INTRODUCTION
Neospora caninum is a major cause of infectious bovine abortion worldwide and causes important economic losses to the cattle industry (Dubey et al. Reference Dubey, Schares and Ortega-Mora2007). In spite of the world-wide distribution and broad host range of this parasite, the number of isolates of N. caninum described to date is very limited. Many attempts have been made to obtain viable N. caninum isolates by bioassays in mice or directly in cell culture; however, the isolation in vitro of this parasite is arduous and often unsuccessful. Since the first isolation of the parasite N. caninum from dogs in 1988 (Dubey et al. Reference Dubey, Hattel, Lindsay and Topper1988), isolation from bovine and other intermediate hosts, such as sheep, water buffaloes, and white-tailed deer, has been reported from several countries. In total, no more than 65 isolates have been reported (Dubey et al. Reference Dubey, Schares and Ortega-Mora2007).
Biological characterization among these isolates has revealed genetic diversity (Schock et al. Reference Schock, Innes, Yamane, Latham and Wastling2001; Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Pedraza-Díaz, Gómez-Bautista and Ortega-Mora2006) and significant variation in in vivo pathogenicity (Atkinson et al. Reference Atkinson, Harper, Ryce, Morrison and Ellis1999; Schock et al. Reference Schock, Innes, Yamane, Latham and Wastling2001; Miller et al. Reference Miller, Quinn, Windsor and Ellis2002; Collantes-Fernández et al. Reference Collantes-Fernández, López-Pérez, Álvarez-García and Ortega-Mora2006) and in in vitro growth characteristics (Schock et al. Reference Schock, Innes, Yamane, Latham and Wastling2001; Pérez-Zaballos et al. Reference Pérez-Zaballos, Ortega-Mora, Álvarez-Garcia, Collantes-Fernández, Navarro-Lozano, García-Villada and Costas2005). These differences could influence the clinical outcome of the disease and could be directly related with other important epidemiological features, such as the potential for transmission. The isolate populations analysed in these studies were usually obtained from symptomatic dogs and cattle, stillborn calves or abortions and could therefore be biased towards more virulent isolates. Thus, new isolates from healthy animals should be obtained and biologically characterized in order to investigate the characteristics of N. caninum isolates from infected cattle that are clinically healthy.
In this report, we describe an improved technique to isolate N. caninum from congenitally infected calves. In addition, we genetically characterize 9 new N. caninum isolates that were obtained from calves from different regions of Spain.
MATERIALS AND METHODS
Naturally infected calves, serological analysis, and sample collection
Between 2003 and 2006, 14 calves that originated from 7 Holstein-Friesian dairy herds from different regions in Spain were selected for isolation (Table 1).
Table 1. Description of calves used for Neospora caninum isolation in this study

* Dairy herd prevalence analysed by ELISA (CIVTEST).
# CT13 and CT16 calves were born twins.
& Brain tissues were submitted to laboratory when bovine spongiform encephalopathy was discarded.
a Healthy.
b Neospora prevalence not reported.
c Reproductive data not available.
d Calves from Navarra originated from 2 dairy herds. Number in parentheses identifies dairy herd.
Prior to sacrifice of the calves, congenital Neospora infection was confirmed by indirect fluorescent antibody test (IFAT) analysis of a sample of pre-colostral serum, as described by Álvarez-García et al. (Reference Álvarez-García, Pereira-Bueno, Gómez-Bautista and Ortega-Mora2002). The pre-colostral sera were diluted 2-fold, starting at 1:125 or 1:100, and titrated. Calves were used for isolation when their pre-colostral titres were positive by IFAT (>1:250) to N. caninum.
Calf CT3 was a stillbirth. Except for CT17 and CT18, all calves remained clinically healthy at the time of sacrifice, and no abnormalities were observed at necropsy. Calves CT17 and CT18 were born weak, underweight, and unable to rise. They died between 24 h and 36 h after birth; necropsy was immediately performed and no macroscopic lesions were noticed (Table 1).
During necropsy, brain and lungs were removed aseptically, kept at 4°C, and sent to our laboratory. After reception of samples, 5–15 g of different brain portions were immediately placed in PBS buffer containing 2% antibiotic-antimycotic solution (Gibco BRL, Paisley, UK) and maintained at 4°C until they were used for mouse inoculation. Furthermore, tissue specimens were fixed in 10% neutral buffered formalin for processing by routine histological methods.
