times within each stimulus train. For each change to a new stimulus train, the first stimulus constitutes a deviant tone, differing from the preceding train of standards in both pitch and duration. The deviant tone then becomes the new standard, repeating three, eight, or 33 times until the next deviant appears. Note: S, standard stimulus; D, deviant stimulus.
Specific standard and deviant trials were averaged to generate ERP waveforms and corresponding deviant-standard difference waves, and all components (RP, DN, MMN) were measured by calculating the mean amplitude between 100 and 200 ms at electrode Fz from their respective ERPs. RPs were assessed in ERPs derived from standard trials in sequence positions 3, 8, or 33 within local trains of standard repetitions, resulting in 270 trials for the third standard (RP3), 180 trials for the eighth standard (RP8), and 90 trials for the 33rd standard (RP33). Likewise, DNs were assessed in ERPs derived from deviant trials occurring after three (DN3), eight (DN8), or 33 (DN33) standard repetitions, with each ERP comprising 90 trials. MMNs associated with different numbers of standard repetitions preceding the deviant stimulus (MMN3, MMN8, MMN33) were assessed in corresponding deviant-standard difference waves (e.g. MMN3 = DN3 − RP3). ‘Memory trace’ indices for RP, DN, and MMN were calculated as the amplitude difference between components associated with long v. short trains of standard repetitions (e.g. DNMT = DN33 − DN3) for use in correlational analyses. In addition, overall mean RP, DN, and MMN were measured from ERP averages of all standards, all deviants, and their corresponding difference waves. Additional information about the EEG procedures are in online Supplementary Materials.
Data analysis plan
The groups were compared on demographic, clinical, and cognitive variables using χ2 and independent sample t tests. MMN, RP, and DN amplitudes were analyzed separately using 2 (Group; SZ, HC) × 3 (Standard Repetition; 3, 8, 33) × 2 (Time; Time 1, Time 2) mixed models. In these models, memory trace effects were assessed using two a priori orthogonal reverse Helmert contrasts for standard repetitions: (1) intermediate (8) v. short (3), and (2) long (33) v. mean of intermediate and short (8,3). Overall MMN amplitude was assessed using a 2 (Group; SZ, HC) × 2 (Time; Time 1, Time 2) mixed model. Using hierarchical linear regression models, MCCB cognitive measures were each regressed on a MMN index (MMNMT, RPMT, DNMT, overall MMN amplitude), Group, and the MMN index × Group interaction to test for significant regression line slope differences between the groups. Significant slope differences were followed up with bivariate correlations within each group. When the MMN index × Group interaction was not significant, it was dropped from the model, and the common slope was then tested for significance. Relationships of clinical and functioning measures with MMN indices were assessed in the SZ group with bivariate correlations. For each family of regression or correlation analyses (cognitive, clinical, functioning), correction for multiple tests was imposed using Bonferroni correction (family wise-corrected α set to p < 0.05). Finally, ICC (3,1) coefficients (Shrout and Fleiss, Reference Shrout and Fleiss1979) were calculated to assess test-retest reliability of the roving standard indices.
Results
Demographic, clinical, cognitive, and functioning data are presented in Table 1. The groups were well matched on age, gender, race, and ethnicity. The level of personal education was significantly lower, and the level of parental education was marginally lower, in SZ compared with HC.
Means of the roving standard MMN indices are presented by group in Table 2. The number of trials included in ERP averages after artifact rejection are presented in online Supplementary Materials. Grand average waveforms for RP, DN, and MMN and topographic maps across the three standard repetition train lengths for each group are presented in Figs 2 and 3. Average amplitudes for the MMN, RP, and DN associated with short, intermediate, and long trains of standard repetitions for each group are plotted in Fig. 4. Descriptive data and mixed model results are presented in Tables 2 and 3.

Fig. 2. (a) MMN averaged waveforms (electrode Fz), and (b) MMN topographic maps, collapsed across Time 1 and Time 2. Note: HC, healthy control group; SZ, schizophrenia group; MMN, mismatch negativity. Shaded box denotes 100–200 ms time window.

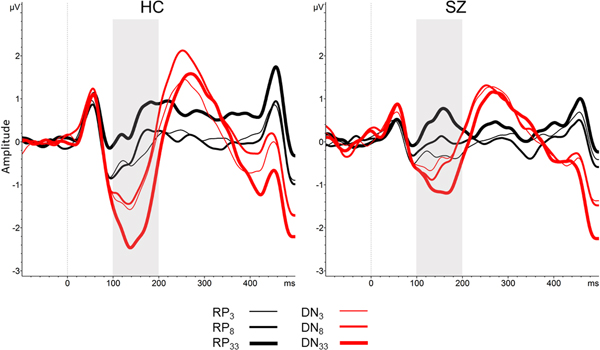
Fig. 3. RP and DN averaged waveforms (electrode Fz) collapsed across Time 1 and Time 2. Note: HC, healthy control group; SZ, schizophrenia group; RP, repetition positivity; DN, deviant negativity. Shaded box denotes 100–200 ms time window.

