INTRODUCTION
Cellular differentiation is a process in which the morphology and physiology of a cell is transformed dramatically. Cell differentiation is generally studied in the context of developmental biology where less specialized cells become specialized ones, leading to the formation of tissues and organs. However, this is an evolutionary conserved mechanism. Several cell types are known to differentiate in order to evade death due to genome instability or to overcome hostile environments as observed in the unicellular pathogen evasion of environmental, physiological and pharmacological stresses respectively. When faced with harsh conditions, amoebae and other protists commonly switch their phenotype to form cysts, a process called ‘encystation’; however, little is known of the underlying mechanisms that mediate encystation. In general, the present drug regimen is targeted against the functional aspects of parasites, rather than their structure. Being dormant, cysts have little or no metabolic activities making the available drugs futile and thus presenting a therapeutic nightmare. For example, treatment against Acanthamoeba keratitis involves aggressive application of topical drugs for up to a year and even then recurrence can occur (Martinez and Visvesvara, Reference Martinez and Visvesvara1997; Marciano-Cabral and Cabral, Reference Marciano-Cabral and Cabral2003; Khan, Reference Khan2006; Visvesvara et al. Reference Visvesvara, Moura and Schuster2007). The ability to switch phenotype is rather like the two-faced drama, ‘Dr Jekyll and Mr Hyde’. Such a dramatic shift in phenotype is fascinating and complex, yet it involves a highly orchestrated set of events. A complete understanding of the molecular processes leading to encystation can help us find new targets for chemotherapy.
LIFE CYCLE OF ACANTHAMOEBA SPP
Acanthamoeba undergoes 2 stages during its life cycle; an active trophozoite stage during which Acanthamoeba reproduces, and a dormant cyst stage (Fig. 1). The trophozoite stage dominates when the growth conditions are optimal i.e. abundant food supply, neutral pH, appropriate temperature (i.e. 30°C) and osmolarity between 50 and 80 mOsmol. Notably, Acanthamoeba exhibits growth at a variety of temperatures, pH, osmolarity and hydrostatic pressures. Acanthamoeba reproduces asexually by binary fission, resulting in 2 genetically identical daughter cells (Byers et al. Reference Byers, Kim, King and Hugo1991). Most of the cellular contents are synthesized continuously so the cell mass gradually increases as the cell approaches division leading to mitosis and cytokinesis. In axenic cultures, Acanthamoeba shows a typical exponential growth phase, followed by a period of reduced growth rate, called the ‘growth deceleration phase’, and finally the stationary phase during which no further increase in cell density occurs (Band and Mohrlok, Reference Band and Mohrlok1973; Stevens and Pachler, Reference Stevens and Pachler1973). Later studies have revealed that the majority of Acanthamoeba cells of the stationary phase culture develop into cysts (Stohr et al. Reference Stohr, Bommert, Schulze and Jantzen1987). Overall, it can be concluded that trophozoites are generated in the mitotic division cycle, whereas cells committed at late G2 phase of the cell cycle develop into cysts in response to adverse conditions such as (i.e. lack of food, hyper- or hypo-osmolarity, extremes in temperature and pH, high cell densities, and chemicals) (Neff and Neff, Reference Neff and Neff1969; Band and Mohrlok, Reference Band and Mohrlok1973; Stohr et al. Reference Stohr, Bommert, Schulze and Jantzen1987; Jantzen and Schulze, Reference Jantzen and Schulze1988). Encystation is accompanied by morphological changes, termination of cell growth, and biochemical differences. In simple terms, the trophozoite becomes metabolically inactive (minimal metabolic activity) after an initial burst of metabolic activity, and encloses itself within a resistant shell. The cellular levels of RNA, proteins, triacylglycerides and glycogen declines substantially during the encystation process resulting in decreased cellular volume and dry weight (Weisman, Reference Weisman1976). In addition, excess food, water and particulate matter is expelled with a decrease in cytoplasmic mass and the trophozoite condenses itself into a double-walled cyst with the wall serving only as a shell to help survive hostile conditions. The two walls are 5–20 μm in diameter. The outer cyst wall, the ectocyst, is wrinkled with folds and ripples and contains proteins and lipids. The inner cyst wall, the endocyst, contains cellulose and hence stains positive using the Periodic acid-Schiff reagent. Both walls meet at pores or ostioles that are covered by convex-concave plugs or opercula. The cyst walls contain at least 2 major products that are not detected in the trophozoite stage; cellulose (Tomlinson and Jones, Reference Tomlinson and Jones1962) and an acid-insoluble protein-containing material (Neff and Neff, Reference Neff and Neff1969). The former has been reported to be the major component of the inner wall of the cyst (also known as the endocyst). Enzymes for the synthesis and breakdown of cellulose have been identified in Acanthamoeba, which are likely to participate in encystation and excystation (Anderson et al. Reference Anderson, Watkins, Samuelson, Spencer, Majoros, Gray and Loftus2005). Later studies have identified a cyst-specific protein of 21 kDa that is absent in the trophozoite stage of Acanthamoeba. The expression of the cyst-specific protein-21 mRNA is restricted to the early stages of encystation (Hirukawa et al. Reference Hirukawa, Nakato, Izumi, Tsuruhara and Tomino1998). Cysts are viable airborne cells that can withstand prolonged starvation, desiccation, cold, heat, and thus, may help spread Acanthamoeba in the environment and/or carry these pathogens to susceptible hosts. The cysts can remain viable for decades while maintaining their pathogenicity, thus presenting a role in the transmission of Acanthamoeba infections (Mazur et al. Reference Mazur, Hadas and Iwanicka1995; Sriram et al. Reference Sriram, Shoff, Booton, Fuerst and Visvesvara2008).