Bioassay for N. caninum in nude mice
Positive PCR brain portions (see below) were homogenized, filtered in sterile gauze, and centrifuged at 1350 g for 15 min. The supernatant was discarded, and 0·4 ml of the sediment suspended in PBS with 2% antibiotic-antimycotic solution (Gibco BRL) (equivalent to 5 g of cerebral tissue) was inoculated intraperitoneally into 2–4 female BALB/c athymic nude mice (Charles River, Barcelona, Spain). Mice were maintained in the Animal House of Universidad Complutense of Madrid with irradiated feed and water ad libitum. Mice were examined daily for the development of clinical signs of neosporosis. Animals showing clinical signs, such as emaciation and lethargy, were sacrificed and their peritoneal cavity flushes and brains were aseptically collected. Peritoneal fluids were used for in vitro cultivation (see below) and their brains were used for in vivo maintenance of the isolates until the cell culture isolation and cryopreservation of the parasite was achieved (see below). Thus, a fraction of fresh brain was homogenized in PBS with antibiotics by passing through a descending series of needles (20 to 25 G) and immediately inoculated to other athymic mice. In addition, a sample of this homogenate was tested for the presence of DNA from the parasite by specific nested-PCR (see below).
Isolation in cell culture
For cell inoculations, the mouse's peritoneal cavity was flushed with 8 ml of Dulbecco's Minimum Essential Medium (DMEM) supplemented with 2% antibiotic-antimycotic solution (Gibco BRL), 10 mm HEPES, and 2% equine serum. The peritoneal wash was immediately inoculated onto a 24 h cell monolayer culture of African green monkey kidney cells MARC-145 (25 cm2) and incubated at 37°C in a 5% CO2 humidified incubator. The medium was changed after 24 h. To keep the total amount of cultured cells to a minimum, blind passages of isolation cultures were made at 4 to 7-day intervals until the parasite was observed microscopically. After that time, passages were made every 4 days and the amount of tachyzoites was determined by Trypan blue exclusion, followed by counting in a Neubauer chamber. On each passage, the cultures were scraped, passed by 25 G needle, and inoculated onto a new monolayer cell culture. All isolates were maintained in MARC-145 cell culture for more than 2 months. Moreover, the isolates were cryopreserved in liquid nitrogen.
DNA extraction and PCR parasite detection
Genomic DNA was extracted from pellets of 108 tachyzoites of N. caninum and from 20 mg of tissue using the GenomicPrep cell and tissue DNA isolation kit (Amersham Biosciences, Uppsala, Sweden), following manufacturer's protocols. All samples were pre-treated with proteinase K (100 μg/ml; Sigma) at 55°C overnight.
Detection of DNA from the parasite was carried out by a nested-PCR on the internal transcribed spacer (ITS-1) region of N. caninum, as described by Buxton et al. (Reference Buxton, Maley, Wright, Thomson, Rae and Innes1998). To avoid the carryover of contaminating nucleic acids, each step of the procedure was performed in a different room and aerosol-resistant pipette tips were used. In each batch of PCR amplifications, DNA from N. caninum tachyzoites was used as positive controls. To identify false-positive results, negative control reactions (reactions without template and extractions of DNA from negative brain) were added to each set of PCRs. Secondary amplification product was visualized as a 249 bp band by electrophoresis in 1·5% agarose/ethidium bromide gel in 0·5X TBE buffer.
Genetic characterization of N. caninum isolates
Fifty nanograms of genomic DNA from purified tachyzoites were used to amplify and sequence 13 microsatellite markers with the primers and PCR conditions as described by Regidor-Cerrillo et al. (Reference Regidor-Cerrillo, Pedraza-Díaz, Gómez-Bautista and Ortega-Mora2006). The nucleotide sequences of the microsatellites analysed in this study have been deposited in the GenBank database under Accession numbers EU816095–EU816191.
