Introduction
Oxidation–reduction reactions play a significant role in providing energy for microorganisms. Microbial metabolic activities influence the equilibrium between matter and energy in the biosphere. Since microorganisms have a broad range of metabolic capacity, they consume a variety of elements and minerals to extract energy through electron exchange processes. Microbes are also responsible for different types of geochemical processes in nature, such as weathering of rocks and the formation of new minerals (Roh and Moon, Reference Roh and Moon2001). Furthermore, they also participate in a variety of geochemical processes such as the formation of ore deposits (Nealson and Myers, Reference Nealson and Myers1990; Juniper et al., Reference Juniper, Martineu, Sarrazin and Gelinas1995) and cycling of organic matter (Lovley et al., Reference Lovley, Phillips and Lonergan1991; Nealson and Saffarini, Reference Nealson and Saffarini1994).
Iron is a versatile and essential energy source for many microorganisms, generating energy by oxidation and reduction reactions in either a soluble or solid phase. It is regarded as the second most redox-active substance on Earth, having three primary oxidation states of Fe(0), Fe(II) and Fe(III) (Esther et al., Reference Esther, Sukla, Pradhan and Panda2015). Before the 19th century, Fe(III) reduction was considered an abiotic process (Allison and Scarseth, Reference Allison and Scarseth1942). However, with the identification of enzymatically reducing Fe(III) bacteria, the role of living organisms in iron biogeochemical cycling was considered to be an important environmental process (Lovley et al., Reference Lovley, Phillips and Lonergan1991; Esther et al., Reference Esther, Sukla, Pradhan and Panda2015). Iron-reducing bacteria (IRB) can reduce various types of iron oxides, resulting in amorphous to crystalline phase changes (Weber et al., Reference Weber, Achenbach and Coates2006). Additionally, IRB are considered to be biocatalysts that do not get consumed in the chemical reaction (Paul et al., Reference Paul, Sangeetha, Deepika and Buddolla2019). IRB accept electrons for the oxidation of hydrogen and organic matter and reduce iron to a variety of forms such as ferrihydrite and hydrous ferric oxides (Fe2O3⋅nH2O) (Fredrickson et al., Reference Fredrickson, Zachara, Kennedy, Dong, Onstott, Hinman and Li1998), lepidocrocite (γ-FeOOH) (Ona-Nguema et al., Reference Ona-Nguema, Abdelmoula, Jorand, Benali, Géhin, Block and Génin2002), goethite (α-FeOOH) (Liu et al., Reference Liu, Kota, Zachara, Fredrickson and Brinkman2001), hematite (α-Fe2O3) and magnetite (Kostka and Nealson, Reference Kostka and Nealson1995). Generally, IRB exist in anaerobic environments and transform crystalline or amorphous Fe(III) oxides into crystalline Fe(II) oxide phases such as magnetite (Fe3O4), siderite (FeCO3), vivianite (Fe3(PO4)⋅2H2O) and iron sulphide (FeS) (Lovley et al., Reference Lovley, Giovannoni, White, Champine, Phillips, Gorby and Goodwin1993; Zachara et al., Reference Zachara, Kukkadapu, Fredrickson, Gorby and Smith2002).
Similarly, iron-oxidizing bacteria (IOB) also play a significant role in the iron cycle in sediments both in the past and present. For example, deposition of banded iron formations (BIF) in the Archean and Palaeoproterozoic oceans through the formation of Fe(OH)3 can be attributed to these microorganisms (Planavsky et al., Reference Planavsky, Bekker, Rouxel, Kamber, Hofmann, Knudsen and Lyons2010). During early Earth, IOB were considered anoxygenic photoautotroph, which could perform Fe(II)-based photosynthesis, which occurred likely using highly oxidized species such as oxygen or nitrate (Kappler and Newman, Reference Kappler and Newman2004). Photoautotrophic Fe(II) oxidation has been suggested as a possible way for producing BIF, which were deposited during the late Archean through early Proterozoic (2.7–1.9 Ga) (Konhauser et al., Reference Konhauser, Hamade, Raiswell, Morris, Ferris, Southam and Canfield2002). Modern-day Fe(II) oxidation driven by photoautotrophic bacteria leads to CO2 fixation by using light as the primary energy source (Widdel et al., Reference Widdel, Schnell, Heising, Ehrenreich, Assmus and Schink1993).
As a result of redox processes, iron may form minerals such as nontronite, ferric citrate (as Fe3+ reduction), vivianite and siderite (as Fe2+ oxidation) (Zhang et al., Reference Zhang, Dong, Jiang, Kukkadapu, Kim, Eberl and Xu2009). The redox reactions of Fe(II) and Fe(III) play a fundamental role in modern environmental biogeochemistry and are also considered to have been critical biogeochemical processes on early Earth (Weber et al., Reference Weber, Achenbach and Coates2006). Microorganisms involved in these iron redox reductions are comprised of both archaeal and bacterial domains. They can metabolically exploit the favourable redox potential between the Fe(III)/Fe(II) couple and various electron donors or acceptors. Generally, mineral surfaces provide a suitable host for aerobic or anaerobic microorganisms, where they appear as scattered individual cells, as colonies, or as biofilms on mineral surfaces (Hutchens, Reference Hutchens2009).
Microbially enhanced weathering leaves unique biosignatures or fingerprints on the minerals, and these are the clues that we intend to follow for extraplanetary research although the living organism itself may have disintegrated, these signatures can be indirectly be used as clues to pinpoint that life could have existed. For instance, fossilized microbial biofilms (Westall et al., Reference Westall, Steele, Toporski, Walsh, Allen, Guidry, Mckay, Gibson and Chafetz2000) and some mineral acids or organic biomarkers such as amino acids, lipids and nucleic acids (Röling et al., Reference Röling, Aerts, Patty, Ten Kate, Ehrenfreund and Direito2015) are considered as persistent biomarkers on Earth and potentially use as proxies to investigate the extraterrestrial life. Furthermore, previous research by Kolo et al. (Reference Kolo, Konhauser, Krumbein, Ingelgem, Hubin and Claeys2009) observed adhesion of microbial-like structures to hematite surfaces by using scanning electron microscopy (SEM) images. Thus, microscopic analysis could confirm these microbial–mineral interactions, including transmission electron microscopy (Ransom et al., Reference Ransom, Bennett, Baerwald, Hulbert and Burkett1999) and SEM-energy dispersive X-ray (EDX) spectroscopy (Rogers and Bennett, Reference Rogers and Bennett2004).
At the turn of the 20th century, scientists started to explore other planets and extraterrestrial objects for possible life sources. Among the various contenders, Mars is considered as one of the most astrobiological important terrestrial planets where life could have existed in the past and still may be preserved today. The formation of Mars was very similar to that of Earth (Jakosky, Reference Jakosky1998), with both planets being initially wet and warm. This similarity has led many scientists to believe that early Mars could have supported microbial life forms similar to those on Earth (Jakosky, Reference Jakosky1996). However, it is now widely believed that the existence of any Martian surface biota would be unlikely because Mars has been faced with extreme environmental challenges and inhospitable conditions on the surface (Clark, Reference Clark1998; McCollom, Reference Mccollom2006). Among those factors, highly oxidizing atmospheric species and extremely oxidized compounds in the soil are significant because of their potential to damage organic material. Apart from this, extreme ultraviolet irradiation, the absence of both liquid water and organic materials and higher ionizing radiation may have created an adverse surficial environment on Mars (Clark, Reference Clark1998). If life originated in the early Martian environment, it is conceivable that traces of early life could potentially be preserved and exist in the Martian subsurface (McKay and Stoker, Reference Mckay and Stoker1989). However, scientific controversies continue to exist about the existence of life on Mars, that life might have evolved and still may have managed to exist in some of the more habitable niches on the planet (Nealson, Reference Nealson1997).
