Introduction
Alien species are important resources for increasing the genetic diversity of bread wheat (Triticum aestivum L.) (Mujeeb-Kazi and Kimber, Reference Mujeeb-Kazi and Kimber1985). The cultivated einkorn T. monococcum L. subsp. monococcum (2n = 2x = 14, genome AmAm) is the first cultivated wheat. It is closely related to Triticum urartu Tumanian ex Gandilyan (2n = 2x = 14, AuAu) which is the A genome donor progenitor of hexaploid bread wheat (Dvorák et al., Reference Dvorák, Terlizzi, Zhang and Resta1993). T. monococcum ssp. monococcum has useful traits for bread wheat improvement, such as high-protein content (Tranquilli et al., Reference Tranquilli, Cuniberti, Gianibelli, Bullrich, Larroque, MacRitchie and Dubcovsky2002), diverse Glu-A1mx alleles (Li et al., Reference Li, Li, Zeng, Zhao, Chen, Kou, Ning, Yuan, Zheng, Liu and Zhang2016, Reference Li, Li, Chen, Liu, Kou, Ning, Yuan, Hao, Liu and Zhang2017), tolerance to cold stress (Aslan et al., Reference Aslan, Ordu and Zencirci2016), resistance to preharvest sprouting (Sodkiewicz, Reference Sodkiewicz2002) and high resistance to diseases (Mikhova, Reference Mikhova1988; Hussien et al., Reference Hussien, Bowden, Gill and Cox1998; Chhuneja et al., Reference Chhuneja, Kaur, Garg, Ghai, Kaur, Prashar, Bains, Goel, Keller, Dhaliwal and Singh2008; Rouse and Jin, Reference Rouse and Jin2011a, Reference Rouse and Jinb; Schmolke et al., Reference Schmolke, Mohler, Hartl, Zeller, Sai and Hsam2012; Zaharieva and Monneveux, Reference Zaharieva and Monneveux2014). Moreover, its high tocol and carotenoid contents make it a promising source for functional food production (Brandolini et al., Reference Brandolini, Hidalgo and Moscaritolo2008).
The application of cultivated einkorn in bread wheat breeding is greatly limited by its poor crossability and the sterile F1 hybrids produced by its direct cross with bread wheat (The and Baker, Reference The and Baker1975; Cox et al., Reference Cox, Harrell, Chen and Gill1991; Plamenov et al., Reference Plamenov, Belchev, Kiryakova and Spetsov2009). Post-syngamic hybridization barriers resulting in embryo abortion and failure of endosperm development make direct transfer of useful genes from einkorn to bread wheat difficult (Bhagyalakshmi et al., Reference Bhagyalakshmi, Vinod, Kumar, Arumugachamy, Prabhakaran and Raveendran2008). An alternative approach for introgressing traits from a diploid species into hexaploid wheat is to create amphiploids between diploid and tetraploid species which are then subsequently crossed with cultivated wheat (Dorofeev et al., Reference Dorofeev, Udachin, Semenova, Novikova, Grazhdaninova, Shitova, Merezhko and Filatenko1987).
There are two main methods for synthetic amphiploid production using T. monococcum. One method is by producing Triticum timococcum or synthetic Triticum zhukovskyi (2n = 6x = 42, AtAtGGAmAm) by crossing Triticum timopheevii and T. monococcum (Kostov, Reference Kostov1936; Cao et al., Reference Cao, Armstrong and Fedak2000; Goncharov et al., Reference Goncharov, Bannikova and Kawahara2007). New T. timococcum lines were recently developed in order to introgress useful genes for conventional and organic wheat breeding (Mikó et al., Reference Mikó, Megyeri, Farkas, Molnár and Molnár-Láng2015). The second is by Triticum turgidum–T. monococcum amphiploid (AABBAmAm, 2n = 6x = 42) production which combines useful einkorn genes with tetraploid T. turgidum wheat, usually T. turgidum ssp. durum (Dorofeev et al., Reference Dorofeev, Udachin, Semenova, Novikova, Grazhdaninova, Shitova, Merezhko and Filatenko1987; Gill et al., Reference Gill, Dhaliwal and Multani1988; Mujeeb-Kazi and Hettel, Reference Mujeeb-Kazi and Hettel1995; Watanabe et al., Reference Watanabe, Kobayashi and Furuta1997; Cakmak et al., Reference Cakmak, Cakmak, Eker, Ozdemir, Watanabe and Braun1999; Megyeri et al., Reference Megyeri, Mikó, Molnár and Kovács2011).