Genetic profiles for these microsatellite markers were obtained by DNA fragment analysis, except for the MS3, using a set of nested-PCRs developed for each genetic marker (Pedraza-Díaz et al., manuscript in preparation). The primary reaction included 5 ng of genomic DNA that was extracted from purified isolates and 200–400 ng of DNA that was extracted from calf brain samples. Then, 5 μl of a 1:5 dilution of the PCR product was added to the secondary amplification reaction mixture. To avoid carryover of contaminating nucleic acids, control measures were carried out, as previously described. PCR products were visualized under UV light in 2% NuSieve 3:1-agarose/ethidium bromide gel (Nu-Sieve 3:1, Cambrex BioScience, USA). Finally, 13·5 μl of HiDi formamide (Sigma-Aldrich, St Louis, MO) and 0·5 μl of Gene Scan-500 (LIZ) Size Standards (Applied Biosystems, Foster City, CA) were added to 1 μl of PCR product DNA solution from each sample. The size of the fluorescent PCR-products was determined using a 48-capillary 3730 DNA analyser (Applied Biosystems) at the Unidad Genómica del Parque Científico de Madrid and analysed with GeneMapper® Software V 3.5.
Histopathological analysis
Different sections from brain and lung from calves were processed by routine histological methods. Tissues were fixed in 10% neutral buffered formalin and dehydrated through graded alcohols before being embedded in paraffin wax and stained with Haematoxylin and Eosin (H&E). Analysis was based on the observation of lesions consistent with N. caninum infection (Pereira-Bueno et al. Reference Pereira-Bueno, Quintanilla-Gozalo, Pérez-Pérez, Espi-Felgueroso, Álvarez-García, Collantes-Fernández and Ortega-Mora2003). Lesions were classified into one of the following 4 categories: none detected (0), mild (1), moderate (2), or severe (3).
Isolate nomenclature
Isolates were sequentially designated as Nc-Spain and a number, according to the order of in vitro isolation and cryopreservation in liquid nitrogen.
RESULTS
Serological, histological, and PCR results from calves
The pre-colostral titre for specific IgG antibodies to N. caninum by IFAT was analysed from all calves, except for the CT18 calf because its pre-colostral serum was not collected. Pre-colostral antibody titres varied from 1:250 to 1:3200 (Table 2).
Table 2. Results by IFAT, nested-PCR and H&E staining of calves used for isolation

§ PCR-positive brain sections/total number of brain sections analysed.
* Bacterial proliferation in blood vessels of the lung.
# Heifer CT16 showed an IFAT titre 1:200 when sacrificed.
a Percentage of positive samples from all samples tested.
b Severity grade of the lesion (0=none detected, 1=mild, 2=moderate, 3=severe).
c Number of brain sections with characteristic lesions/total number of brain sections analysed.
d Only 1 section from lung tissue was analysed.
e Lung sample not collected.
f Negative result.
g PCR analysis from concentrate tissue.
h Pre-colostral sera not collected.
Histological lesions associated with N. caninum infection were examined in tissue samples (brain and lung) from all infected calves. The main lesions observed in the brain were foci of gliosis without necrosis and perivascular cuffing with mononuclear cells; in the lungs, the main lesions observed were intersticial pneumonia and bronchopneumonia (Table 2). Most lesions were very mild and diffusely observed in all bovine sections. The calf CT2 lesions of the lung, however, were graded as severe or moderate. Tissue cysts or tachyzoite-like stages were not found in any bovine tissue section examined.
N. caninum infection in lung and brain tissues was confirmed by PCR. All brains were positive by ITS-1 nested PCR. Heifer CT16, which was sacrificed 20 months after birth, showed an IFAT titre of 1:200 and was negative when a PCR test was performed directly on DNA samples that were extracted from 20 mg of different brain sections. Neospora DNA, however, was later detected in the sediment that was obtained in the preparation of brain samples for mice inoculation, where the tissue cysts of parasite could be concentrated. Neospora was predominantly detected in brain tissues; the frequency of detection varied from 20% to 100% (Table 2).
Bioassay in nude mice
Mice inoculated with infected calf brain tissues became sick 4 to 164 days after the inoculation. The clinical signs observed were emaciation and various neurological signs, including ataxia, paralysis of hind limbs and forelimbs, flexed or hyper-extended, and lethargy (Table 3). Mice inoculated with samples from CT6 and CT12 calves and CT16 heifer did not develop clinical signs of neosporosis in the 6 months following inoculation. Mice inoculated with CT3 samples suddenly died 24 h after inoculation. These deaths were probably due to a bacterial infection since histopathological analysis detected bacterial proliferation in calf tissues.
