INTRODUCTION
The establishment of symbiotic associations of marine animals with large, bottom-dwelling invertebrates is a common and widely distributed phenomenon in marine ecosystems. Among the known echinoderm hosts, the comatulid crinoids (i.e. comatulids or feather stars) harbour the richest and most specific symbiotic assemblages (Deheyn et al., Reference Deheyn, Lyskin and Eeckhaut2006). They consist of specialized species of animals: polychaetes, myzostomids, gastropods, crustaceans, brittle stars and fish (Zmarzly, Reference Zmarzly1984; Morton & Mladenov, Reference Morton, Mladenov and Morton1992; Huang et al., Reference Huang, Rittschof and Jeng2005), that remain during most or all their life on their host while non-specialized species simply occur on comatulids accidentally (Britayev & Mekhova, Reference Britayev and Mekhova2011).
Traditionally, the settlement of planktonic larvae has been considered as the starting point of any symbiotic assemblage (Marsden, Reference Marsden1987; Preston & Doherty, Reference Preston and Doherty1990; Pernet, Reference Pernet2000). However, migrations of post-settled juveniles and adults may also play an important role (Thiel et al., Reference Thiel, Zander and Baeza2003; De Bruyn et al., Reference De Bruyn, Rigaud, David and De Ridder2009). There are several possible motives for migration, among them: food limitation related to symbionts' growth (Castro, Reference Castro1978), foraging for food outside the host (Castro, Reference Castro1978), intraspecific or interspecific competition (Castro, Reference Castro1978; Britayev, Reference Britayev1991), searching for mating partners (Wirtz & Diesel, Reference Wirtz and Diesel1983; Britayev & Mekhova, Reference Britayev and Mekhova2014) and to find hosts that provide better protection (Thiel et al., Reference Thiel, Zander and Baeza2003). However, movement between hosts is very risky due to a high level of predation pressure in tropical ecosystems (e.g. Glynn et al., Reference Glynn, Stewart and McCosker1972), and increases mortality of migrating symbionts (Yanagisawa & Hamaishi, Reference Yanagisawa and Hamaishi1986). Thus, we may expect that short-distance migrations are less risky for symbionts than long-distance ones.
Nevertheless, our previous studies demonstrated successful migrations of species associated with the comatulid crinoid Himerometra robustipinna (Carpenter, 1881) both short-distance (tens of centimetres) and relatively long-distance (several metres) (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012). In the latter case the substrate between the site with depopulated crinoids, and rocks that harboured native crinoids, had numerous shelters for migrating symbionts (consisting of rubble, pebbles and fragments of corals). These results raise several questions. How do symbionts cover these long distances – by crawling on the substrate, or by drifting in the water column with the appropriate currents? Does the substrate have an influence on the symbionts' long-distance host-to-host migration ability? Does the method of migration (crawling versus drifting) differ in different groups of symbionts?
In this paper we attempted to check the ability of symbionts to complete long-distance migrations, and to respond to these questions by means of two in situ experiments with a depopulated crinoid host Himerometra robustipinna.
MATERIALS AND METHODS
Study localities
Field experiments and crinoids sampling were carried out in Nhatrang Bay (South China Sea, South Vietnam), in the vicinity of the Research and Experimental Station ‘Dambay’ belonging to the Russian-Vietnamese Tropical Center, located at the south-eastern part of Tre Island (12°11′25.35″N 109°20′28.26″E). The underwater landscape is characterized by the presence of dead fragments of coral reef of different sizes, elevated 1–2 m above a sandy bottom at depth 1.5–3 m close to the rocky area. Both coral fragments and rocks are suitable substrates for crinoids. This area is bordering seaward with a sandy bottom without crinoids.
Studied organisms, their collection and treatment
Himerometra robustipinna is a widely distributed species in the Indo-West Pacific inhabiting shallow-water habitats. It is very common in the Bay of Nhatrang and easily distinguishable from other comatulids due to its bright-red colour. These are the reasons why we selected it for recolonization experiments in this and previous experimental treatments (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012). Symbiotic associated fauna includes 11 species of animals: polychaetes, myzostomids, gastropods, galatheids, ophiuroids (one species in each taxon), and six species of shrimps (Mekhova & Britayev, Reference Mekhova, Britayev, Britayev and Pavlov2012).