Fig. 4. Average amplitude for (a) MMN and (b) RP to standards and DN to deviants associated with different numbers of standard repetitions in SZ and HC. Note: MMN, mismatch negativity; RP, repetition positivity; DN, deviant negativity; HC, healthy control group; SZ, schizophrenia group.
Table 2. Descriptive statistics for roving standard MMN indices and mixed model analyses

SZ, schizophrenia group; HC, healthy control group; RP, repetition positivity; DN, deviant negativity
Table 3. Mixed model analyses

SZ, schizophrenia group; HC, healthy control group; RP, repetition positivity; DN, deviant negativity
Mixed model analyses of roving standard ERP indices
The mixed model analysis of MMN amplitudes revealed a significant main effect of Group, with SZ patients showing smaller (i.e. less negative) MMN than HC participants. This effect interacted significantly with Time, with the MMN amplitude deficit in SZ patients, relative to HC, being larger at Time 2 than at Time 1. Despite this, neither the increase in MMN amplitude over time in HC [F (1,147.25) = 2.81, p = 0.10], nor the decrease in MMN amplitude over time in SZ [F (1,203.29) = 2.20, p = 0.14], were significant. Two reverse Helmert contrasts among levels of the Standard Repetition factor were used to assess the MMN memory trace effect. The MMN3 v. MMN8 contrast was not significant, nor did it significantly interact with Group, Time, or with Group × Time. However, the MMN3,8 v. MMN33 contrast was highly significant (p < 0.001) reflecting a marked increase in MMN amplitude (i.e. increased negativity) when deviants were preceded by long trains of standard repetitions, relative to the average MMN associated with short and intermediate trains of standard repetitions. While this MMN memory trace effect appeared to be larger in the HC than in the SZ group (see Fig. 3a), the difference was not significant (p = 0.10). Furthermore, the MMN3,8 v. MMN33 contrast effect did not significantly interact with Time or with Group × Time. No other main effects or interactions were significant.
Next, RP and DN across the three Standard Repetition train lengths and two Time points in SZ and HC were tested in two separate mixed model analyses. For RP, memory trace effects were significant for both reverse Helmert contrasts, indicating that RP amplitude modestly (p = 0.02) increased in positivity for the eighth standard relative to the third standard in repeating standard stimulus trains, and strongly (p < 0.001) increased for the 33rd standard relative to the mean of the third and eighth standards. These memory trace effects on the RP did not significantly interact with Group, Time, or Group × Time.
For DN, there was a significant main effect of Group (p = 0.04), with HC exhibiting a larger (i.e. more negative) DN amplitude than SZ. This effect did not significantly interact with Time, nor was the main effect of Time significant. Memory trace effects on DN were not significant for the DN3 v. DN8 contrast, nor did this contrast significantly interact with Group, Time, or Group × Time. However, when the mean of DN3 and DN8 were contrasted with DN33, a strong memory trace effect was evident (p < 0.001). This significant memory trace effect on DN showed a trend interaction with Group (p = 0.07), with the increase in amplitude (i.e. increased negativity) of DN33, relative to DN3,8, being larger in HC [t(141.54) = −4.87, p < 0.001] than in SZ [t(189.67) = −2.80, p = 0.06].
For overall MMN amplitude (i.e. based on ERPs derived from all deviants and standards), there was a significant main effect of Group (p = 0.04), with HC exhibiting larger (i.e. more negative) MMN amplitude compared with SZ. In addition, a significant Group × Time interaction (p = 0.04) was present, indicating that the SZ deficit in overall MMN amplitude relative to HC was greater at Time 2 than at Time 1. Despite this interaction, neither the slight increase in MMN amplitude in HC over time [F (1,27.98) = 2.89, p = 0.10], nor the slight decrease in MMN amplitude in SZ over time [F (1,33.61) = 1.89, p = 0.19], was significant.
Cognitive, clinical, and functioning relationships with roving standard MMN indices
The regression analyses and correlation matrices are presented in online Supplementary Materials. Although a few differential associations between the memory trace indices and MCCB variables were found in SZ and HC, none survived correction for multiple tests. Similarly, correlations of clinical or community functioning with MMN indices in the SZ group were not significant after correction for multiple tests.
Test-retest reliability of roving standard ERP indices
Test-retest reliability coefficients (ICCs) for the roving standard ERP indices are presented in Table 4. In brief, the ICCs indicated good-to-moderate reliabilities for overall MMN, DN, and RP. However, when looking at the values for the separate short, intermediate, and long standard trains, the reliabilities were moderate to poor. Finally, the reliabilities were poor for memory trace effects.