Table 3. Bioassay in nude mice

§ Number of nude mice clinically affected/total number of nude mice inoculated.
* Day of sacrifice by severity of symptoms.
a Mice succumbed at 24 h by a bacterial infection.
b Healthy, clinical signs not shown.
c Clinical signs observed after mice succumbed to infection.
The isolates were maintained in vivo by inoculating 1/3 or 1/4 of brain tissues into uninfected nude mice. Since carcases of mice inoculated with CT17 calf tissues were found autolysed, they were not processed for isolation. Identical clinical signs were observed in mice used to maintain the isolates. Although these signs were observed over a long period (ranging from 6–5 days post-inoculation for CT13 samples to 49–108 days for CT5), the majority of clinical signs were observed at 25–50 days post-inoculation. Nested-PCR was applied to murine brain samples that were infected with calf tissues (including mice inoculated with CT17 samples) to confirm the presence of N. caninum. All tested samples extracted from clinically affected mice were positive. Asymptomatic mice were sacrificed 6 months post-inoculation and detection by PCR was performed on brain samples. These were all negative.
Parasite isolation
Peritoneal cavity washes collected from infected nude mice inoculated with calf and mouse brain tissues prior to necropsy were used for isolation in cell culture. The majority of isolates were obtained in cell culture from mice inoculated with calf tissues, but 1 or 2 mice passages were needed for the isolation from samples CT2, CT10 and CT11.
Cytopathology was identified in infected MARC-145 cells from 4 to 36 days after inoculation of the flask with the peritoneal fluid. Few Neospora-like intracellular tachyzoites and/or parasite vacuoles were observed. After first visualization of the parasite, the number of tachyzoites increased with successive passages infecting the majority of cells in the monolayer. The number of passages needed to achieve 106 tachyzoites per ml ranged from 3 to 13 (Table 4).
Table 4. Parasite isolation in cell culture
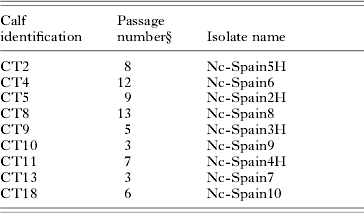
§ Total passages in cell culture until count >106 tachyzoites/ml.
Genetic characterization
Alleles for each of the 13 microsatellites analysed in this study were amplified and sequenced from all the isolates obtained, except for Nc-Spain10. Instead, Nc-Spain10 was analysed by DNA fragment analysis. Only the MS10 marker was subsequently sequenced. Most of the alleles detected in this study were previously identified in the analysis of 9 worldwide isolates and the Spanish Nc-Spain1H isolate included in previous studies (Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Pedraza-Díaz, Gómez-Bautista and Ortega-Mora2006; Rojo-Montejo manuscript in preparation). New alleles were identified to MS2, MS5, MS7, MS8, MS10 and MS21 microsatellite markers (Tables 5 and 6). Multi-locus analysis of the 9 isolates showed 8 different profiles. The pair Nc-Spain3H–Nc-Spain4H and Nc-Spain10 with regard to the Nc-Spain1H isolate (Rojo-Montejo et al., manuscript in preparation) showed identical genetic patterns. The remaining 6 isolates, however, displayed a unique profile. Moreover, profiles from Nc-Spain7 and Nc-Spain9 isolates only differed in the MS10 marker and were the closest genetically.
Table 5. Summary of microsatellite alleles obtained

* Not checked by DNA fragment analysis.
# Isolate analysed exclusively with DNA fragment analysis. Only MS10 marker was sequenced.
a New alleles identified.
b Microsatellite marker not analysed.
c Two alleles for MS5 marker were detected in CT17 sample.
Table 6. Description and assignment of new alleles

Identical multi-locus profiles were obtained from purified tachyzoites from each isolate and from each of the corresponding PCR-positive calf samples used for isolation when DNA fragment analysis was applied. The CT17 sample was also analysed, showing a unique profile with the detection of new alleles to MS5, MS6A and MS8 markers (Tables 5 and 6). Moreover, 2 alleles were detected for the MS5 from the CT17 sample, and only 1 allele was detected for the other microsatellite markers.