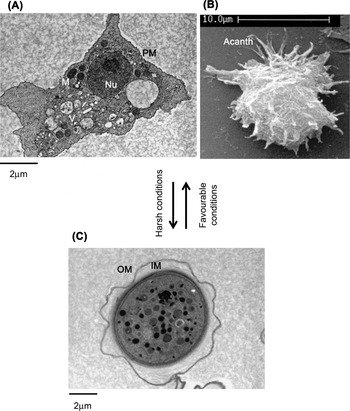
Fig. 1. Life cycle of Acanthamoeba. Under harsh conditions, Acanthamoeba trophozoites transform into the resistant cyst form, while favourable conditions permit vegetative growth. (A) Transmission electron micrograph of trophozoite stage of Acanthamoeba. (B) Scanning electron micrograph of Acanthamoeba trophozoite showing spike-like acanthopodia. (C) Transmission electron micrograph of Acanthamoeba cyst. Nu, nucleus; V, vacuoles; M, mitochondria; PM, plasma membrane; Acanth, acanthopodia; OU, outer membrane; IM, inner membrane.
ENCYSTATION AT THE MORPHOLOGICAL-LEVEL
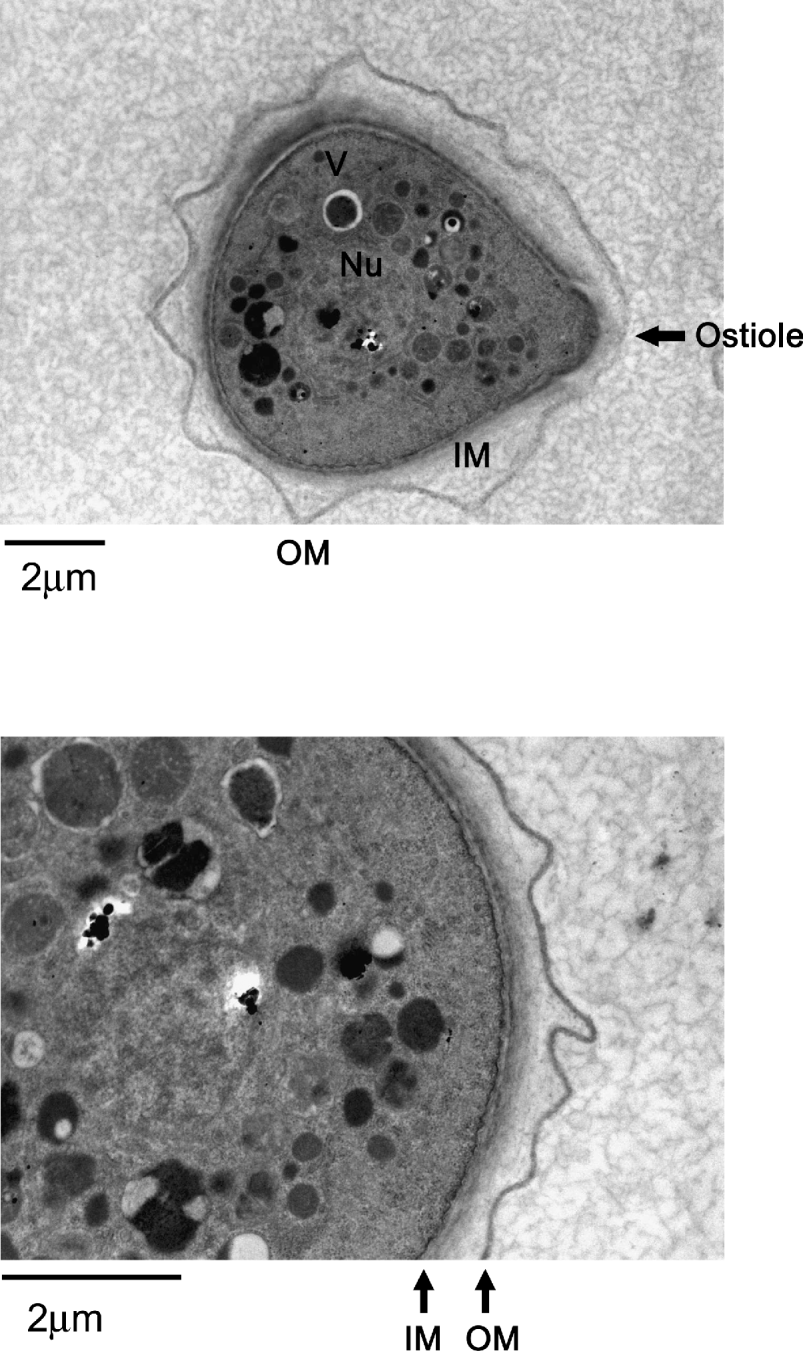
The morphological and intracellular changes of encystation are observed within a few hours after inoculating Acanthamoeba trophozoites into nutrient-free medium. This is evident by the accumulation of organelles around the nucleus, which become significant with time. There is an absence of any major organelles outside this circular region, which became prominent with time. By 48 h, a double-walled cyst is observed with many intracellular organelles including mitochondria left out in the discarded region of the cell. This subsequently leads to the formation of a complete double-walled cyst. Notably, the reduction in cell surface area (loss of cell membrane) and cell volume (intracellular contents) is