Among the various pieces of evidence used to indicate the presence of life on Mars, reports of iron-rich hematite as a significant component in the Martian surface and subsurface and its association with water makes the planet highly relevant in the search for life (Allen et al., Reference Allen, Westall and Schelble2001). Throughout Mars space missions, scientists have proven the abundance of hematite on the Martian surface. Particularly, the Thermal Emission Spectrometer instrument on the Mars Global Surveyor has been used for mapping the mineralogical compositions of the Martian surface and found grey crystalline hematite (α-Fe2O3) at the Sinus Meridiani, Aram Chaos and in other numerous scattered locations throughout Valles Martians in Mars (Christensen et al., Reference Christensen, Bandfield, Smith, Hamilton and Clark2000b; Christensen et al., Reference Christensen, Morris, Lane, Bandfield and Malin2001). Furthermore, the geological data gathered from Viking landing sites prove the occurrence of hematite (α-Fe2O3) as both superparamagnetic (nanocrystalline) hematite and larger diameter hematite particles (Morris et al., Reference Morris, Agresti, Lauer, Newcomb, Shelfer and Murali1989). Since hematite is also one of the most common minerals on the Earth's surface, the investigation of possible life traces on hematite may reveal clues about the dormant life on Mars. This study attempts to investigate microbial-induced weathering and re-precipitation characteristics of weathered hematite samples collected from Sri Lanka using a biogeochemical approach. The goals of this study are three-fold: (a) characterize and correlate elemental concentrations to the morphological features observed in weathered hematite samples, (b) propose potential mechanisms for bacterial weathering of hematite and (c) identify the possible microorganisms that are involved in the weathering process.
Materials and methods
Study area and sample collection
Weathered hematite samples were collected on 25 August 2016 from a road cutting at the premises of the University of Peradeniya (7.267512°N, 80.606914°E), Sri Lanka (Fig. 1(a)). This road cutting is located on the Charnockitic rock outcrop. The climate in this region is tropical and receives a significant amount of annual rainfall (2083 mm) with a yearly average temperature of 24.5°C (Climate-Data.Org, 2020). For this study, two types of samples were aseptically collected: (1) a weathered hematite rock (HR) sample from a depth of 0.6–0.9 m below the surface, weighing approximately 1.5 kg (Fig. 1(b)), and (2) five different haematitic soil (HS) samples along a vertical section of the outcrop profile at uniform intervals of 0.3 m (Fig. 1(b)). When collecting the soil samples, the uppermost soil layer was removed to avoid contamination of humus materials (from decayed plant and animal materials). All the samples were stored under room temperature (20°C) and pressure (1 atm) in separate plastic containers before the analyses were carried out.

Fig. 1. (a) Geological map of Sri Lanka showing litho-tectonic divisions and the primary sampling location at Peradeniya. (b) Sampled soil profile showing the different HS layers and yellow circle represent sampling points in the HR.
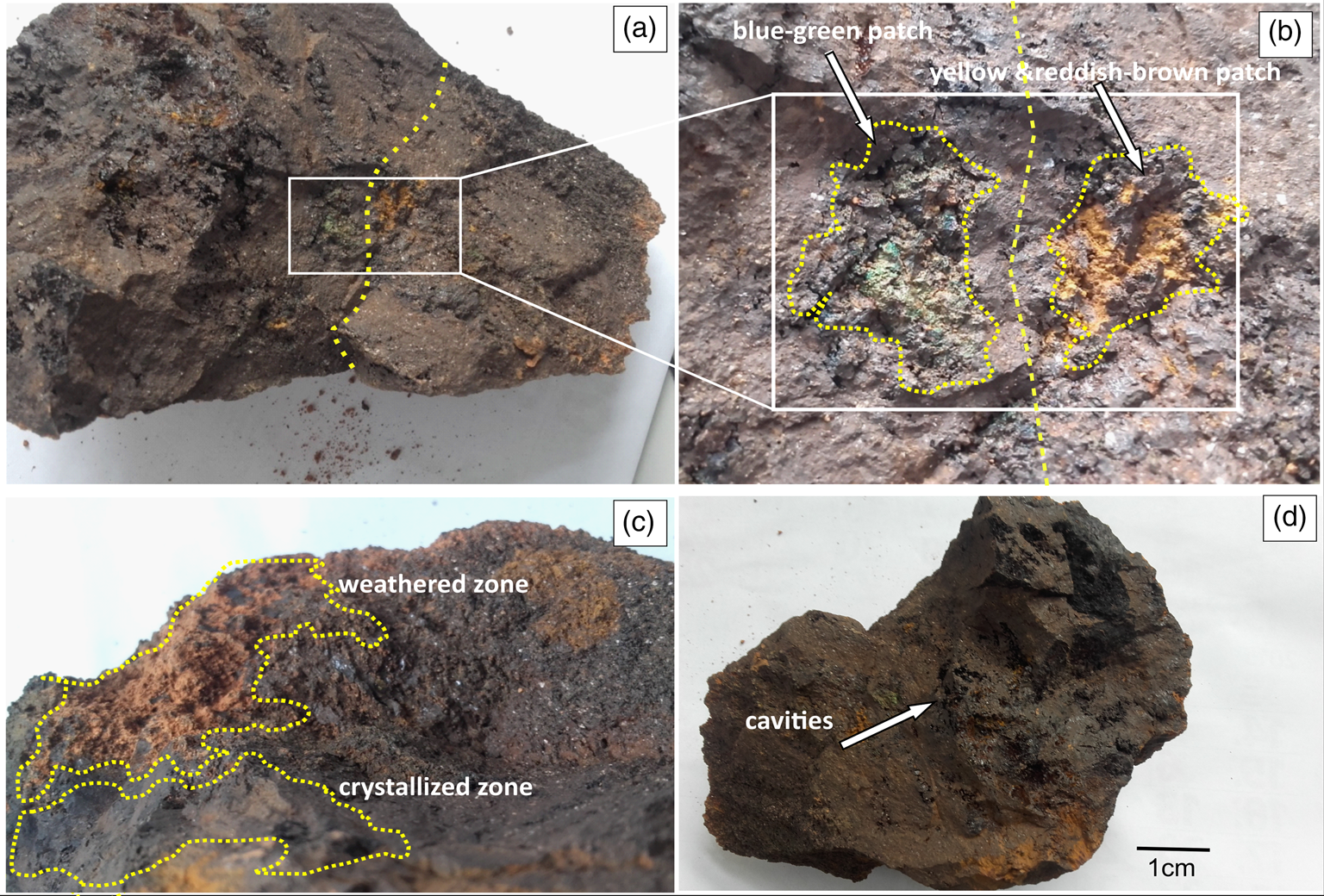
Fig. 2. Different morphological features on HR sample. (a) hematite sample showing parent weathered zone (right side of the dashed line within the rectangle) and crystallized zone (left side), (b) the blue-green granular and yellow and reddish-brown patches between weathered and crystallized zone, (c) highly weathered, granular, textured zone with yellow-brown patches and developed crystals of hematite and (d) an image showing cavities on the selected specimen.