DISCUSSION
This study describes the isolation and genetic characterization of N. caninum from 9 Holstein-Friesian calves from Spain. Eight of them were isolated from asymptomatic calves and 1 was isolated from a clinically affected calf. Our results are important because most of the bovine isolates worldwide have been obtained from clinically affected animals and only 6 isolates have been obtained from healthy animals. Of the 6 isolates from healthy animals, 2 were isolated from adult cows (Sawada et al. Reference Sawada, Kondo, Tomioka, Park, Morita, Shimada and Umemura2000; Okeoma et al. Reference Okeoma, Williamson, Pomroy, Stowell and Gillespie2004) and 4 were isolated from congenitally infected calves (Yamane et al. Reference Yamane, Kokuho, Shimura, Eto, Shibahara, Haritani, Ouchi, Sverlow and Conrad1997; Piergili Fioretti et al. Reference Piergili Fioretti, Rosignoli, Ricci, Moretti, Pasquali and Polidori2000; Miller et al. Reference Miller, Quinn, Windsor and Ellis2002; Okeoma et al. Reference Okeoma, Williamson, Pomroy, Stowell and Gillespie2004). Thus, little is known about the biological variability of N. caninum, especially among isolates from healthy animals that could display a natural reduced virulence. Moreover, the number of described bovine isolates of N. caninum remains small because the isolation process is difficult. To date, only 1 isolate from a foetus had been described in Spain (Canada et al. Reference Canada, Meireles, Mezo, Gónzalez-Warleta, Correia da Costa, Sreekumar, Hill, Miska and Dubey2004) and another one designed as Nc-Spain1H was recently obtained in our laboratory from an asymptomatic calf (Rojo-Montejo et al., manuscript in preparation).
There is no standardized method to isolate N. caninum from bovine tissues. Initial attempts to isolate viable N. caninum in cell culture were largely unsuccessful because autolysed aborted fetuses were used. Therefore isolation of N. caninum from fresh neural tissues of congenitally infected, live calves might be easier since the viability of the parasite is not endangered by the autolysis of neural tissues, although in addition the parasite load could be low. These might be the reasons why Conrad et al. (Reference Conrad, Barr, Sverlow, Anderson, Daft, Kinde, Dubey, Munson and Ardans1993) isolated N. caninum from only 2 of 49 fetuses confirmed by histology and Yamane et al. (Reference Yamane, Kokuho, Shimura, Eto, Shibahara, Haritani, Ouchi, Sverlow and Conrad1997) isolated N. caninum from only 1 of 7 calves whose pre-colostral sera were positive by IFAT when bovine tissues were directly inoculated to cell monolayer.
In this study, pre-colostral sera from asymptomatic calves used for isolation were previously analysed by IFAT to confirm the congenital exposure to N. caninum. Calves with pre-colostral Neospora titres >1:200 were considered positives, but the majority of titres detected were ⩽1:1600. Two calves showed a titre of 1:2000, and only the clinically affected CT17 calf showed a pre-colostral titre 1:3200. In contrast, previous studies of isolation from asymptomatic calves found that pre-colostral titres were ⩾1:2000 by IFAT (Yamane et al. Reference Yamane, Kokuho, Shimura, Eto, Shibahara, Haritani, Ouchi, Sverlow and Conrad1997; Piergili Fioretti et al. Reference Piergili Fioretti, Rosignoli, Ricci, Moretti, Pasquali and Polidori2000; Okeoma et al. Reference Okeoma, Williamson, Pomroy, Stowell and Gillespie2004).
Following histological analysis with H&E staining of brain tissues revealed compatible lesions with protozoal infection in the brain and lungs, but the parasite was not detected. Lesions were generally graded as mild or not observed, even in 2 symptomatic calves (CT17 and CT18). Thus, clinical signs showed by CT17 and CT18 calves can not be directly attributed to Neospora infection because the presence of other abortifacient agents, such as viruses or bacteria, were not examined. Similar histological patterns have been reported in the limited number of infected healthy calves examined (Miller et al. Reference Miller, Quinn, Windsor and Ellis2002; Piergili Fioretti et al. Reference Piergili Fioretti, Rosignoli, Ricci, Moretti, Pasquali and Polidori2000; Okeoma et al. Reference Okeoma, Williamson, Pomroy, Stowell and Gillespie2004).