more than 50% (Bowers and Korn, Reference Bowers and Korn1969). In the dormant cyst form, Acanthamoeba is able to monitor environmental conditions via a thin membranous ostiole (Fig. 2). Under favourable conditions, the ostiole membrane disintegrates and Acanthamoeba emerges in the vegetative trophozoite form. The dense cytoplasm of encysted cells suggests that volume change occurs partially as a result of dehydration. Under the scanning electron microscope, the morphological changes are visible and include thickening or shortening of the acanthopodia until the surface is covered with short stubby processes, followed by the development of thick, interconnecting ridges that characterize the surface of the mature cyst.
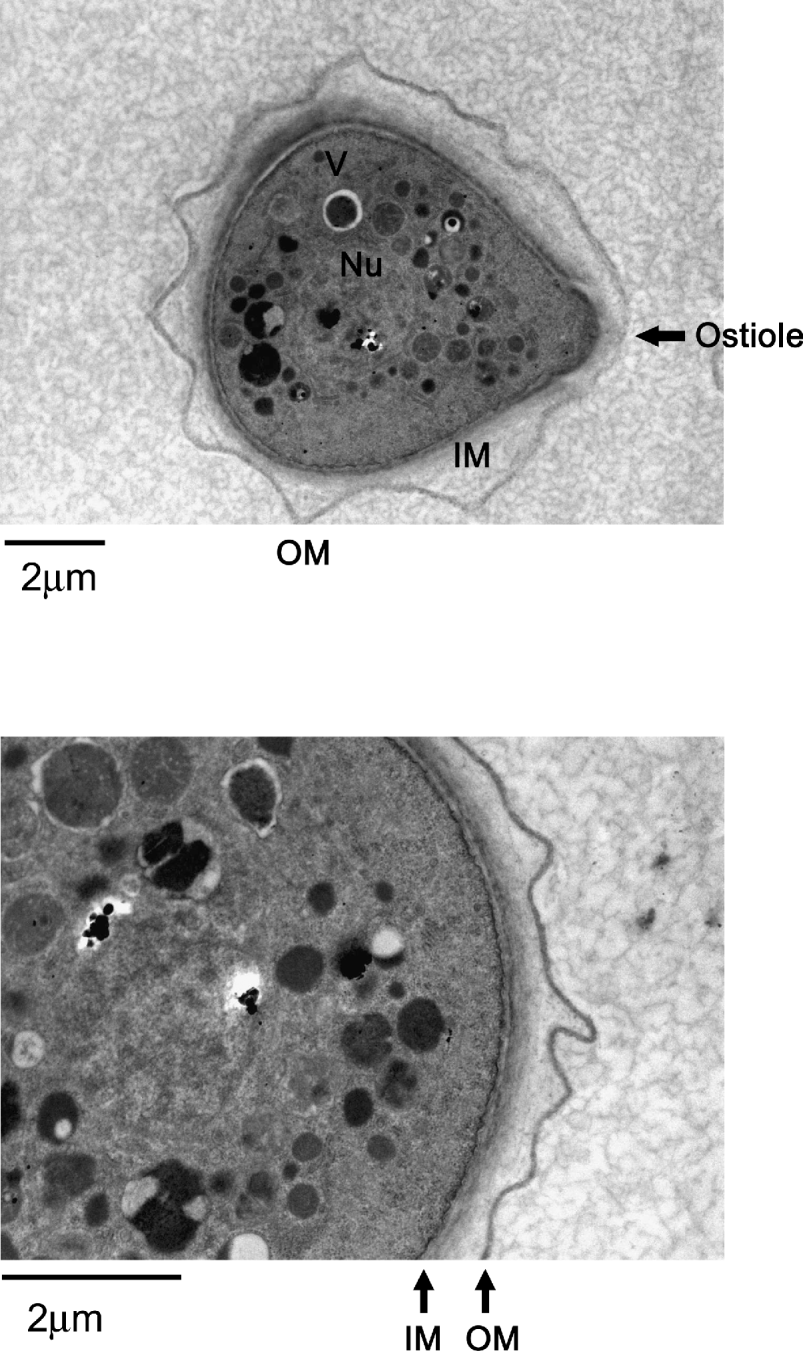
Fig. 2. Representative transmission electron micrographs showing a complete double-walled cyst of Acanthamoeba castellanii belonging to the T4 genotype (American Type Culture Collection, ATCC 50492). Encystment was induced by inoculating trophozoites on non-nutrient agar plates. Samples were collected after 7 days of incubation and processed for TEM. V, vacuoles; Nu, nucleus; IM, inner membrane; OU, outer membrane; arrow shows an ostiole. The results are representative of 3 independent experiments and in each experiment 20 random sections of the slide were selected for observation.