In the present study, we have developed 56 T. turgidum–T. monococcum amphiploids using 31 T. turgidum accessions from five subspecies. This article reports the development, molecular cytogenetic identification and the agronomic trait evaluation of these new synthetic T. turgidum–T. monococcum amphiploids.
Materials and methods
Plant materials
Sixty T. turgidum and 83 T. monococcum accessions with diverse geographic origins (Zhang et al., Reference Zhang, Yan, Dai, Chen, Yuan, Zheng and Liu2008; Li et al., Reference Li, Li, Zeng, Zhao, Chen, Kou, Ning, Yuan, Zheng, Liu and Zhang2016) were used in this study. Lines with PI or CItr prefixes were kindly provided by USDA-ARS, USA while AS lines were obtained from the Sichuan Agricultural University. These T. turgidum lines were derived from either subspecies dicoccon (26 lines), durum (three lines), turanicum (six lines), turgidum (24 lines) or persicum (one line) (Van Slageren, Reference Van Slageren1994). All 83 T. monococcum accessions used were T. monococcum ssp. monococcum (Zhang et al., Reference Zhang, Yan, Dai, Chen, Yuan, Zheng and Liu2008; Li et al., Reference Li, Li, Zeng, Zhao, Chen, Kou, Ning, Yuan, Zheng, Liu and Zhang2016).
Hybridization
Hybridization between these species was undertaken using T. turgidum as the female parent and T. monococcum as the male parent. Reciprocal crosses were not attempted since einkorn cytoplasm induces male sterility (The and Baker, Reference The and Baker1975). Crosses were made under field conditions in the 2013–2014 crop season. Emasculation and pollination were done as previously described by Zhang et al. (Reference Zhang, Yan, Dai, Chen, Yuan, Zheng and Liu2008). No embryo rescue or hormone treatment was applied for the production of F1 seeds. The spikes were harvested and the number of seeds set per spike counted approximately 20 d after pollination. Crossability was expressed as the percentage of seed set per floret pollination for each line.
Chromosome doubling by colchicine treatment
F1 seeds were germinated in Petri dishes and the root tips analysed cytologically prior to planting. Hybrid F1 plants were chromosome doubled by colchicine treatment at the three-tiller stage according to Cao et al. (Reference Cao, Armstrong and Fedak2000) and then transplanted in the field at the Wenjiang Experimental Station of Sichuan Agricultural University. Treated F1 plants were self-fertilized and the seed set (percentage of selfed seed set per self-pollenated floret) for each plant calculated.
Cytological observation
Cytological observation on chromosome number in root-tip cells and chromosome pairing in pollen-mother cells (PMCs) were done as previously described by Zhang et al. (Reference Zhang, Yen, Zheng and Liu2007). For meiotic analysis, at least 50 PMCs were observed for each synthetic amphiploid. Univalent (I), bivalents (II), trivalents (III), quadrivalents (IV) and pentavalents (V) were counted and their average numbers were calculated.
Multicolour fluorescence in situ hybridization (FISH) was carried out according to Tang et al. (Reference Tang, Yang and Fu2014) and Zhao et al. (Reference Zhao, Ning, Yu, Hao, Zhang, Yuan, Zheng and Liu2016). For multicolour FISH, synthetic oligonucleotides Oligo-pSc119.2-1, Oligo-pTa71-2, Oligo-pTa535-1 and (AAC)5 were used as probes to detect FISH signals in order to differentiate individual chromosomes of T. turgidum and T. monococcum in newly synthesized T. turgidum–T. monococcum amphiploids. Probe Oligo-pSc119.2-1 preferentially paints tandem repeats on B-genome chromosomes, Oligo-pTa71-2 is largely specific for the sub-terminal regions of 1BS and 6BS, Oligo-pTa535-1 preferentially paints tandem repeats on the Am- and A-genome chromosomes, while (AAC)5 is largely specific for the 6Am chromosome (Megyeri et al., Reference Megyeri, Farkas, Varga, Kovács, Molnár-Láng and Molnár2012, Reference Megyeri, Mikó, Farkas, Molnár-Láng and Molnár2017; Tang et al., Reference Tang, Yang and Fu2014; Zeng et al., Reference Zeng, Luo, Li, Chen, Zhang, Ning, Yuan, Zheng, Hao and Liu2016). All probes were synthesized and labelled with FAM or Tamra (TSINGKE Biological Technology Company, Chengdu, China). Hybridization signals were observed using an Olympus BX-63 epifluorescence microscope and the images were photographed using a Photometric SenSys Olympus DP70 CCD camera (Olympus, Tokyo). Raw images were processed using Photoshop ver. 7.1 (Adobe Systems Incorporated, San Jose, CA, USA). Individual chromosomes of amphiploids were compared with the karyotypes of the previously published FISH patterns of T. turgidum (Zeng et al., Reference Zeng, Luo, Li, Chen, Zhang, Ning, Yuan, Zheng, Hao and Liu2016) and T. monococcum (Megyeri et al., Reference Megyeri, Farkas, Varga, Kovács, Molnár-Láng and Molnár2012; Mikó et al., Reference Mikó, Megyeri, Farkas, Molnár and Molnár-Láng2015).