Nude mice were used to increase the success of isolation in cell culture because the parasite load expected in brain tissues from asymptomatic calves could be low. The inoculation in immunocompromised mice as a previous step is more efficient than the direct inoculation of infected tissues onto cell culture for isolation of N. caninum. Chemically immunocompromised mice, athymic mice, or gamma interferon knockout mice have been used to isolate N. caninum from intermediate hosts (Yamane et al. Reference Yamane, Shibahara, Kokuho, Shimura, Hamaoka, Haritani, Conrad, Park, Sawada and Umemura1998; Piergili Fioretti et al. Reference Piergili Fioretti, Rosignoli, Ricci, Moretti, Pasquali and Polidori2000; Koyama et al. Reference Koyama, Kobayashi, Omata, Yamada, Furuoka, Maeda, Matsui, Saito and Mikami2001; Miller et al. Reference Miller, Quinn, Windsor and Ellis2002; Canada et al. Reference Canada, Meireles, Mezo, Gónzalez-Warleta, Correia da Costa, Sreekumar, Hill, Miska and Dubey2004; Vianna et al. Reference Vianna, Sreekumar, Miska, Hill and Dubey2005). Nude mice lack T-cell response, which facilitates the proliferation of the parasite in their tissues and increases the chance of successful isolation in cell culture (Yamane et al. Reference Yamane, Shibahara, Kokuho, Shimura, Hamaoka, Haritani, Conrad, Park, Sawada and Umemura1998). Moreover, prior to mice inoculation, PCR analysis of brain tissues from calves collected for isolation confirmed the presence of the parasite and ruled out isolation failure due to inoculation of uninfected brain samples. In previous studies of isolation of N. caninum, nude mice inoculated with brain calf samples showed clinical signs until 180 days post-inoculation (Yamane et al. Reference Yamane, Shibahara, Kokuho, Shimura, Hamaoka, Haritani, Conrad, Park, Sawada and Umemura1998). In this study, clinical signs usually developed earlier, as nude mice infected with calf tissues became sick between 4 and 164 days post-inoculation. The appearance of clinical signs and the number of infected mice were variable, which could be directly related to the inoculation doses. All inoculated mice succumbed to infection when they were infected with calf samples in which the parasite's DNA was detected in a higher number of samples by PCR, indicating a higher parasite load in their brains although direct quantitative analyses as real-time PCR were not applied. In addition, in subsequent passages in nude mice, similar clinical signs were observed although they appeared earlier, probably due to a higher dose of parasite in the same amount of inoculated tissue. On the other hand, isolation in vivo from calves CT6, CT12, and CT16 probably failed due to a low parasite load in their brain samples. For example, CT12 and CT16 calves had the lowest percentage of detection of parasite by PCR in their brain samples. In fact, the parasite was only detected when a concentrate of brain sample from heifer CT16 was used. Even so, the influence of virulence inherent to the isolate cannot be discarded. Other studies using a standardized experimental model must be conducted to establish the pathogenicity of these isolates and to determine its effect on the outcome of infection, at least in this nude mice model.
Most isolation methods include the inoculation of trypsin- or non–trypsin-treated brain homogenates onto the cell culture, which is usually incubated for a short time because prolonged incubation can be toxic to the cell monolayer and can reduce the chance of isolation in vitro. For this reason, we did not inoculate the brain tissues. Instead, the peritoneal fluids from sick mice were directly inoculated to cell culture, which can be maintained on the monolayer for 24 h without toxic results.