ENCYSTATION AT THE ULTRASTRUCTURAL LEVEL
Nuclear activity
The nuclear volume decreases to at least 40%, while the nucleolar volume decreases to about 75% associated with a partial expulsion of the peripheral chromatin (Volkonsky, Reference Volkonsky1931). In the mature cysts, the nucleolus is seen as a large, dense droplet. The functional activity within the nucleus and of the nucleolar proteins is important for encystation. For example, when Acanthamoeba trophozoites are halved using microsurgical procedures, the nucleated halves encyst but the non-nucleated halves do not form cysts suggesting that nuclear activity (i.e. DNA synthesis) is required during the encystation process (Roti Roti and Stevens, Reference Roti Roti and Stevens1974, Reference Roti Roti and Stevens1975; Martin and Byers, Reference Martin and Byers1976).
Golgi complex
The changes in the Golgi complex are observed both at the level of form and distribution. The Golgi complex of the trophozoite occurs as large aggregates of cisternal stacks, but occurs as smaller aggregates in the encysting cells, accompanied by the appearance of dense staining material within the Golgi complex that are also present on the cell surface. This dense staining content of the Golgi complex gradually diminishes at the time the endocyst is being formed and is usually absent in the mature cysts. The volume fraction of the Golgi complex in encysting cells increases about 6-fold early on in encystation and thereafter returns to its original volume fraction of about 0·5% (Bowers and Korn, Reference Bowers and Korn1969).
Phagocytic and pinocytic activity
Once Acanthamoeba cells enter the stationary phase, phagocytic activity ceases, while the pinocytic activity is halved (Chambers and Thompson, Reference Chambers and Thompson1976). The reduced pinocytic activity remains sensitive to respiratory inhibitors. The water expulsion vesicles remain free of any dense content throughout encystation but are often partially collapsed. Early in encystation, large numbers of vacuoles are observed in the encysting cells and many lysosomes and peroxisomes are present in the mature cysts (Muller, Reference Muller1969; Muller and Moller, Reference Muller and Moller1969). The digestive vacuoles disappear during later stages of encystation and their contents are discharged.
Respiration
The respiration rates of whole cells and isolated mitochondria as well as the intracellular ATP levels of cells diminish (after an initial burst in metabolism) throughout the course of encystation. Significant changes are observed within the mitochondria. Within 1–2 h of induced encystation, coiled lamellate structures appear within mitochondria that are formed by compaction and by coiling of tubular entities that lie in an enlarged space between cristae. The lamellar bodies are lost in the later stages of encystation. The mitochondria of dehydrated cysts are much more condensed and still contain the tubular cristae but are poorly defined relative to those in the trophozoites. The mitochondria are incorporated into autolysosomes but remain at a constant fraction of the cell cytoplasm (Bowers and Korn, Reference Bowers and Korn1969).
Glycogen stores
Among macromolecules, glycogen is the most rapidly degraded molecule during encystation. In the initial phase of encystation, cellular levels of glycogen decrease by one half to two thirds within 4 h and then fall to 10–20% of the original amount. Lysosomal hydrolases are probably most likely responsible for the rapid degradation of glycogen. Both lysosomes and peroxisomes have been isolated from Acanthamoeba (Muller, Reference Muller1969; Muller and Moller, Reference Muller and Moller1969) that contain various enzymes including α-glycosidases, amylase, β-galactosidase, β-N-acetyl-glucosaminidase, β-glucuronidase, protease, phosphatase, acid hydrolase, RNAse, and DNAse (Weisman, Reference Weisman1976). The autolysed material is used as a source of precursors for cell wall synthesis or as a source of energy for the cell that has no external food source (Bowers and Korn, Reference Bowers and Korn1969). Hydrolytic degradation of glycogen would ultimately yield glucose, which must be phosphorylated by ATP for its conversion to glucose-1-phosphate prior to UDP-glucose (uridine diphosphate glucose) and cellulose formation. In addition, glycogen phosphorylase breaks down glycogen to yield glucose-1-phosphate directly (Verma and Raizada, Reference Verma and Raizada1975). The role of glycogen phosphorylase in Acanthamoeba encystation was shown by silencing the catalytic domain of the glycogen phosphorylase using the RNA interference method. It was shown that glycogen phosphorylase enzyme is required for cyst wall assembly, mainly for the formation of the endocyst (inner cell wall) of Acanthamoeba (Lorenzo-Morales et al. Reference Lorenzo-Morales, Kliescikova, Martinez-Carretero, De Pablos, Profotova, Nohynkova, Osuna and Valladares2008).