Specimens of Himerometra robustipinna were hand-collected at 5–15 m depth using scuba equipment, gently pulled away from the substrate, and placed in individual zip-lock plastic bags to avoid loss of symbionts during transportation to the boat. After that crinoids were successively washed in an isotonic solution of magnesium chloride and immersion of clove oil in the seawater (2 ml L−1). Washouts were sieved through a 1.0 mm mesh, and crinoids were checked by eye for attached macrosymbionts. All found organisms were fixed in 70% alcohol solution for further identification and counting. The smallest size group treated for polychaetes, galatheids and shrimps was 3–5 mm in length, while for molluscs and ophiuroids all specimens were included in the analysis, since they were easily detected (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012). Each host was tagged using a small numbered plastic plate and placed on the experimental substrate. This treatment led to autotomy of visceral mass in ~60% of treated crinoids. However, no mortality of crinoids was recorded and all specimens treated after 7 days' exposure had well-developed visceral masses, which is in accordance with data on its regeneration rate completed in 4–7 days (Dolmatov et al., Reference Dolmatov, Bobrovskaya and Girich2014).
Experiments
Two sets of in situ experiments were performed: exposure of depopulated crinoids (1) on stony ‘islands’ isolated from native crinoid assemblages by sandy substrate, and (2) in cages suspended in the water column. In set 1 four artificial ‘islands’ 1.0–1.3 m in diameter were constructed from boulders on the sandy bottom at 2.5–3.0 m depth, 2.5–5.0 m away from fragments of dead coral reef and rocks harbouring the native crinoid assemblages (Figure 1A). We arranged 12–15 specimens of Himerometra robustipinna on each ‘island’ and exposed these for 1, 2, 3 and 4 weeks to assess whether substrate has an influence on the symbionts' long-distance migrations. Comatulids are animals that can move; therefore we checked areas adjacent to the ‘islands’ to return back specimens moving away on the second and third days of exposure. Nevertheless, nine of 53 crinoids were missed during exposure, and the final number of animals on each ‘island’ varied from 9 to 13.
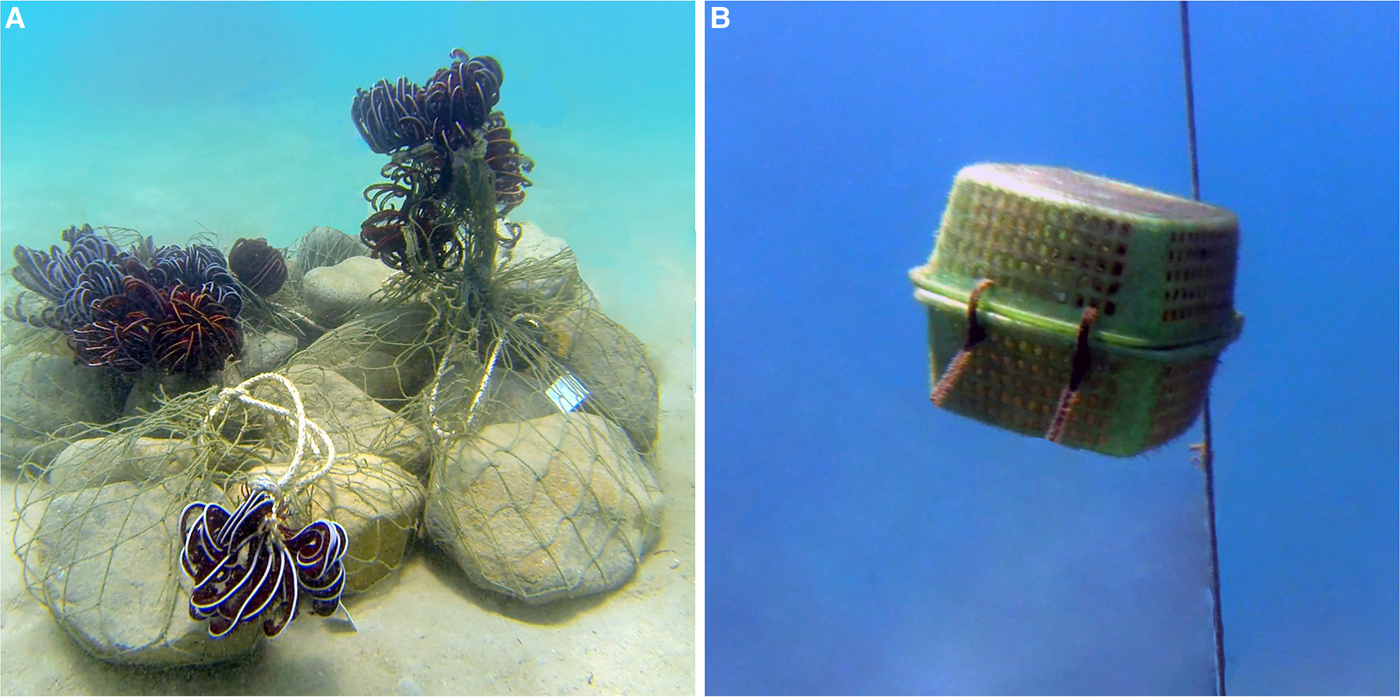
Fig. 1. (A) artificial ‘island’ with crinoids; (B) cage with crinoid suspended in the water column.