Analytical methods
Selected HR fragments were separated from the parent rock, including specimens with (i) highly weathered zones, (ii) crystallized zones and (iii) blue-green granular textured zones (Fig. 2). Micro-morphological and chemical characteristics of selected samples were observed through SEM (Zeiss Evo LS 15, Jena, Germany) equipped with an EDX detector (X-act; Oxford Instruments, Abingdon, United Kingdom). Initial SEM analyses were carried out on 30 August 2016 (5 days after sample collection) to observe the growth pattern of the minerals. Following this initial analysis, the samples were stored under ambient conditions as mentioned before while being exposed to the atmosphere, and the same locations in the minerals were re-analysed after 30 days (29 September 2016) to monitor possible alterations.
X-ray diffraction (XRD) analyses were carried out on bulk samples (both rock and soil) collected along the soil profile to determine the variation of elemental compositions of weathered HR with depth. The samples were sieved using a 4 mm sieve and fragments larger than 4 mm were collected for further analysis. The soil samples were then powdered using an agate mortar and pestle for XRD analyses (Siemens D5000; Germany). To evaluate the bulk composition of soil and rock samples, inductively coupled plasma-mass spectrometry (ICP-MS; Thermo Fisher ICapQ; Bremen, Germany) analyses were performed for three different selected sample sets: (i) soil sample collected from the third soil profile, (ii) freshly broken hematite fragment from the HR and (iii) weathered hematite fragment collected from the same profile as HR. Samples were acid digested with 5 ml HNO3, 3 ml of HCl and 3 ml HF using a microwave digester (CEM-Mars-6; Mathews, USA) equipped with Essyprep high-pressure vessels. Digestion was carried out at a constant temperature of 180°C for 20 min, following which the sample was centrifuged, filtered and volumerized. Trace elements in the digested solutions were analysed for Li, Be, V, Cr, Mn, Co, Ni Cu, Zn, Ga, As, Se, Rb, Sr, Ba, Tl and Pb using ICP-MS with appropriate quality controls. Calibrations of the instrument were carried out using multi-element standards (AccuStandard®, Connecticut, USA).
DNA extraction and sequencing
Two different HR samples were analysed to determine the microbial diversity in the collected rock: (i) aseptically collected 1.0 g of HR fragments were soaked overnight in 50 μl of sterile nuclease-free water (DNA sample 1), and (ii) 1.0 g HR were crushed and placed in 50 μl of sterile nuclease-free water overnight (DNA sample 2). Samples used for the DNA extraction were collected from the same parent rock used for collecting samples for SEM analysis. After overnight soaking in sterile nuclease-free water, the bacteria living within the HR fragments were collected onto a 0.22 μm filter membrane using a sterilized, reusable filter funnel attached to a vacuum system. Microbial DNA was extracted from the filter membrane using the MoBiO laboratories Power Water DNA isolation kit (Germantown, Maryland, USA) according to the manufacturer's instructions. DNA was quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA), and subsequently, the samples were shipped to the Massey Genome Service, Massey University, Palmerston North, New Zealand, for sequencing. Quality checking of samples was conducted at Massey University by Qubit Fluorometric Quantization. Culture-independent 16S amplicon sequencing technology was used to characterize the microbial population associated with hematite. The following forward and reverse primers along with the highlighted adapter sequence were used in polymerase chain reaction (PCR) to amplify the V3–V4 region of the 16S rRNA gene:
Forward primer V3: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGAGGCAGCAG
Reverse primers V4: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACAAGGGTATCTAATCC
For the PCR reaction, 1 μl of genomic DNA (5 ng μl−1) was added to 17 μl of AccuPrimePfxSuperMix (Invitrogen™), and 1 μl each of the barcoded forward and reverse V3–V4 amplicon primers were added and the PCR was run at 95°C for 2 min, then for 30 cycles at 95°C for 20 s, at 55°C for 15 s, at 72°C for 5 min followed by a final extension at 72°C for 10 min. The library concentration was quantified by Qubit HS assay (Invitrogen™ number: Q32854) and the full library size of ~630 bp was verified on a Perkin Elmer LabChip® GX Touch HT instrument using the DNA High Sensitivity LabChip® assay. The libraries were pooled by equal molarity. The pooled library was diluted to 8 pM and PhiX was diluted to 12.5 pM with ice-cold HT-1. Finally, 800 μl of the pooled library and 200 μl of PhiX were combined to provide a calculated spike of 20% PhiX. Samples were mixed and 600 μl was loaded into a thawed Illumina MiSeq V2 cartridge for sequencing on the Illumina MiSeq platform using paired-end sequencing with 250 nucleotide read length. The resulting amplicons were visualized in an agarose gel and PCR-purified samples were pooled sequenced on the MiSeq platform. Prior to QC trimming, the reads were joined with flash.
Results and discussion
Visual and microscopic morphological characterization
Our observations and analyses revealed unique morphological and chemical features that helped interpret the biogeochemical processes occurring in the hematite-rich soil system. The first morphologically distinct zone in the HR sample showed highly weathered, yellow-brown patches with granular texture (Fig. 2(a) and (b)). The discrete coloration in these highly weathered zones possibly occurred due to the oxidation of Fe(0) or Fe(II) to Fe(III) as explained by Pérez-Guzmán et al. (Reference Pérez-Guzmán, Bogner and Lower2010). Generally, oxidation of Fe(II) to Fe(III) occurs under neutral pH conditions, which leads to the formation of minerals such as goethite (α-FeOOH), akaganeite (β-FeOOH), hematite (α-Fe2O3, β-Fe2O3) or magnetite (Fe3O4) (Weber et al., Reference Weber, Achenbach and Coates2006; Pérez-Guzmán et al., Reference Pérez-Guzmán, Bogner and Lower2010). The observed yellow-brown weak granular texture indicated the alteration of the initial mineralogical composition of Fe(0) into Fe2O3 or equivalent mineralogical form such as maghemite (γ-Fe2O3, ɛ-Fe2O3), hematite (α-Fe2O3, β-Fe2O3), etc. In the second zone, well-developed crystals were observed adjacent to the weathered yellow-brown patches (Fig. 2(c)). These zones indicated the areas where the parent rock material had not undergone weathering. However, these zones could have provided potential mineral resources for iron-consuming bacteria and could have been subjected to biological weathering at later stage.