SDS-PAGE analysis
Seed protein extraction and sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) were undertaken as described by Yan et al. (Reference Yan, Wan, Liu, Zheng and Wang2002). Detection of Glu-A1mx proteins of T. monococcum was as described by Li et al. (Reference Li, Li, Zeng, Zhao, Chen, Kou, Ning, Yuan, Zheng, Liu and Zhang2016). Bread wheat cultivars Chuanyu 12 (subunit 1, 7 + 8, 5 + 10), Longfumai 1 (2*, 7 + 8, 5 + 10) and Chinese Spring (null, 7 + 8, 2 + 12) were used as reference standards for comparing the electrophoretic mobility of HMW-GSs.
Stripe rust resistance evaluation
Field evaluation for stripe rust resistance was conducted both at seedling and adult plant stages at the Wenjiang Experimental Station of Sichuan Agricultural University in the 2015–2016 crop season. Lines were grown as individual plants spaced 10 cm apart in 2 m rows with 30 cm between rows. The highly rust-susceptible spreader variety SY95-71 was planted on each side of each experimental row. A stripe rust epidemic was initiated 6 weeks after planting by inoculating plants with urediniospores mixtures that included current predominant Chinese stripe rust races such as CYR32, CYR33 and CYR34. Rust isolates were provided by the Research Institute of Plant Protection, Gansu Academy of Agricultural Sciences. Stripe rust infection type (IT) on individual plants was recorded three times at 10 d intervals. Disease notes were taken when the flag leaves of the susceptible check SY95-71 were heavily infected. For each plant, the IT produced was estimated on a 1–9 scale (Wellings and Bariana, Reference Wellings and Bariana2004) with the highest IT recorded used as the resistance type of the line. Plant ITs were divided into seven classes: highly resistant (1–2), resistant (3), moderately resistant (4), intermediate (5), moderately susceptible (6–7), susceptible (8) and highly susceptible (9).
Results
The crossability of T. turgidum with T. monococcum
Two hundred and sixty-four hybridization combinations were undertaken by crossing 60 T. turgidum lines, belonging to five subspecies, with 83 T. monococcum accessions (online Supplementary Tables S1–S3). From the 10,810 florets pollinated, 1983 seeds were obtained. The mean crossability of the 264 combinations was 18.34% and ranged from 0 to 89.29% depending upon the cross. Many (90.73%, 923/1017) of the hybrid seeds produced germinated and produce plants. Amongst the 264 T. turgidum × T. monococcum combinations, 34.47% failed to produce seeds and 6.44% had crossabilities <5%, while 9.47%, 9.09, 7.95, 7.95, 5.68 and 12.88% of combinations had crossabilities of 5–10, 10–15, 15–20, 20–25, 25–30 and 30–50%, respectively. One hundred and fifty-six combinations had crossabilities >5% and are listed in online Supplementary Table S1. A total of 6.06% of combinations had crossability frequencies >50% and all these latter highly compatible combinations were obtained from crosses between T. turgidum subspecies turgidum and dicoccon with T. monococcum. Of the five T. turgidum subspecies investigated, the persicum and durum subspecies exhibited highest crossability (>30%), while dicoccon had the lowest crossability with 11.90% (online Supplementary Table S2). Amongst the 83 T. monococcum accessions, PI352486, PI352484 and PI355517 showed the highest crossability (>50%).