Table 4. Test-retest reliability coefficients for roving standard MMN indices

*p < 0.05, **p < 0.01; ICC, intra-class correlation, using two-way mixed single measures consistency formula (ICC 3,1 from Shrout and Fleiss, Reference Shrout and Fleiss1979); SZ, schizophrenia group; HC, healthy control group; MMN, mismatch negativity; RP, repetition positivity; DN, deviant negativity. MT, memory trace effect, defined for each component by subtracting value associated with repeated standard 3 from value associated with repeated standard 33. Overall MMN, RP, and DN measures are based on ERP averages of all standards, all deviants, and the resulting ERP deviant-standard difference waves.
Discussion
Using a roving standard MMN paradigm, with trains of 3, 8, or 33 standards before a deviant that heralded the onset of a new standard train, a significant memory trace effect was evident for MMN in both HC and SZ groups, with MMN amplitude being substantially larger (i.e. more negative) when deviants were preceded by 33 v. three or eight standards. This memory trace effect is thought to reflect the build-up of a stronger memory trace for the standard, and a stronger prediction that it will recur, as the number of standard repetitions increase, and a correspondingly larger prediction error signal to deviants preceded by longer trains of standards. Although this memory trace effect tended to be smaller in the SZ patients than in the HC controls, this difference was not significant. Moreover, MMN did not show a significant memory trace effect in either group when deviants were preceded by eight standards relative to three standards.
An advantage of the roving standard MMN paradigm is its ability to disentangle the two processes that contribute to MMN: (1) the build-up of the memory trace for the standard and the development of the prior expectation that it will recur, reflected by the RP response to repeated standard sounds, and (2) the prediction error signal elicited by stimuli that violate prior expectations, reflected by the DN response to infrequent deviant sounds. A corresponding limitation of the roving standard paradigm is that it does not equate the number of trials for the various repetition sequence lengths for RP. Our results showed the predicted memory trace effect on the RP, with its amplitude modestly but significantly increasing to the eighth repeated standard relative to the third, and a more prominent increase to the 33rd standard. These effects were equally evident in both groups. While the standard repetition train lengths used in this study (i.e. 3, 8, and 33) were similar to those used in prior roving standard MMN paradigms (e.g. Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004), a memory trace effect on the DN and the MMN was not evident in either group when comparing deviants preceded by three v. eight standards. However, a memory trace effect was seen in increased negativity for deviants preceded by 33 standards relative to eight or three standards, and this effect was attenuated at a trend level in SZ relative to HC participants.
In addition to these memory trace effects, our results replicated the expected reduction in overall MMN amplitude in SZ patients, relative to HC, both in the overall MMN measured from ERP averages of all standards and deviants, and in the standard repetition analysis. Based on the separate analyses of MMN's constituents (i.e. DN and RP), the SZ patient MMN deficit can be attributed to a significant overall reduction in DN, but not RP, amplitude. Overall, our findings support the idea that SZ patients show aberrant prediction error signaling, and they show a trend toward reduced strengthening of this prediction error signal with more standard repetitions. Furthermore, our findings with RP suggest that SZ patients do not have deficits in forming memory traces of, or establishing and building expectations for, recurring auditory stimuli. The overall reduction in amplitude of the DN and MMN in this SZ sample are congruent with the findings of Baldeweg et al. (Reference Baldeweg, Klugman, Gruzelier and Hirsch2004). However, the studies differ in that Baldeweg et al. (Reference Baldeweg, Klugman, Gruzelier and Hirsch2004) found that the memory trace was diminished for RP and relatively intact for DN in SZ, whereas we found the opposite pattern. The roving standard paradigm used by Baldeweg et al. (Reference Baldeweg, Klugman, Gruzelier and Hirsch2004) differed from the one used in the current study with regard to the type of deviant and the number of repetitions of the standard, which may contribute to the different pattern of findings. It is also possible that the poor reliability of the roving standard indices may contribute to the different findings across studies.
Previous studies have reported significant relationships between MMN and symptoms, cognitive performance, and functional outcome in SZ (Baldeweg et al., Reference Baldeweg, Klugman, Gruzelier and Hirsch2004; Light and Braff, Reference Light and Braff2005; Kiang et al., Reference Kiang, Light, Prugh, Coulson, Braff and Kutas2007; Wynn et al., Reference Wynn, Sugar, Horan, Kern and Green2010; Friedman et al., Reference Friedman, Sehatpour, Dias, Perrin and Javitt2012; Baldeweg and Hirsch, Reference Baldeweg and Hirsch2015; Light et al., Reference Light, Swerdlow, Thomas, Calkins, Green, Greenwood, Gur, Gur, Lazzeroni, Nuechterlein, Pela, Radant, Seidman, Sharp, Siever, Silverman, Sprock, Stone, Sugar, Tsuang, Tsuang, Braff and Turetsky2015; Hamilton et al., Reference Hamilton, Perez, Ford, Roach, Jaeger and Mathalon2017). We expected to find similar correlations with the predictive coding indices derived from this roving standard paradigm. However, while we found some moderately sized associations within groups, none of the correlations survived correction for multiple tests. One could speculate that predictive coding-related measures from the roving standard MMN paradigm might be more relevant to shorter term mechanisms of neural plasticity that support new learning, rather than stable cognitive abilities that may be learned over a lifetime and may be more closely related to daily functioning.