The third zone showed unique blue-green and granular regions compared to the crystallized and highly weathered zones (Fig. 2(b)). The majority of the granular areas were located within the highly weathered zone, and a few were located at the boundary between the highly weathered zone and crystallized zone. Formation of blue-green coloration occurred possibly due to the formation of Fe(II) oxides as a product of biogeochemical reduction of Fe(III) (equation (1)) or oxidation of Fe(0) into Fe(II) with the presence of O2 and water as shown in equations (2) and (3) (Pérez-Guzmán et al., Reference Pérez-Guzmán, Bogner and Lower2010):
Generally, Fe(III) oxides are stable under anoxic and neutral or alkaline pH settings. Under favourable aqueous or moist environments, the green rust would appear as hydroxide and would contain a combination of Fe(II) and Fe(III) as formula Fex3+Fey2+(OH)3x+2y−z(A−); where A− = Cl− or ½SO42− (Pérez-Guzmán et al., Reference Pérez-Guzmán, Bogner and Lower2010). However, as equation (3) denotes, when these mineral surfaces were exposed to oxygen, Fe(II) started to oxidize into Fe(III) rapidly, with a half-life of several minutes (Morgan and Stumm, Reference Morgan and Stumm1996). Therefore, the appearance of greenish patches was less pronounced compared to the presence of yellow-brown rust in the collected samples. Furthermore, the development of cavities was identified in the HR sample (Fig. 2(d)), which could be a result of dissolution during the weathering process. The experimental conditions in our analysis would have limited the growth of anaerobic IRB. This effect is important to consider, particularly during processing of the samples for bacterial analysis. We collected and processed the samples under atmospheric conditions (i.e. an open system). Therefore, hematite samples would have come in contact with atmospheric oxygen and would have eliminated or minimized IRB from the system (samples). Hence, we were not able to detect major IRB such as strains from Geobacter and Shewanella that have been reported in previous studies (Croal et al., Reference Croal, Gralnick, Malasarn and Newman2004; Roden, Reference Roden2006; Crosby et al., Reference Crosby, Roden, Johnson and Beard2007). However, we did detect six iron-reducing genera that belonged to the phyla of Actinobacteria and Firmicutes, which were also reported by Gupta et al. (Reference Gupta, Dutta, Sarkar, Panigrahi and Sar2018) and Zhang et al. (Reference Zhang, Zeng, Liu, Chen, Guo, Ma, Dong and Huang2019).
SEM analysis
Field emission spectroscopic analyses were carried out specifically for samples separated from the blue-green granular textured zone to examine both chemical and morphological characteristics. Four distinct and important morphologic features were initially identified by using high-resolution SEM images as globular structures, growth layers, nuclear components and nuclei. Figure 3(a) shows a broader view of morphological features of the blue-green textured zone, before the incubation period of 30 days. Re-analysis of the samples after storing under ambient conditions for 30 days showed continuous growth and enlargement of the growth layers (Fig. 3(b), (c) and (d)).

Fig. 3. SEM images showing the characteristic morphological features of blue-green, granular patches seen in Fig. 2(b): (a) globular morphology and well-developed vesicular spaces and cavities; (b) globular structures covered with three growth layers (1, 2 and 3); (c) magnified version of (b), the propagation of globular-like structures from the core region of the nuclei colonies and (d) a closer look at the ‘nuclei’ identified as the primary stage of growth layers with remnant EPS-like structures.
Observed globular morphology and well-developed vesicular spaces on the HR sample suggested the dissolution of weathered materials (Fig. 3(a)). These morphologies were likely influenced by bacterial weathering activities, such as enzymatic Fe(III) reduction. For example, the reduction of Fe(III) would produce highly soluble Fe(II), which can easily remove iron from the parent rock (Lovley, Reference Lovley1997). With the influence of acidic pH conditions from rainwater, Fe(III) could be turned into Fe(II) hydroxides (Pérez-Guzmán et al., Reference Pérez-Guzmán, Bogner and Lower2010). Since Fe(II) hydroxides would be in a soluble phase, they could easily be leached out from the system (i.e. parent rock) and added into the soil profile. The removal of iron oxides from the parent rock structure would be enhanced by the formation of cavities or vesicles (Fig. 3(a)). Additionally, the breakdown of the initial parent rock would provide a freshly exposed surface that would allow bacteria to further scavenge for metals and other energy sources in the primary rock by forming vesicles and tunnels. This concept is discussed later in the section ‘Intensive biogeochemical weathering mechanism’.
Formation of vesicular spaces or dissolution features (Fig. 3(a)) indicated a breakdown of the parent rock through weathering, and layered globular structures of different sizes showed the continuous growth patterns of the mineral assemblages with intermittent stages (Fig. 3(b) and (c)). The first two outer layers (layers 1 and 2) were relatively thin, whereas the inner layer (layer 3) was thicker by comparison to the outer layers (Fig. 3(b) and (c)). The inner, third layer represented a unique, fibrous, acicular texture, which developed perpendicular to the nuclear component. On the contrary, the fibrous material appeared to be forming the new layer (layer 3) parallel to the existing layers (Fig. 3(c), layers 1 and 2). Each fibrous ‘bundle’ appeared to originate from a nuclear component. Figure 3(c) and (d) shows the specific morphological feature identified as ‘nuclei’ throughout this study. The middle part of Fig. 3(c) shows the propagation of globular-like structures identified as ‘nuclei colonies’, which contained a large cluster of nuclei. This component also had well-developed globular structures, and the surface of each structure contained the smaller nuclei. These nuclei served as possible templates or starting points for the continued growth of the globular structures.
Factors such as geography, climatic conditions (i.e. precipitation and temperature) and fluctuation of the groundwater table of the region could potentially favour bacterial growth and weathering rate of the parent rock. The hematite deposit described in this study was collected from a road outcrop. The location is not in direct contact with any stream or any other potential external water from surface flow. Therefore, only groundwater or precipitation, which after percolation, would form the in-situ fluids. Consequently, groundwater table fluctuations can change the geochemical conditions, which can affect the microbial population. In our context, redox conditions and dissolved oxygen would be of particular interest because of their influence on iron oxidation state, and in turn, the mineralogy and microbial population. Additionally, this outcrop is located in a tropical region that receives a considerable amount of rainwater during the rainy season, which would enhance the weathering conditions. These groundwater changes may lead to fluctuations under oxic conditions, providing favourable conditions for (hematite) weathering. A previous study by Meng et al. (Reference Meng, Zuo, Wang, Li, Du, Liu and Chen2020) showed the temporal variation of redox species due to seasonal groundwater fluctuations during the wet and dry seasons while identifying the relationship between groundwater and microbial communities in the redox dynamic zone.
The weathering of the parent rock occurred primarily due to the oxidation of iron, Fe(0) into Fe(II) and further into Fe(III) through microbial/chemical oxidation or microbial reduction of Fe(III) into Fe(II) (Fig. 4). The SEM-EDX image presented in Fig. 4 (left side) is a section obtained from Fig. 5(c), and its chemical compositions are presented in SI Table 1. The average chemical composition of the nuclei region indicated higher weight% of iron (74.2%) and lower weight% of oxygen (24.2%), whereas the growth layers showed lower weight% of iron (59.2%) and higher weight% of oxygen (33.5%). Consequently, deterioration of outermost growth layers would provide access to water and oxygen molecules to get into the inner layers and later to the nuclei region. Recent studies have shown the potential implications of water (Cabrol et al., Reference Cabrol, Wynn-Williams, Crawford and Grin2001) and oxygen (Shekhtman, Reference Shekhtman2019) availability in the Martian environment, which allows for the possibility of the survival of microbial communities in the subsurface beneath the oxidized zone. Evidence from various sources, including Martian meteorites (Greenwood et al., Reference Greenwood, Warren and Rubin2000), imagery of large Amazonian palaeo lakes (Cabrol and Grin, Reference Cabrol and Grin1999) and implications from an atmosphere evolution model (Haberle et al., Reference Haberle, Mckay, Schaeffer, Joshi, Cabrol and Grin2000) and Viking mission findings (Cabrol et al., Reference Cabrol, Wynn-Williams, Crawford and Grin2001) indicate the existence of hydrological activities on Martian environment.