Cytoskeleton
The cytoplasmic microtubules can be observed at all stages of encystation.
During outer wall (i.e. ectocyst) deposition, the cytoplasmic microtubules seem to be confined to the central region of Acanthamoeba. Later, during inner wall (i.e. endocyst) deposition, microtubules are found in all regions of the cell. Actin synthesis is reduced about 2-fold during early stages of encystation while actin protein synthesis is completely shut down during the late stages of encystation, suggesting the translational regulation of actin synthesis (Jantzen, Reference Jantzen1981; Byers et al. Reference Byers, Kim, King and Hugo1991). This is supported by findings of actin turnover during encystation. For example, during the exponential growth phase, no actin degradation was observed; however, as Acanthamoeba entered the stationary phase, a dramatic increase in the amount of actin was observed (Rubin Reference Rubin and Maherand Maher, 1976).
Cell wall synthesis
The cyst wall consists of a laminar, fibrous outer layer, (i.e. ectocyst), and an inner layer of fine fibrils (i.e. endocyst) both of which are stained using Periodic acid-Schiff's suggesting the presence of glycans. Both layers are normally separated by a space, except at certain points where they form opercula in the centre of ostioles (exit points for excysting Acanthamoeba trophozoite). The ectocyst is the first to be synthesized in the encysting cells and appears as an amorphous, discontinuous layer about 10 μm thick just outside the plasma membrane (Bowers and Korn, Reference Bowers and Korn1969). The ectocyst terminates in a loose fibrous layer and the entire ectocyst is 0·3–0·5 μm in thickness and fibrils appear to be less than 50 Å in diameter. The endocyst differs from the ectocyst in texture and appears finely granular but as for the ectocyst, the fibrils are less than 50 Å in diameter. The ectocyst is thinner in the operculum than elsewhere in the wall whereas the endocyst is generally thicker than elsewhere in the wall. During encystation, there is no consistent or well-defined space between the plasma membrane and the endocyst; but in the mature cyst there is a space of about 0·1μm between the endocyst and the plasma membrane. The endocyst is shown to possess cellulose that accounts for nearly one third of the dry weight of the cyst wall (Tomlinson and Jones, Reference Tomlinson and Jones1962). The inaction of cellulase against cysts indicates that the cellulose exists as an inner and inaccessible layer of the wall (Neff and Neff, Reference Neff and Neff1969; Barrett and Alexander, Reference Barrett and Alexander1977). The ectocyst consists of polysaccharides and proteins, among which is a 70 kDa protein (Rubin et al. Reference Rubin, Hill, Hepworth and Boehmer1976; Barrett and Alexander, Reference Barrett and Alexander1977). The 70 kDa protein is synthesized during the encystation process and represents 15% of the dry weight of the mature cysts (Neff and Neff, Reference Neff and Neff1969). The presence of hydroxyproline in the ectocyst is reported (Bauer, Reference Bauer1967). On this basis, it is suggested that the two walls are also different in their chemical composition.
Overall, the cyst wall composition for A. castellanii belonging to the T4 genotype has been shown to contain 33% protein, 4–6% lipid, 35% carbohydrates (mostly cellulose), 8% ash, and 20% unidentified materials (Neff and Neff, Reference Neff and Neff1969; Weisman, Reference Weisman1976), although it is anticipated that the cyst wall composition varies between isolates belonging to different species/genotypes (Weisman, Reference Weisman1976; Khan, Reference Khan2006). The precursor of cellulose is glucose, the source of which is glycogen. Glycogen is a storage polysaccharide and consists of glucose repeating subunits linked by α(1→4) glycosidic bonds. Glycogen is converted into glucose-1-phosphate by glycogen phosphorylase (Weisman, Reference Weisman1976; Lorenzo-Morales et al. Reference Lorenzo-Morales, Kliescikova, Martinez-Carretero, De Pablos, Profotova, Nohynkova, Osuna and Valladares2008). Next, glucose-1-phosphate uridylyltransferase (or UDP-glucose pyrophosphorylase) enzyme synthesizes UDP-glucose from glucose-1-phosphate and UTP as follows (Weisman, Reference Weisman1976):
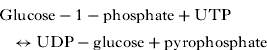
The glucose subunits from UDP-glucose are incorporated into the cell wall as β(1→4)-glucans (i.e. cellulose) (Weisman, Reference Weisman1976). The level of UDP-glucose increases 5-fold within 5 h of the induction of encystation. However, the specific activity of UDP-glucose pyrophosphorylase, which catalyses the synthesis of UDP-glucose remained relatively constant during the 20 h after the induction of encystation but rapidly decreased thereafter (Rudick and Weisman, Reference Rudick and Weisman1974). Overall, UDP-glucose is used as a glucosyl donor in the synthesis of cellulose (Weisman, Reference Weisman1976). In contrast, during the developmental programmes of Dictyostelium discoideum, there is a 10-fold increase in the UDP-glucose pyrophosphorylase specific activity (William and Loomis, Reference William, Loomis and 1969). However, the activity of β-glucan synthetase increases some 30-fold, whose function is to catalyse the formation of β(1→4) glucan (cellulose), similar to the plant cells (in contrast, fungal cell wall cellulose can contain either β(1→3) glucan or β(1→4) glucan). Both alkali-soluble and alkali-insoluble β-glucan occur in the Acanthamoeba cyst walls (Weisman, Reference Weisman1976). This results in the formation of cellulose, i.e. rigid, linear rods that aggregate laterally into microfibrils (25 nm in diameter and composed of 2000 cellulose chains). Thus encystation is associated with the appearance of the β-glucan synthetase, cyst wall cellulose and the formation of the mature cyst (Weisman, Reference Weisman1976).