Production of T. turgidum–T. monococcum amphiploids
Randomly selected hybrid seeds were germinated to produce F1 plants. The F1 seeds from 163 crosses germinated with the germination rate of 90.37% (913/1010) and produced vigorous F1 plants (online Supplementary Table S4). Chromosome number of root-tip cells was used for hybrid confirmation with 21 chromosomes (triploid) present in hybrids (online Supplementary Fig. S1). Between one and five F1 plants from each of the 163 crosses were chromosome doubled by colchicine treatment at the three-tiller stage. Treated plants from 70 of these crosses successfully generated selfed seed (S1) (online Supplementary Table S4) although seed was viable from 56 synthetic amphiploids only. The chromosome number of root-tip cells with 2n = 42 confirmed the success of chromosome doubling (online Supplementary Fig. S2). These 56 viable lines were produced from crosses involving 31 T. turgidum lines and 31 T. monococcum accessions (online Supplementary Table S4). Progeny from viable synthetic amphiploids grew vigorously (online Supplementary Fig. S3) and some of them produced more than 30 spikelets (Fig. 1).

Fig. 1. Examples of spike morphology of amphiploids. (1) Syn-TAM-1, (2) Syn-TAM-2, (3) Syn-TAM-3, (4) Syn-TAM-4, (5) Syn-TAM-5, (6) Syn-TAM-6, (7) Syn-TAM-10, (8) Syn-TAM-11, (9) Syn-TAM-13, (10) Syn-TAM-14, (11) Syn-TAM-15, (12) Syn-TAM-25, (13) Syn-TAM-26, (14) Syn-TAM-27, (15) Syn-TAM-28, (16) Syn-TAM-29, (17) Syn-TAM-33, (18) Syn-TAM-35, (19) Syn-TAM-37, (20) Syn-TAM-38, (21) Syn-TAM-39, (22) Syn-TAM-41, (23) Syn-TAM-42, (24) Syn-TAM-43, (25) Syn-TAM-44, (26) Syn-TAM-46.
Chromosome observations in amphiploids
S3 progeny from nine amphiploids were observed to contain around 40–42 chromosomes (Table 1). Plants from these nine lines that contained 42 chromosomes were used for multicolour FISH using probes Oligo-pSc119.2-1, Oligo-pTa71-2, Oligo-pTa535-1 and (AAC)5 that are largely specific for the B genome, sub-terminal regions of 1BS and 6BS, Am and A genomes, and chromosome 6Am, respectively. The red coloured Oligo-pSc119.2-1 probe gave strong signals on all the B genome chromosomes and weaker signals at the terminal end of the short or long arm on three A chromosomes (2A, 4A and 5A) (Fig. 2). The yellow coloured Oligo-pTa71-2 probe produced strong signals at the sub-terminal regions of chromosomes 1BS, 6BS (Fig. 2). The green coloured Oligo-pTa535-1 probe, which hybridized mainly to chromosomes of the Am and A genomes (Fig. 2), could distinguish these two karyotypes with the inclusion of probe (AAC)5 which identifies chromosome 6Am. The probe (AAC)5 gave strong signals on chromosome 6Am (Fig. 2(a)), which was different from signals on the other Am chromosomes. The probe (AAC)5 gave no signals on chromosome 6A of the examined tetraploid parent. Combining these four probes successfully discriminated the entire 42 chromosomes of synthetic T. turgidum–T. monococcum amphiploids (Fig. 2(a)).