Fig. 4. Schematic diagram showing possible oxidation and reduction mechanism of native Fe and illustration of growth layers.
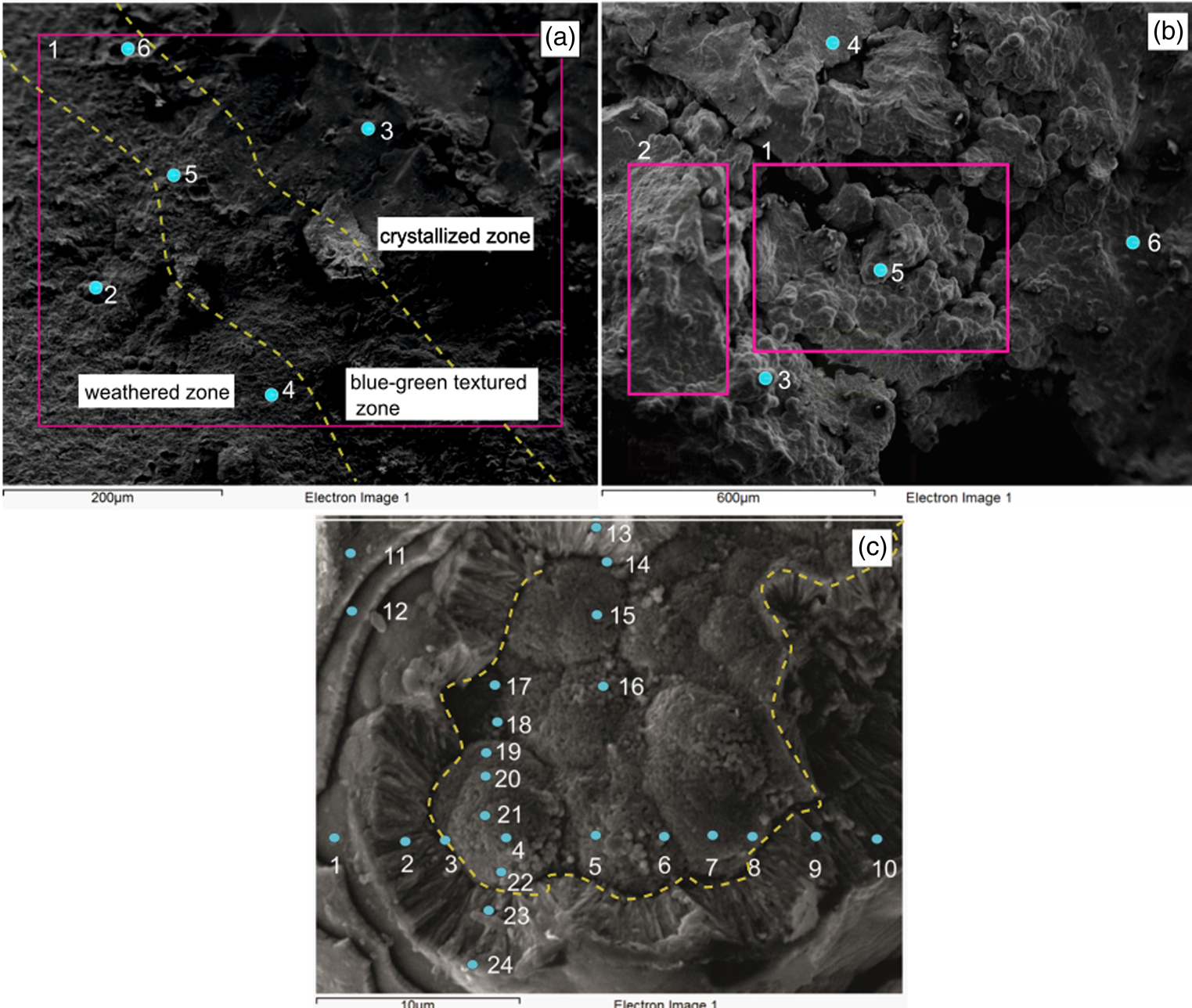
Fig. 5. SEM-EDX analysis carried out in the blue-green granular section seen in Fig. 2. (a) Highly weathered zone (left), crystallized zone (right) and blue-green textured zone; (b) a closer overview of the surface which represents the globules and well-developed cavities and (c) the numbered points indicate the locations where SEM-EDX analysis was carried out for the ‘nuclei’ region and growth layers.
Furthermore, previous studies hypothesized that living organisms may have been trapped in the Martian regolith under dormant conditions (e.g. permafrost bacteria) and could reproduce seasonal or cyclical favourable conditions (Cabrol et al., Reference Cabrol, Wynn-Williams, Crawford and Grin2001). A recent study conducted above the surface of Gale Crater on Mars showed significant seasonal and interannual fluctuations of oxygen suggesting an unknown atmospheric or surface mechanism related to oxygen formation (Trainer et al., Reference Trainer, Wong, Mcconnochie, Franz, Atreya, Conrad, Lefèvre, Mahaffy, Malespin and Manning2019; Cabrol et al., Reference Cabrol, Wynn-Williams, Crawford and Grin2001). Given the recent findings, preferable hydrology and atmospheric conditions (i.e. oxygen), we can hypothesize that liquid water and oxygen circulation could provide potential habitat for microbial communities.
Additionally, we observed potential signatures of extracellular polymeric substance (EPS)-like structures in the SEM-EDX images (Fig. 3(d) and SI Figs. 2 and 3). Generally, EPS is considered as a component of the microorganisms' immediate environment and is composed of a combination of polysaccharides, proteins, nucleic acid and lipids (Hong et al., Reference Hong, Chen, Rong, Cai, Dai and Huang2013). EPS helps microorganisms to survive in various environmental settings based on the availability of preferable conditions (Flemming and Schaule, Reference Flemming, Schaule, Heitz, Sand and Flemming1996). Consequently, the morphological features we noted as EPS had fibrous fabric and contained traces of carbon and phosphorus in EDX images, suggesting the presence of EPS structures (SI Tables 1, 3 and 4) (Fredrickson et al., Reference Fredrickson, Zachara, Kennedy, Dong, Onstott, Hinman and Li1998; Guangyin and Youcai, Reference Guangyin, Youcai, Guangyin and Youcai2017). The presence of phosphorous would indicate breakdown products of cell components and its accumulation in the surrounding environment where EPS would be present (Fredrickson et al., Reference Fredrickson, Zachara, Kennedy, Dong, Onstott, Hinman and Li1998).