ENCYSTATION AT THE MOLECULAR LEVEL
RNA and protein synthesis
The RNA levels decrease 50% during encystation. There is a decline of 70–80% of the rRNA within 1 h of transfer into the non-nutrient medium (Schulze and Jantzen, Reference Schulze and Jantzen1982), while rRNA synthesis could not be detected after the cells had been incubated in the non-nutrient medium for 7 h. The amount of RNA polymerase I, II, III activities from whole cells remains constant during encystation (Stevens and Pachler, Reference Stevens and Pachler1973; Detke and Paule, Reference Detke and Paule1978, 1979). The presence of actinomycin or cycloheximide, inhibitors of RNA and protein synthesis, respectively, prevents the formation of mature cysts, suggesting that synthesis of biomolecules is crucial for the encystation process (Jantzen, Reference Jantzen1981).
Lipids
Acanthamoeba shows a significant decrease in the surface area during encystation (more than 65%). A large reduction in cell surface implies a significant loss of the cell membrane. In addition, smooth endoplasmic reticulum is also absent that is normally present in the trophozoite. The levels of lysophosphatidylcholine, lysophos-phatidylethanolamine, phosphatidylcholine, phosphatidylethanolamine and alkali-stable phospholipids I, and II are significantly lower in the mature cysts of Acanthamoeba, compared with the trophozoites. The major constituent sterols of trophozoites, ergosterol, 7-dehydrostigmasterol and 7,22,25-tride-hydrostigmasterol are not detected in the cysts (Mehdi and Garg, Reference Mehdi and Garg1987).
Other molecular changes
During encystation, Acanthamoeba releases polyphosphate into the encystation medium (Deslauriers et al. Reference Deslauriers, Jarrell, Byrd and Smith1980). The immature cysts show detectable levels of nucleotide diphosphate and triphosphate similar to the vegetative cells, while mature cysts only excreted polyphosphate as well as a component which has a chemical shift of a phosphodiester (Deslauriers et al. Reference Deslauriers, Jarrell, Byrd and Smith1980). The levels of cyclic AMP rise to 2 to 3-fold within 6–8 h (due to changes in adenylate cyclase), and then reduce to levels similar to the exponential growth phase levels. The adenylate cyclase activity of Acanthamoeba during exponential growth phase is generally low but rises 2 to 4-fold during the stationary phase and reaches to peak at the time when cysts are detectable in the cultures (Achar and Weisman, Reference Achar and Weisman1980). Cyclic AMP acts via protein kinase-mediated modification of enzymes and proteins and may influence transcriptional-, translational-, and post-translational processes. The role of cyclic AMP in encystation is thought to be mediated via glycogen degradation by converting glycogen phosphorylase to the active form but this remains to be determined. Interestingly, it is reported that cyclic AMP stimulates the phospholipase A activity of Acanthamoeba (Hax et al. Reference Hax, Demel, Spies, Vossenberg and Linnemans1974). Other signalling molecules involved in encystation include 21 kinds of PKC-like genes that are highly expressed during encystation (Moon et al. Reference Moon, Chung, Hong and Kong2011). This is not surprising, as previous studies have shown that the activity of PKC proteins is very important in the encystation process of several organisms. For instance, Dictyostelium discoideum requires autophosphorylation of myosin II heavy chain-specific protein kinase C for its activation and membrane dissociation (Dembinsky et al. Reference Dembinsky, Rubin and Ravid1997) and has a single diacylglycerol kinase gene with similarity to mammalian theta isoforms (De La Roche et al. Reference De La Roche, Smith, Carrasco, Merida, Licate, Cote and Egelhoff2002). Giardia duodenalis expresses several PKC isoforms – beta, delta, epsilon, theta and zeta (Bazan-Tejeda et al. Reference Bazan-Tejeda, Arguello-Garcia, Bermudez-Cruz, Robles-Flores and Ortega-Pierres2007).