MMN from traditional (i.e. non-roving) paradigms have previously been shown to have moderate-to-good test-retest reliability in SZ (Light and Braff, Reference Light and Braff2005; Hall et al., Reference Hall, Schulze, Rijsdijk, Picchioni, Ettinger, Bramon, Freedman, Murray and Sham2006; Lew et al., Reference Lew, Gray and Poole2007; Light et al., Reference Light, Swerdlow, Rissling, Radant, Sugar, Sprock, Pela, Geyer and Braff2012; Biagianti et al., Reference Biagianti, Roach, Fisher, Loewy, Ford, Vinogradov and Mathalon2017). The ICCs for the overall MMN derived in our study is comparable to some of these estimates. This paper represents the first report of test-retest reliability of MMN, RP, and DN components, as well as the corresponding memory trace effects, derived from a roving standard MMN paradigm in SZ and HC. Within the two groups, the roving ERP indices for RP, DN, and MMN at each of the three repetition sequences showed poor-to-moderate test-retest reliability. Notably, there was a general tendency for reliability to improve as the number of standard presentations in a sequence increased, and correspondingly, as the amplitudes of the components increased. The memory trace effects, i.e. the difference in ERP amplitude between the longest and shortest repetition sequences for MMN, RP, and DN, showed particularly poor reliability. Possibly, the memory trace effects are more susceptible to state-related influences and noise than the component amplitudes from which they are derived.
Even though the paradigm included more trials of the short sequences (i.e. three and eight), even this might not have been enough to establish high levels of reliability because shorter sequences would be expected to have more noise than longer sequences. This potential benefit of increasing trial numbers is consistent with the observation that the overall MMN, RP, and DN, derived from all available standard and deviant trials, achieved moderate-to-strong reliability. However, the potential impact on testing session length and subject burden does present a limitation for the number of long sequence trials that can be included in a testing session. Moreover, it is not possible to equate the number of trials presented to the subject for the various repetition sequence lengths for RP. It is also possible that the tactile distractor task used in this study may have impacted the reliability estimates of the MMN indices, as MMN latency and amplitude can be influenced by distractor task attentional demands and modality (Rissling et al., Reference Rissling, Park, Young, Rissling, Sugar, Sprock, Mathias, Pela, Sharp, Braff and Light2013). Further study of the impact of altering task parameters, including the number of standard repetitions in the sequence, the type of deviants, and the type of distractor task, on psychometric properties is warranted.
In conclusion, our results demonstrate the potential utility of roving standard MMN paradigms and applications of the predictive coding framework to the study of SZ. These data demonstrate that MMN deficits in SZ patients can be understood to reflect deficient prediction error signaling, rather than reduced memory trace formation for the standard. The reliability analyses suggest that further refinements to roving standard paradigms will be needed to yield adequate reliability for roving standard ERP indices if they are to be used in longitudinal studies or clinical trials.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718004087.
Acknowledgements
We thank the research subjects for participating in this study. We gratefully acknowledge Ana Cecilia Myers, M.S. for coordinating the study, Michelle Dolinsky, B.A. for subject recruitment efforts, Courtney Fazli, B.S., Gabrielle Pascual, B.S., and Nora Polon, B.A. for their assistance with data collection.
Financial support
This study was supported by funding from FORUM Pharmaceuticals, Inc. (PIs: Green & Marder), the Veterans Administration VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC), the Veterans Administration Research Enhancement Award Program (REAP) on Enhancing Community Integration for Homeless Veterans. Dr McCleery is currently supported by a career development award from NIH (K23MH108829), and previously by an NIH institutional training fellowship (T32MH096682).
Conflict of interest
Dr McCleery has received compensation from MedAvante-Prophase, Inc. for clinical assessment services. Dr Mathalon serves as a consultant for Boehringer Ingelheim and Takeda. Dr Marder has been a consultant for FORUM, Allergan, Lundbeck, Roche, Otsuka, Merck, Teva, Takeda, and Newron. He has received research support from FORUM and Neurocrine. Dr Green has been a consultant to AiCure, Lundbeck, and Takeda, and he is on the scientific board of Cadent. He has received research funds from FORUM. Dr Wynn, Dr Hellemann, and Mr Roach report no disclosures.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.