Bovine and Vero cell lines have been used to isolate and efficiently grow N. caninum in cell cultures. In this study, the MARC-145 cell clone was used because this cell host can maintain N. caninum (Pérez-Zaballos et al. Reference Pérez-Zaballos, Ortega-Mora, Álvarez-Garcia, Collantes-Fernández, Navarro-Lozano, García-Villada and Costas2005). Tachyzoites were visualized in cell culture before 40 days post-inoculation in all isolates. In previous studies, tachyzoites may not have been visible microscopically until 60 days after inoculation with the homogenate (Dubey and Schares, Reference Dubey and Schares2006). The amount of time required for the parasite to be visualized in cell culture could be affected by the density of tachyzoites in the peritoneal cavity fluid and the proliferation and/or adaptation of parasites in vitro. In fact, differences in adaptation to cell culture among isolates were observed, as a different number of cell subpassages were needed to produce a similar amount of tachyzoites for each isolate. Three passages were carried out for Nc-Spain7 and Nc-Spain9, while 13 passages were needed for Nc-Spain8. Furthermore, differences in growth rate in vitro among Neospora isolates have been described (Schock et al. Reference Schock, Innes, Yamane, Latham and Wastling2001). Although Neospora does not show a cell culture preference, a recent study found that isolates were not able to grow in vitro (Vianna et al. Reference Vianna, Sreekumar, Miska, Hill and Dubey2005). New in vitro assays are needed to verify the differences in proliferation rate of these isolates. It is possible that these differences are related to the virulence of protozoan pathogens, as is the case for Toxoplasma gondii (Saeij et al. Reference Saeij, Boyle and Boothroyd2005).
Using an isolation method similar to the one used in our present study, Yamane et al. (Reference Yamane, Shibahara, Kokuho, Shimura, Hamaoka, Haritani, Conrad, Park, Sawada and Umemura1998) reported a 100% success rate. They obtained 4 isolates from 3 congenitally infected calves and 1 stillbirth calf. The authors, however, did not mention any other isolation attempt or provide additional clinical data (clinically healthy or affected). Our isolation procedure was effective, as only 5 of the 14 isolation attempts in cell culture failed. Two of them were due to factors that were not related to the method (from CT3 and CT17 calves). The other 3 failures (CT6, CT12, and CT16) may have been due to the reduced load of the parasite in brain samples since the frequency of detection of the parasite by PCR was very low.
For genetic characterization, 13 microsatellite markers with a high-resolution genotyping power (Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Pedraza-Díaz, Gómez-Bautista and Ortega-Mora2006) were applied to the 9 N. caninum isolates and CT17 calf sample. New alleles were detected and specific multi-locus profiles were obtained for the majority of isolates and CT17 calf, confirming the high discrimination power of these genetic markers. The fact that the isolates with the same genetic pattern (Nc-Spain3H–Nc-Spain4H pair and Nc-Spain10 with regard to Nc-Spain1H; Rojo-Montejo et al., manuscript in preparation) and those with the closest genetic profile (Nc-Spain7–Nc-Spain9) were obtained from calves from the same herd may indicate a relationship between geographical origin and genetic profile. Further analysis should be performed with a wider panel of isolates to ensure the suitability of these genetic tools as virulence markers. These genetic tools can also be used in studies of population structure and epidemiology in N. caninum. The higher polymorphism of these markers, the DNA fragment analysis and the identical profiles obtained for calf brain tissues and the isolates enable us to verify that calves were only infected by the corresponding isolate obtained in vitro. In fact, in our study, mixed infections were not detected when microsatellites were evaluated in calf tissues when isolation was achieved. Two alleles for microsatellite MS5, however, were detected in calf CT17. To date, mixed infections have not been reported because the available genetic tools lacked the level of discrimination exhibited by these microsatellite markers.
This report describes a suitable isolation method for N. caninum. We obtained 9 new isolates; 8 of them were isolated from clinically healthy calves. To date, only 1 isolate from a fetus (Canada et al. Reference Canada, Meireles, Mezo, Gónzalez-Warleta, Correia da Costa, Sreekumar, Hill, Miska and Dubey2004) and 1 from an asymptomatic calf using this method has been described (Rojo-Montejo et al., manuscript in preparation) in Spain. All isolates were identified as a member of Neospora spp., based on the ITS-1 sequence and the microsatellite analysis. Future characterization in vitro and in vivo studies will allow us to examine the effect of biological variability among isolates from healthy or clinically affected animals on the outcome of infection.
We would like to thank the dairy farm veterinarians for samples and data collection and the SALUVET group. This work was supported by the Spanish Ministry of Science and Technology and Hipra Laboratories S.A. (PETRI 95-0777.OP) and the INIA (RTA04-047-C2). Nc-Spain7 isolate has been patented by the SALUVET group (P200701978).