In addition to the aforementioned, the activities of isocitrate lyase, glycolate and maleate are involved in encystation. This is shown by the findings that the inhibitors of isocitrate lyase, glycolate and maleate inhibited encystation by 18–67% (Mehdi and Garg, Reference Mehdi and Garg1987). The activity of isocitrate lyase in the encysting cells steadily increased up to 24 h of encystation but the activity of isocitrate dehydrogenase steadily decreased up to 32 h of encystation and thereafter no activity was detected.
Encystation is characterized by a decrease in acid phosphatase, deoxyribonuclease and acid ribonuclease with acid ribonuclease decreasing most rapidly while acid phosphatase decreases least rapidly (Martin and Byers, Reference Martin and Byers1976). Acanthamoeba also possesses phenol oxidase activity. The localization of phenol oxidase is in the cytoplasm of encysting Acanthamoeba and in the outer cyst wall, but whether this enzyme plays a role in encystation remains to be determined (Sykes and Band, Reference Sykes and Band1985).
Encystation is associated with the upregulation of genes that encode proteins with homology to xylose isomerase, Na P-type ATPase, and subtilisin-like serine protease (Moon et al. Reference Moon, Chung, Hong and Kong2007). In addition, cyst-specific protein 21, protein kinase C, proteasome and heat shock protein, as well as several genes like cullin 4, autophage protein 8 and ubiquitin-conjugating enzymes were identified and found to be related to encystation (Moon et al. Reference Moon, Chung, Hong, Ahn and Kong2008).
Calcium-binding sites and encystation
During the vegetative trophozoite stage, Acanthamoeba binds to calcium as shown by the appearance of calcium deposits on the cell surface membrane (Sobota and Przelecka, Reference Sobota and Przelecka1981a, Reference Sobota and Przeleckab). During the early stages of encystation, calcium is observed on the cell surface as large deposits (70–80 nm) (Sobota and Przelecka, Reference Sobota and Przelecka1981a, Reference Sobota and Przeleckab). Later in differentiation, calcium deposits (170 nm) appear in mitochondria while no deposits are observed on the plasma membrane (Sobota and Przelecka, Reference Sobota and Przelecka1981a, Reference Sobota and Przeleckab). Although the role of calcium deposits in encystation remains unknown, it is suggested that the plasma membrane or the mitochondria may be involved in storage of excess cellular calcium during various stages of the life cycle of Acanthamoeba (Sobota and Przelecka, Reference Sobota and Przelecka1981a, Reference Sobota and Przeleckab).
EXCYSTATION
The cysts possess pores known as ostioles, identified by the presence of an operculum surrounded by a circular ridge that is evident on the surface of the mature cysts. Ostioles are possibly used to monitor environmental changes. The trophozoites emerge from the cysts under favourable conditions leaving behind the outer shell. In the pre-emergent stage, a contractile vacuole becomes evident and moves towards the wall. The Acanthamoeba trophozoite then pulls away from the endocyst and moves freely within the cyst wall. The first indication of the emergence of trophozoites is visible by scanning electron microscopy as the appearance of a cytoplasmic bud pushing through the ostioles, from which the operculum had been removed (Chambers and Thompson, Reference Chambers and Thompson1976; Dudley et al. Reference Dudley, Alsam and Khan2008). The emerging cytoplasmic bud does not possess the long acanthopodia, which are characteristics of the trophozoites. These acanthopodia do not appear until the trophozoites have completely excysted. The hole through which the trophozoites emerge is apparent on the surfaces of the empty cyst walls, otherwise surfaces of the empty cyst walls are indistinguishable from those of mature cysts and exhibit sculptured interconnecting ridges surrounded by shallow craters (Chambers and Thompson, Reference Chambers and Thompson1976). Thus the Acanthamoeba trophozoites emerge without digestion of the cyst wall. The emerged trophozoites actively reproduce as described above, and so complete the cycle.