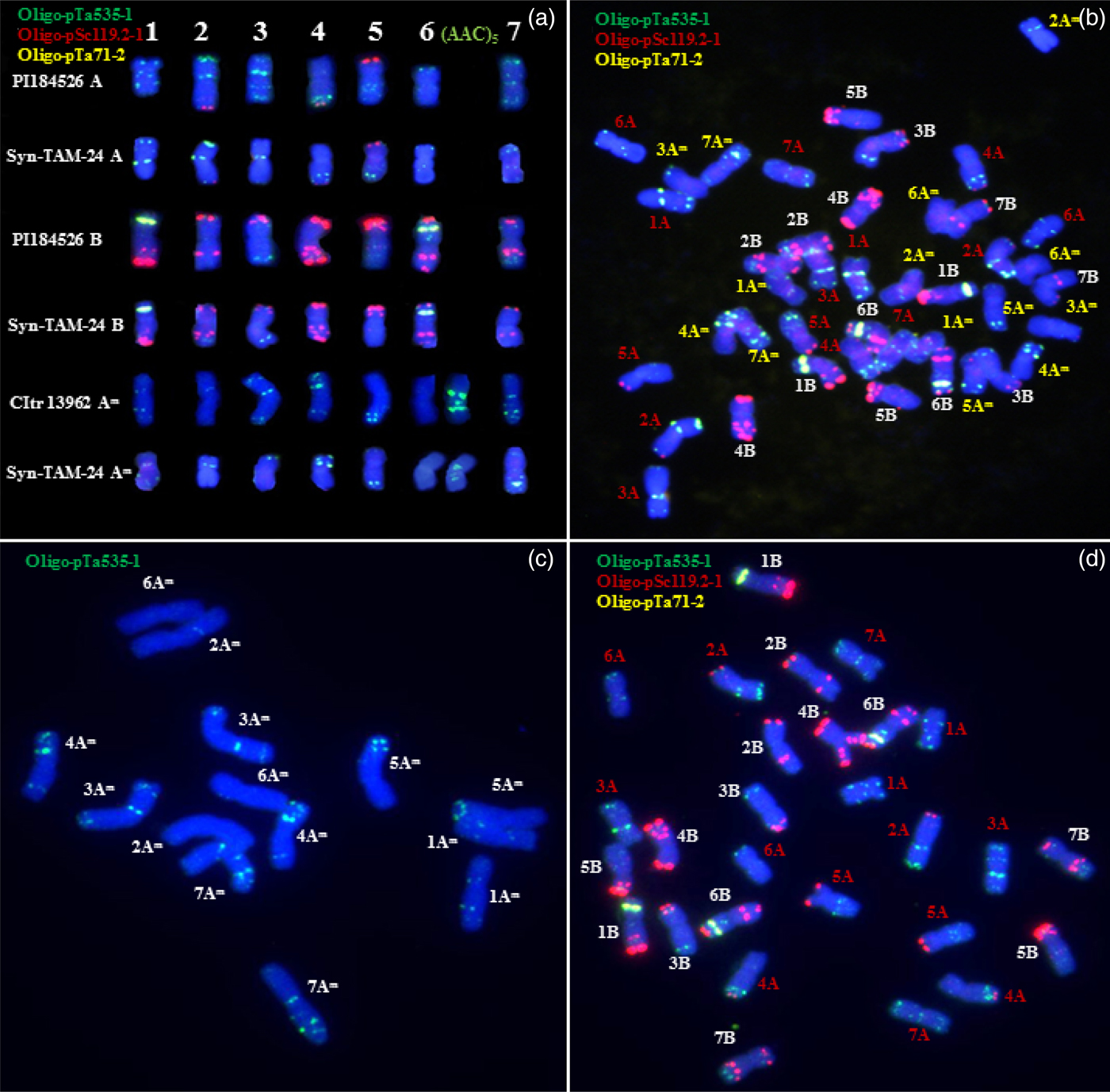
Fig. 2. Examples of FISH identification using four synthetic oligonucleotides probes of Oligo-pSc119.2-1 (red), Oligo-pTa535-1 (green), Oligo-pTa71-2 (yellow) and (AAC)5 (green). FISH karyotypes of A, B, Am genomes in Syn-TAM-24 and its parents (a), a cell of Syn-TAM-24 (b), T. monococcum ssp. monococcum CItr13962 (c), and T. turgidum ssp. turanicum PI184526 (d).
Table 1. Chromosome number distribution and chromosome pairing of T. turgidum–T. monococcum amphiploids

a I, univalent; II, bivalent; III, trivalent; IV, quadrivalent; V, pentavalent.
S3 plants with 42 chromosomes and analysed by FISH were also used for meiotic analysis of chromosome pairing in PMCs at metaphase I (Table 1, online Supplementary Fig. S4). Most of the 42 chromosomes paired as bivalents, while a low number of trivalents, quadrivalents and pentavalents were also observed. The presence of these multi-valents suggests that pairing between Am and A chromosomes occurred, while pentavalents may be a consequence of chromosome rearrangements such as translocation.