Furthermore, EPS matrices are considered mediators of mineral precipitation and dissolution in natural environments (Yin et al., Reference Yin, Weitzel, Jiménez-López, Griesshaber, Fernández-Díaz, Rodríguez-Navarro, Ziegler and Schmahl2020). Hence, we surmise that bacterial polymers/EPS may have accelerated the hematite weathering process and influenced the biomineralization in the microenvironments (Williams, Reference Williams, Mann, Webb and Williams1989). We used indirect morphological and chemical composition evidence to characterize the features as EPS; however, more accurate techniques, such as biofilm extraction and culturing would be necessary to confirm its presence. Additionally, 16S rRNA gene results showed evidence of EPS relationship to bacterial communities at the genus level. Mainly, we observed Acinetobacter, Bacillus and Paenibacillus from DNA analysis of HR samples, which indicates a potential relationship with EPS and the development of biofilms (Wu et al., Reference Wu, Cai, Jing, Niu, Ji, Ashry, Gao and Huang2019) (see the section ‘Bacterial diversity analysis’). However, with 16S rRNA amplicon sequencing, we could only get to the genus level. To identify specific genes for EPS production, whole genome sequencing and gene expression studies would be needed.
From an astrobiological perspective, traces of the microbial communities would have been present on Mars but were unable to survive to present-day due to the unfavourable conditions. The presence of those microorganisms could have left biosignatures preserved as fossil biochemicals in Martian regolith (Gilichinsky and Wagener, Reference Gilichinsky and Wagener1995; Soina et al., Reference Soina, Vorobiova, Zvyagintsev and Gilichinsky1995). Investigation of Martian biofilms formed possibly by excreting EPS would provide implications about the life that existed on Mars. Due to the similar environmental settings on early Earth and Mars, microbial biofilms could have been a common feature of early life on both planets. Therefore, biofilms are considered to be a useful tool to study early extraterrestrial life (Westall et al., Reference Westall, Steele, Toporski, Walsh, Allen, Guidry, Mckay, Gibson and Chafetz2000). Previous studies have reported terrestrial fossil biofilms from Recent to the Early Archaean (3.5 b.y.) when similar environmental conditions would have existed both on early Earth and Mars. Moreover, experimental results obtained by the simulated Martian environment/regolith showed evidence of EPS formation (Westall et al., Reference Westall, Steele, Toporski, Walsh, Allen, Guidry, Mckay, Gibson and Chafetz2000; Feyh and Szewzyk, Reference Feyh and Szewzyk2010). In this study, we also observed EPS-like structures that could have undergone microbial weathering of hematite. Hence, we suggest that evidence of the existence of biofilms/EPS as a reliable implication for extraterrestrial life.
Even though the current Martian environment does not have oxygen, microorganisms from the Martian subsurface could have obtained oxygen from silicate minerals. However, in this study, we were not able to specifically analyse that hypothesis. One of the limitations of our study was that we were unable to directly document the activity of microorganisms and had to rely on indirect clues, such as geochemical and mineralogical changes. Future study will focus on investigating similar samples using environmental-SEM, which can help visualize microorganisms and their interaction with the minerals. This would help to characterize the working relationship between microbes, EPS and minerals more precisely.
Commonly, microbes aggregate as multicellular aggregates glued together by EPS (Fig. 3(d)) and are located at oxidation–reduction interfaces (Gao et al., Reference Gao, Lu, Liu, Li, Li, Song, Wang, Zhang and Zhu2019), whereas the adhesion properties of EPS could directly affect the microbially induced leaching processes (Zhu et al., Reference Zhu, Li, Jiao, Jiang, Sand, Xia, Liu, Qin, Qiu and Hu2012). Hence, we expect the formation of EPS-like structures to transport iron and other trace elements from the core region of the rock to the soil profile during the microbial weathering process. Particularly, we observed a unique anomaly of trace metal variations both in soil and rock samples, which possibly may have occurred due to microbial weathering with the presence of EPS-like structures. This concept is further discussed in the section ‘Elemental analysis’.
EDX elemental analysis
EDX analysis was carried out to determine the chemical properties associated with the features observed in the blue-green granular textured zone of the HR sample. With the acquired SEM image of a relatively large surface area, three different morphological zones were identified; a highly weathered zone, a crystallized zone and a blue-green textured zone (Fig. 5(a)). In Fig. 5(a), the diagonal portion (the area demarcated between the dashed lines) of the image represents the ‘blue-green textured zone’ whereas the left side of the diagonal portion represents the weathered zone, and the right side of the diagonal portion represents the crystalized zone of the rock sample (Table 1). The general elemental composition ( weight percentage) of the area within the section outlined by the box in Fig. 5(a) (spectrum 1) indicated that about 58.4% of the surface area contained iron, whereas 34.4, 6.2 and 1.0% contained oxygen, carbon and silicon, respectively (Table 1). The overall analysis of EDX spectra showed that the weathered zone, crystalized zone and blue-green textured zone were dominated by iron and oxygen with lower amounts of carbon, silicon and phosphorous.
Table 1. Results of EDX represents the general elemental compositions analysed for different parts of the HR sample

Sp. represents spectrum.
A broader overview of the blue-green textured zone is given in Fig. 5(b). The presence of globular surfaces indicated that microbial activities were dominant in these regions and showed a low weight percentage of iron (37.9 ± 3.3%) and high weight percentage of oxygen (44.7 ± 1.6%), together with carbon (9.9 ± 0.7%), aluminium (3.9 ± 0.6%) and silicon (3.3 ± 0.6%) (Fig. 5(b), spectra 1 and 2; Table 2). The unaltered freshly broken hematite surfaces (Fig. 5(b), spectra 4 and 6) showed relatively high iron (72.2 ± 5.2%) and low oxygen (24.4 ± 2.0%), aluminium (1.2 ± 1.7%) and silicon (1.3 ± 0.2%) contents (Table 2). This anomaly in the chemical composition between the weathered and freshly broken surfaces may have occurred due to geochemical alterations induced by the presence of iron-metabolizing bacteria in the HR sample.
Table 2. Results of EDX represents the general elemental compositions analysed for different parts of the HR sample

Sp. represents spectrum.
EDX analysis was carried out on the growth layers and nuclei colonies to study the elemental variation for each point (Fig. 5(c); SI Table 1). The elemental compositions in the nuclei colony regions were similar at every point analysed, especially for iron and oxygen contents. The iron content of the nuclei region was higher (74.3 ± 6.4%) compared to that of the growth layers (59.1 ± 12.6%). In contrast, oxygen values were higher in growth layers (33.5 ± 7.5%) compared to the nuclei region (24.2 ± 4.1%) (SI Table 1). However, the average weight percentages of carbon, aluminium and silicon in the growth layers were 5.4 ± 3.7%, 0.76 ± 1.6% and 1.1 ± 1.3%, respectively, whereas these weight percentages were not as high in the nuclei region (carbon = 0.9 ± 2.2%, aluminium = 0.1 ± 0.1% and silicon = 0.4 ± 0.67%). The chemical properties of growth layers showed different elemental variations compared to the nuclei region. Most of the chemical data in the growth layer region showed an inverse relationship between iron and oxygen compared to the nuclei region. Furthermore, the calculated ratio between iron and oxygen indicated that iron content was higher in nuclei colonies (Fe : O = 3.2) compared to growth layers (Fe : O = 2.0). In the core region, the nuclei colonies were prominent and began to consume Fe(0), whereas in the growth layers, the exposed minerals were subjected to microbial oxidation and converted to Fe(II) and Fe(III) (equations (1) and (3)):
The IRB convert Fe(III) into the soluble Fe(II) form, which becomes commonly available in soil as Fe(OH)2 (equations (4) and (5)). The reduction of Fe(III) to Fe(II) would be comparatively slower than the oxidation of Fe(II) to Fe(III) and would occur in subsurface anoxic environments (Esther et al., Reference Esther, Sukla, Pradhan and Panda2015). As rainwater percolates into the soil layer, more oxygen is introduced into the system (equation (2)), which oxidizes Fe(II) to Fe(III) and gets re-precipitated as Fe2O3 developing a reddish-brown coloration. Hence, the oxidation process could be potentially catalysed with the help of fresh oxygen (equations (2) and (3)). Overall, EDX elemental analysis suggests different morphological features (i.e. nuclear region, globular structures and growth layers) contain unique chemical compositions that occur possibly due to microbial interactions in the parent rock.