REGULATORS OF THE LIFE CYCLE
Both the encystation and excystation processes require active macromolecule synthesis and can be blocked by cycloheximide (a protein synthesis inhibitor), actinomycin D, and 5-bromodeoxyuridine (that can be incorporated into the DNA in place of thymidine) (Roti Roti and Stevens, Reference Roti Roti and Stevens1974). In addition, recent studies have shown that Acanthamoeba serine proteases are crucial in the differentiation of A. castellanii belonging to the T4 genotype. Using selective protease inhibitors, in tandem with siRNA primers, specific to the catalytic site of Acanthamoeba serine proteases, it was observed that the inhibition of serine proteases attenuates Acanthamoeba metamorphosis, as demonstrated by the arrest of both encystation and excystation (Moon et al. Reference Moon, Chung, Hong and Kong2007, Reference Moon, Chung, Hong, Ahn and Kong2008; Dudley et al. Reference Dudley, Alsam and Khan2008). More recently, cysteine proteases are suggested to be involved in Acanthamoeba encystation (Leitsch et al. Reference Leitsch, Köhsler, Marchetti-Deschmann, Deutsch, Allmaier, Duchêne and Walochnik2010). In addition, encystation may involve xylose isomerase, P-type ATPase, and subtilisin-like serine protease (Moon et al. Reference Moon, Chung, Hong and Kong2007). Inhibitors of isocitrate lyase, glycolate and maleate at a concentration of 8·5 to 34 mM inhibited encystation by 18–67% (Mehdi and Garg, Reference Mehdi and Garg1987). Acanthamoeba is shown to possess trehalose-6-phosphate synthase, suggesting that trehalose plays a role in the stress adaptation and regulates encystation (Anderson et al. Reference Anderson, Watkins, Samuelson, Spencer, Majoros, Gray and Loftus2005). The detection and response to a number of stress conditions is likely to be accomplished with a large set of signal transduction histidine kinases, and a set of putative receptor serine/threonine kinases, similar to those found in Entamoeba histolytica, has been identified in Acanthamoeba (Anderson et al. Reference Anderson, Watkins, Samuelson, Spencer, Majoros, Gray and Loftus2005). Several other molecular determinants have also been shown to be associated with encystation, including protein kinase C, proteasome, heat shock protein, cullin 4, cyst specific protein-21, ubiquitin-conjugating enzymes, and autophage protein 8 (Moon et al. Reference Moon, Chung, Hong and Kong2007, Reference Moon, Chung, Hong, Ahn and Kong2008). For the latter, autophagy is an evolutionally conserved protein degradation pathway in eukaryotes. It plays an essential role during starvation, cellular differentiation, cell death, and aging by eliminating unwanted or unnecessary organelles and recycling the components for re-use. Autophage protein 8 (ATG8), a member of a novel ubiquitin-like protein family, is an essential component of the autophagic machinery. Recent studies have identified and characterized autophagy protein 8 in A. castellanii (Moon et al. Reference Moon, Chung, Hong and Kong2011). Real-time polymerase chain reaction demonstrated that the A. castellanii Atg8 (AcAtg8) gene encoding a 118 amino acid protein was highly expressed during encystation. Fluorescence microscopic analysis following transient transfection of enhanced green fluorescent protein-AcAtg8 revealed small or large vacuolar fluorescent structures in an encysting amoeba. The Atg8 fluorescent structures on the membrane were identified as autophagosomes by co-localization analysis with LysoTracker. Chemically synthesized small interfering RNA against AcAtg8 reduced the encystation efficiency and inhibited autophagosome formation in Acanthamoeba (Moon et al. Reference Moon, Chung, Hong and Kong2011).
Changes in the polyamine levels were observed during the growth of amoeba, suggesting their possible involvement in Acanthamoeba encystation. Acanthamoeba contains several polyamines including, 1,3-diaminopropane, spermidine, spermine, norspermidine, and putrescine. The polyamine found in the greatest concentration in the growing cells is 1,3-diaminopropane, followed by spermidine and a low level of putrescine. Generally, polyamines are considered as growth factors in both eukaryotic organisms as well as prokaryotic cells. The inhibition of polyamines results in amoebistatic effects. These polyamines are significantly decreased in concentration, as Acanthamoeba trophozoites differentiate into cysts. For metabolites of polyamine and S-adenosylmethionine, it was shown that N8-acetylspermidine and acetylspermine are present both in trophozoites and cysts, while acetylcadaverine is present only in growing Acanthamoeba and N1-acetylspermidine only in cysts. S-adenosylmethionine and S-adenosylhomo-cysteine are present in growing trophozoites but S-adenosylmethionine was undetectable or barely detectable in cysts. S-adenosylhomocysteine also decreased in concentration during encystation. The developmental transition from growing Acanthamoeba trophozoites to dormant cysts is characterized metabolically by a threshold adjustment in the concentration of S-adenosylmethionine, S-adenosylhomocysteine, and of the polyamines (especially 1,3-diaminopropane and spemidine (Zhu et al. Reference Zhu, Cumaraswamy and Henney1989). The enzyme, S-adenosyl-L-methionine decarboxylase (approximate molecular weight of 88·8 kDa) is present in Acanthamoeba (Hugo and Byers, Reference Hugo and Byers1993). Inhibition of this enzyme could affect a variety of cell methylation reactions by causing accumulation of its substrate S-adenosylmethionine and could arrest polyamine synthesis by blocking production of decarboxylated S-adenosylmethionine needed for spermidine synthesis. Interestingly, the addition of polyamines to growing cultures of Acanthamoeba blocked the induction of encystation by diamidines, suggesting the involvement of polyamine biosynthesis in Acanthamoeba encystation.
CONCLUSIONS
We envisage that using eukaryotic organisms such as Acanthamoeba as a model organism will elucidate novel mechanisms for how cells (e.g. stem cells, or protists) differentiate. The use of Acanthamoeba as a model organism is not uncommon, as they have been used to determine cellular mechanisms, for example ‘phagocytosis’. In addition, this unicellular organism has been used extensively to understand the molecular biology of ‘motility’. Acanthamoeba does not differ greatly at the ultrastructural level from a mammalian cell, and thus presents an excellent model for cell biology studies. Knowledge gained from such studies will help identify potential targets in the rational development of therapeutic interventions against infections due to this fascinating and important pathogen.