SDS-PAGE analysis
S3 seeds from 56 T. turgidum–T. monococcum amphiploids and their parents were used for SDS-PAGE analysis. The 31 T. monococcum parents of these amphiploids collectively expressed six Glu-A1mx proteins (online Supplementary Table S5; Li et al., Reference Li, Li, Zeng, Zhao, Chen, Kou, Ning, Yuan, Zheng, Liu and Zhang2016). Four of these T. monococcum proteins, Glu-A1m-b, Glu-A1m-c, Glu-A1m-d and Glu-A1m-h, were detected in numerous amphiploids (three, three, 31 and one line, respectively) (Fig. 3). However, this analysis was compromised by the co-migration of different Glu-A1x proteins present in T. turgidum and T. monococcum. Specifically T. monococcum Glu-A1m-c, Glu-A1m-d, Glu-A1m-e and Glu-A1m-f proteins had similar electrophoretic mobility to the Glu-A1x proteins of T. turgidum parents of two, 11, three and two amphiploids, respectively. It was therefore not possible to distinguish the parental origin of Glu-A1 proteins in these lines (online Supplementary Table S5).

Fig. 3. SDS-PAGE profiles of HMW-GSs in some amphiploids and their parents. (1) AS2310, (2) Syn-TAM-38, (3) PI355517 (Glu-A1m-b), (4) AS2637, (5) Syn-TAM-3, (6) CItr13961 (Glu-A1m-c), (7) PI94670, (8) Syn-TAM-8, (9) PI503874 (Glu-A1m-d), (10) AS2334, (11) Syn-TAM-56, (12) PI355521 (Glu-A1m-h), bread wheat CY12, cv. Chuanyu 12 (1, 7 + 8, 5 + 10); LM1, cv. Longfumai 1 (2*, 7 + 8, 5 + 10); and CS, cv. Chinese Spring (7 + 8, 2 + 12). The Glu-A1mx alleles expressed in amphiploids were indicated by white arrows.
Evaluation for stripe rust resistance
Field evaluation of stripe rust resistance showed that 80% of amphiploids (45/56), 74% of tetraploid parents (23/31) and 74% of diploid parents (21/31) were resistant (IT: 1–4) at the seedling stage to the mixed rust inoculum (online Supplementary Table S5). Amongst these plants at the adult stage, 89% (50), 65% (20) and 100% (31) of amphiploids, tetraploid parents and diploid parents were resistant, respectively (IT: 1–4). Forty-five amphiploids (80%) (Fig. 4), 19 tetraploid parents (61%) and 23 diploid parents (74%) were resistant to stripe rust disease at both the seedling and adult plant stages.

Fig. 4. Stripe rust resistance at the adult stage of some amphiploids. (1) The bread wheat check SY95-71, (2) Syn-TAM-1, (3) Syn-TAM-2, (4) Syn-TAM-3, (5) Syn-TAM-4, (6) Syn-TAM-5, (7) Syn-TAM-6, (8) Syn-TAM-10, (9) Syn-TAM-11, (10) Syn-TAM-13, (11) Syn-TAM-14, (12) Syn-TAM-15.
Five amphiploid lines (Syn-TAM-12, Syn-TAM-17, Syn-TAM-22, Syn-TAM-51 and Syn-TAM-53), the tetraploid parent PI221401 and the eight diploid parents (CItr13961, CItr13962, CItr17652, CItr17653, CItr17662, PI265008, PI355521 and PI560726) were susceptible at the seedling stage but resistant at the adult plant stage (online Supplementary Table S5). These lines are potentially useful germplasm sources for incorporating adult plant resistance into breeding programmes.
Reduction of stripe rust resistance was observed for both seedling and adult plant resistance in some amphiploids (online Supplementary Table S5). At the seedling stage, the resistance from three T. monococcum lines (PI503874, PI518452 and PI560727) was completely lost in their amphiploid derivatives (Syn-TAM-8, Syn-TAM-9 and Syn-TAM-23), while the resistance from some lines was partially reduced in their amphiploid. Similar situations were also appeared at the adult plant stage. Some factors such as chromosome absence and suppression under the new amphiploid background could cause resistance loss or reduction.
Discussion
The success or failure of interspecific hybridization largely depends on crossability. Crossability is hence an important factor for developing amphiploids (Megyeri et al., Reference Megyeri, Mikó, Molnár and Kovács2011). Our results demonstrate that crossability between T. turgidum ssp. durum and T. monococcum ssp. monococcum is affected by parental genotypes (The and Baker, Reference The and Baker1975; Gul Kazi et al., Reference Gul Kazi, Rasheed, Bashir, Bux, Aziz Napar and Mujeeb-Kazi2011). In this study, some T. turgidum and T. monococcum genotypes showed high crossability thereby enabling the successful development of new amphiploids, while crosses between other genotypes were unsuccessful.