Elemental analysis
XRD analysis was carried out on the remnants of highly weathered HR fragments that were recovered from each of the soil profile to examine the changes in chemical properties. Tables 3 and 4 show the results of the major elements as oxides and trace metal composition from the top to the bottom soil profile, respectively. Measured elemental composition showed that Fe2O3 content is gradually decreased from the surface to the bottom layers (Fig. 1(b), 1st to 5th soil profile). The highest Fe2O3 content was observed in the first soil profile (29.0 weight%), whereas the lowest content was observed in the 4th soil profile (19.4 weight%). The Fe2O3 content was slightly higher again in the 5th layer (24.8 weight%). This type of anomaly may occur due to leaching of Fe(II) and Fe(III) by rainwater percolation and accumulation of the elements at the bottom. A similar observation was made for MgO, K2O, P2O5 and CaO compositions (Table 3). The upper soil profile was more convenient for aerobic microbial growth because it had more pore spaces allowing air and water to flow. The porosity decreased towards the bottom layer and therefore reduced the weathering capacity. The trace metal data from the X-ray fluorescence (XRF) analysis showed a high concentration (>100 mg kg−1) of trace metals Zr, V and Zn in all soil profiles, whereas Rb was detected in the soil profile with a concentration <10 mg kg−1 (Table 4).
Table 3. Results of XRF represents the major elements as oxides composition analysed sample for soil profile (1 – top; 5 – bottom) by weight%

Table 4. Results of XRF represents the trace metals composition analysed sample for soil profile (1 – top; 5 – bottom)

All concentrations in ppm.
Comparison of ICP-MS data from the digested soil profile (3rd soil profile where the parent rock HR was located), weathered rock and fresh rock showed an interesting trend in trace metal distribution, as explained below. Overall, trace metal detected from the soil profile had higher concentrations compared to weathered rock except for Fe, Be, Tl, Ba and Mn (Fig. 6; SI Table 2). However, the freshly broken rock samples showed much lower trace metal concentration compared to soil and weathered rock samples (Fig. 6). ICP-MS observations for the soil samples suggested that trace metal concentrations were maximum in the soil profile compared to the weathered or fresh rock fragments. This observation suggested that microorganisms were efficient in weathering, particularly in releasing the trapped mineral nutrients from the parent rock (Uroz et al., Reference Uroz, Calvaruso, Turpault and Frey-Klett2009). Furthermore, Uroz et al. (Reference Uroz, Calvaruso, Turpault and Frey-Klett2009) reported that colonization of mineral surface by microbial communities impacted mineral stability and enhanced. Evidence from our study suggests that microorganisms can release trace metals from the core mineral region resulting in higher trace metal concentrations in soil profile compared to fresh and weathered rock, as illustrated in Fig. 4. Furthermore, the formation of EPS and their adhesion properties help to transport iron and other trace elements from the core region of the rock to the soil profile by microbially induced leaching processes (Zhu et al., Reference Zhu, Li, Jiao, Jiang, Sand, Xia, Liu, Qin, Qiu and Hu2012).

Fig. 6. Graph showing the ICP-MS trace metal variation for soil sample, weathered rock and fresh rock fragment from 3rd soil profile.
Bacterial diversity analysis
The bacterial diversity in hematite samples that were analysed using 16S rRNA gene sequencing is presented using a bar graph (Fig. 7) and Krona plot (SI Fig. 1) (Ondov et al., Reference Ondov, Bergman and Phillippy2011). We used two different samples to determine the microbial diversity in the collected HR sample, including (i) DNA sample 1, which was a 1.0 g of HR fragment were soaked overnight in 50 μl of sterile nuclease-free water, and (ii) DNA sample 2, which was a 1.0 g HR were crushed and placed overnight in 50 μl of sterile nuclease-free water. These samples were collected from the same parent rock used for collecting samples for SEM analysis but not from the same samples used for SEM-EDX analysis (due to samples used for SEM analysis were subjected to vacuum and coating). However, both DNA and SEM samples were collected from the blue-green textures zone in the HR.

Fig. 7. Microbial community structure and its relative abundance in HR fragments soaked in nuclease-free water (DNA sample 1) and crushed HR sample mixed well in nuclease-free water (DNA sample 2; from the 3rd soil profile).
Five major bacterial classes were observed from extracted DNA sample 1 (HR fragments soaked in nuclease-free water) including, Actinobacteria (46%), Chloroflexi (17%), Proteobacteria (13%), Acidobacteria (13%), Cyanobacteria (2%) and other minor classes, including Bacteroidetes (1%) and Gemmatimonadetes (0.8%) (Fig. 7). Furthermore, 1.0 g of crushed HR sample that was well-mixed in nuclease-free water (DNA sample 2) showed a relatively higher proportions of taxa, including Proteobacteria (19%), Cyanobacteria (12%), Acidobacteria (11%) and Bacteroidetes (4%) with lower amounts of Actinobacteria (32%) and Chloroflexi (11%) compared to DNA sample 1 (Fig. 7).
The microbial diversity information suggested many members from the iron-metabolizing community. Several Fe(III) reducing bacterial genera were found in both samples 1 and 2 (i.e. relative abundance of DNA sample 1 and DNA sample 2) including Mycobacterium (0.3 and 0.3%), Amycolatopsis (0.1 and 0.1%) and Nocardiodes (0.4 and 0.7%) under the phylum of Actinobacteria (Zhang et al., Reference Zhang, Zeng, Liu, Chen, Guo, Ma, Dong and Huang2019). Additionally, we found iron-reducing families of Clostridiaceae (0.0 and 0.04%), Lachnospiraceae (0.07 and 0.01%), Bacillaceae (0.2 and 0.4%) from the phylum Firmicutes (Gupta et al., Reference Gupta, Dutta, Sarkar, Panigrahi and Sar2018). Observed phylogenetic analysis showed genus Sediminibacterium (0.08 and 0.1%) from the phylum Bacteroidetes (Wang et al., Reference Wang, Hu, Hu, Yang and Qu2012) and genus Pedomicrobium (0.03 and 0.08%) from the phylum Proteobacteria (Braun et al., Reference Braun, Richert and Szewzyk2009) as possible IOB from the analysed samples. About 4% of the taxa from DNA sample 1 and 6% of the taxa from DNA sample 2 could not be identified. Even though notable IRB and IOB were not detected (e.g. Shewanella and Geobacteria), the presence of other iron-metabolizing bacterial communities plays an important role in the weathering of the hematite bearing rock.
Possible mechanisms for microbial growth and influence on identified morphologies
Intensive biogeochemical weathering mechanism
Christensen et al. (Reference Christensen, Bandfield, Clark, Edgett, Hamilton, Hoefen, Kieffer, Kuzmin, Lane and Malin2000a) suggested five mechanisms for the formation of hematite deposits in Martian environment, including (1) direct precipitation from standing, oxygenated, Fe-rich water, (2) precipitation from Fe-rich hydrothermal fluids, (3) low-temperature dissolution and precipitation through mobile groundwater leaching, (4) surface weathering and coatings and (5) thermal oxidation of magnetite-rich lavas. Four of these proposed mechanisms demand water–rock interactions. Particularly, this research proposes a mechanism with low-temperature dissolution and precipitation through mobile groundwater leaching. The tropical climate with plenty of rainfall in the study area enhances the weathering of hematite and provides ideal conditions for microbial growth. As illustrated in Fig. 8, it can be hypothesized that parent rock would have had well-developed crystals together with weakly developed fractures. When these fractured surfaces are exposed to high humidity (i.e. groundwater or rainwater), bacteria and air interact with the minerals and initiate the weathering process (Fig. 8, stage 1). These weak crystal surfaces can create a pathway for bacteria to scavenge and rummage inside the parent rock for both energy-rich materials, but also to establish newer microbial colonies, protected from the elements. With the partially weathered surface formed, the development of bacterial nuclei can be enhanced.

Fig. 8. Schematic diagram of the hypothesized mechanism for microbial weathering.
In the second stage, the size of the bacterial colony can increase by forming the growth layers. Fe(III) reducers will need to come in direct physical contact with Fe(III) oxides and reduce it to Fe(II) (Lovley et al., Reference Lovley, Holmes and Nevin2004). For the IRB to establish contact with the hematite surface, another functional substance (e.g. Fe(III) oxide) is required that works as Fe(III) chelator or electron shuttler (Lovley, Reference Lovley1997). The physical and chemical nature of the newly weathered HR provides a favourable environment for the growth of iron-metabolizing microorganisms. Furthermore, they begin to multiply in these regions while also expanding the nuclei colonies (Fig. 8, stage 2). The formation of growth layers, in turn, allows the microbial colonies to increase in volume.
However, the outermost layers will stop their growth due to onset of limiting favourable conditions, such as the lack of access to the core region, which is rich in iron. The continuous growth of mineral layers would connect each colony and create micro-tunnels (Fig. 8, stage 3). This stage can be considered as the cavity formation stage in the rock, where groundwater percolation enlarges the size of the micro-tunnels. However, groundwater percolation may be responsible for transporting elements which are developed inside the colonies, especially the material from microbial films. Finally, the rapid growth of microorganisms would digest the parent rock and create a new soil profile by changing the initial physical can chemical characteristics.
Relevance to Martian exploration
During the past few decades, Mars landers and rovers gathered in-situ measurements of geochemistry of regolith materials of the Martian surface. For example, XRF analyses of the two Viking landers and alpha proton X-ray spectrometer (APXS) analysis from the Mars Pathfinder mission was able to provide the chemical elements in Martian soil and rocks in larger detail (Rieder et al., Reference Rieder, Economou, Wänke, Turkevich, Crisp, Brückner, Dreibus and Mcsween1997a, Reference Rieder, Wänke, Economou and Turkevich1997b). Knowledge of chemistry and mineralogy of the Martian environment has opened a new window to understand the past physical and chemical weathering processes on Mars (Bell et al., Reference Bell, Mcsween, Crisp, Morris, Murchie, Bridges, Johnson, Britt, Golombek and Moore2000). Cation content and oxidation state, pH and abundance of organic matter in soil are some of the important indicators that need to be investigated as these indicate parameters that undergo recordable changes due to weathering and alteration of parent materials (Bell et al., Reference Bell, Mcsween, Crisp, Morris, Murchie, Bridges, Johnson, Britt, Golombek and Moore2000). Numerous studies link the physical and chemical properties of soil and environmental conditions on Earth as a proxy for Martian simulations (Schwertmann et al., Reference Schwertmann, Taylor, Dixon, Weed, Dixon and Weed1989; Cornell and Schwertmann, Reference Cornell and Schwertmann1996). Particularly, hematite deposits found on Mars are vital for scavenging the traces of early life, and these hematite deposits may prove to be the only large-scale mineralogical evidence for water–rock interactions on early Mars (Allen et al., Reference Allen, Westall and Schelble2001). Even though the present surficial Martian environment is considered inhabitable due to unfavourable environmental conditions, signatures of early biomarkers may still be preserved in the Martian surface or near-surface hematite deposits. It is highly likely that biological material in these samples would have disintegrated while leaving the morphological evidence of previous biogeochemical processes engrained in the minerals. Hence, this evidence is vital for scavenging early life signatures in Martian geological samples and to correlate the mineralogical and morphological (microorganisms) similarities with terrestrial hematite deposits.
Conclusions
This study demonstrates microbial-mediated weathering of terrestrial hematite with unique morphological, chemical and microbial characteristics, as a result of iron metabolism. The parent rock was classified into three different morphological zones as highly weathered zones, crystallized zones and blue-green granular textured zones. Furthermore, four different geomorphologic features in the blue-green granular textured zone (globular structures, growth layers, nuclear components and nuclei) were identified and proposed to be influenced by a microbially induced weathering mechanism. Re-analysed samples after incubation showed a continuous expansion of growth layers and nuclei regions, possibly due to microbially induced iron reduction/oxidation reactions. Observed SEM-EDX data suggested compositional variation among those morphological features; especially, the growth layers indicated low iron content and high oxygen, aluminium, silicon and trace metal content compared to the nuclei region. These observations suggested degradation of iron and transformation of trace metals by microorganisms and their EPS. Results obtained by ICP-MS analysis revealed that trace metal concentrations were higher in soil compared to the parent rock, which provided a biogeochemical evidence indicating microbially induced element leaching from parent rock. Measured major element composition using XRD analysis showed that Fe2O3 content was gradually decreasing from the surface layer to the bottom layers, indicating the influence of environmental factors (i.e. rainwater percolation and porosity of soil layers) for biological weathering. 16S rRNA amplicon sequencing data showed positive results for the availability of microorganisms in the collected sample and proved the weathering of hematite triggered by iron-metabolizing microbial activities.
In the recent past, numerous studies have been carried out on the Earth by linking the physical and chemical properties of soil and environmental conditions to simulate the Martian conditions. Hence, the observations made from the earth materials, their weathering and precipitation process provide potential clues for a biogeochemical role in iron metabolism on early Mars. Mars explorations programmes such as Mars 2020: perseverance are planning to ferry geological samples from Mars and analyse them in terrestrial laboratories to reveal if extraterrestrial life existed there in the past. However, these samples may disintegrate while leaving the morphological evidence intact with minerals. Our research proposes to utilize preserved morphological, mineralogical and microbial traces as potential analogues to study early life on Mars and for interpretations of the biogeochemical role in iron metabolism on Martian environments.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1473550421000124
Acknowledgements
The authors would like to thank Dr Ruwini K. Rupasinghe (University of California, Davis) for DNA extraction from rock samples and Mr Danushka Gamage (University of Peradeniya, Sri Lanka) for providing technical assistance in acquiring SEM images and EDX data.
Conflict of interest
The authors declare that the contents have no conflict of interest towards any individual or any organization.