Resistance suppression can be a problem when transferring resistance from a lower ploidy level (Kema et al., Reference Kema, Lange and van Silfhout1995; Ma et al., Reference Ma, Singh and Mujeeb-Kazi1997; Knott, Reference Knott2000; Ahmed et al., Reference Ahmed, Bux, Rasheed, Gul Kazi, Rauf, Mahmood and Mujeeb-kazi2014). In this study, stripe rust resistance from T. monococcum was probably suppressed in several T. turgidum–T. monococcum amphiploids both at seedling and adult plant stages. However, 50 amphiploids exhibited adult plant resistance to the current predominant Chinese stripe rust races and 45 were resistant at both the seedling and adult plant stages, with some lines carry resistance from both T. turgidum and T. monococcum. These novel amphiploids are promising genetic resources for introducing new wheat stripe rust resistance into breeding programmes.
HMW-GSs are components of the glutenin polymer and play a key role in determining the unique visco-elastic properties of wheat dough (Payne Reference Payne1987). T. monococcum ssp. monococcum is considered a valuable resource for wheat bread-making quality improvement (Tranquilli et al., Reference Tranquilli, Cuniberti, Gianibelli, Bullrich, Larroque, MacRitchie and Dubcovsky2002). Variation at the Glu-A1x locus in common wheat is rare, however, diverse Glu-A1mx alleles are present in T. monococcum ssp. monococcum (Li et al., Reference Li, Li, Zeng, Zhao, Chen, Kou, Ning, Yuan, Zheng, Liu and Zhang2016, Reference Li, Li, Chen, Liu, Kou, Ning, Yuan, Hao, Liu and Zhang2017). In this study, Glu-A1m-b, Glu-A1m-c, Glu-A1m-d and Glu-A1m-h proteins were detected in amphiploid plants that could potentially further improve wheat quality.
Chromosome pairing and recombination between Am and A genomes is essential for transferring genes from T. turgidum–T. monococcum amphiploids into bread wheat. Meiosis of PMCs in hybrids between T. turgidum ssp. dicoccum and T. monococcum was described by Mather (Reference Mather1936) and a maximum of seven configurations found. Meiotic analysis from three T. aestivum/T. monococcum hybrids showed on average five bivalents and 0.16 trivalents per cell (Cox et al., Reference Cox, Harrell, Chen and Gill1991). In our study, multi-valent chromosome pairing was also observed at meiosis in amphiploids. These studies suggest that chromosome pairing does occur between Am and A chromosomes, enabling amphiploids to be used as a ‘bridge’ to transfer useful genes from T. monococcum into bread wheat.
FISH was an effective tool for identifying chromosomes from the A and B genomes of T. turgidum and Am genome of T. monococcum ssp. monococcum (Megyeri et al., Reference Megyeri, Farkas, Varga, Kovács, Molnár-Láng and Molnár2012, Reference Megyeri, Mikó, Farkas, Molnár-Láng and Molnár2017). In this study, the combination of oligonucleotide probes Oligo-pSc119.2-1, Oligo-pTa71-2, Oligo-pTa535-1 and (AAC)5 successfully differentiated individual chromosomes originating from T. turgidum and T. monococcum ssp. monococcum in newly synthesized T. turgidum–T. monococcum amphiploids. These probes can be further used as cytological markers in future breeding with these T. turgidum–T. monococcum amphiploids.
In conclusion, we have produced new T. turgidum–T. monococcum amphiploids that are potentially valuable resources for wheat improvement. Ongoing work will select those T. turgidum–T. monococcum amphiploids lines with useful traits and then introduce these traits into bread wheat followed by backcrossing. It is envisaged that these new traits will make a significant contribution to future wheat improvement.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262118000175.
Acknowledgements
The authors thank Professor Qiuzhen Jia, Gansu Academy of Agricultural Sciences, for providing Chinese stripe rust races. This research was supported by the National Key Research and Development Program (2017YFD0100904), the National Natural Science Foundation of China (31671682, 31671689, 31701426) and the Major International (Regional) Joint Research Project (31661143007).