In set 2, eight completely closed plastic mesh cages (30 × 30 × 20 cm, mesh size 20 × 20 mm) were suspended in the water column (1.0–1.5 m above the bottom, Figure 1B). Depopulated crinoids were placed inside the cages, one per cage. Cages were exposed for 10–11 days, aiming to check whether symbionts were able to disperse through the water column with currents.
After exposure, all experimental hosts were collected and treated in the same way as the crinoids that were collected for the experiments. As the control we employed native symbiotic assemblages washed out from the experimental crinoids.
Data analysis
For comparison of the symbiotic assemblages in experimental sets and in the control we used several indexes: species richness (mean number of species per single infested host), the prevalence (percentage of infested hosts) and abundance (mean number of symbionts per host examined). To check if there are any differences in abundance and mean species richness between groups (experimental sets), we performed an ANOVA. To check which group differs from each other, we used post-hoc (Tukey's Unequal N HSD) test. All statistical analyses were performed in StatSoft Statistica (Version 10).
RESULTS
Set 1. Colonization of crinoids on stony ‘islands’
Depopulated crinoids arranged on the stony ‘islands’ on sandy substrate were rapidly colonized by symbionts. Prevalence was close to 100% observed in the control in all experimental groups varying from 88 to 100% between samples.
There are significant differences among values of symbionts abundance in the control and experimental groups of set 1 according to ANOVA (F (5.95) = 17.598, P ≤ 0.001). Abundance in all groups was lower than in the control (Table 1, Tukey's HSD, P < 0.01), while in the group exposed for 21 days it does not differ significantly from the control (Tukey's HSD, P = 0.051), likely due to the small sample size. Abundance of symbionts in experimental groups increased with exposure (Figure 2).

Fig. 2. Abundance of symbionts in experimental set 1. Experimental time expressed as number of days (x-axis); y-axis – mean number of symbionts per host.
Table 1. Infestation characteristics of crinoid Himerometra robustipinna, in control and experimental groups.

There is a significant difference between groups in species richness according to ANOVA (F = (7.110) = 10.203, P < 0.0001). This index was also significantly lower in experimental groups than in the control (Tukey's HSD, P < 0.01), and did not increase with exposure (Table 1).
Nine species of macrosymbionts were found in association with Himerometra robustipinna in the control sample: polychaetes Paradyte crinoidicola (Potts, 1910), Hypomyzostoma crosslandi (Boulenger, 1913), galatheid Allogalathaea elegans (Adams & White, 1848), pontoniin shrimp Brucecaris tenuis (Bruce, 1969), Periclimenes commensalis Borradaile, 1915, Pontoniopsis comanthi Borradaile, 1915, Palaeomonella pottsi (Borradaile, 1915) and Laomenes nudirostris (Bruce, 1968) (Crustacea: Caridea), ophiuroid Gymnolophus obscura (Ljungman, 1867) (Figure 3). Eight species of symbionts were found in experimental samples, while species composition slightly differed due to disappearance of ophiuroids and myzostomids, and the finding of gastropod Annulobalcis vinarius Dgebuadze, Fedosov & Kanto, 2012. The proportion of different species in experimental samples changed in comparison with the control in favour of polychaetes and shrimps at the expense of galatheids (Table 2).

Fig. 3. Symbionts from different taxa associated with Himerometra robustipinna: (A) Paradyte crinoidicola; (B) Allogalathea elegans s.l.; (C) Annulobalcis vinarius; (D) Laomenes nudirostris. Scale bars: A, B, D = 1 cm; C = 1 mm. Photos: A, B, C by E.S. Mekhova; D by O.V. Savinkin.
Table 2. Symbionts found in association with Himerometra robustipinna and their proportion (%) in the control and experimental sets.

Set 2. Colonization of crinoids in suspended cages
Since one cage during exposure was lost, the number of experimental crinoids reduced to seven. Six of them (86%) were colonized by symbionts. Abundance of symbionts did not differ significantly from that in the control (Tukey's HSD, P = 1.0), while it was significantly higher than in groups with comparable exposure (7 and 14 days) in set 1 (Tukey's HSD, P < 0.01).
Species richness (2.0 ± 0.6) was slightly lower than in the control (2.6 ± 0.9), while it did not differ significantly either from its value (Tukey's HSD, P < 0.89), or from values in groups with comparable exposure (Table 1).
Five species of macrosymbionts were found in association with crionoids in cages. Four of them were observed in the control and experimental groups of set 1 (Table 2), and one species, Pereclimenes diversipes Kemp, 1922 was ‘new’ that has never previously been recorded in association with crinoids in the Bay (Mekhova & Britayev, Reference Mekhova, Britayev, Britayev and Pavlov2012). Its abundance in the samples led to a change in proportion of species in favour of shrimps (Table 2).
DISCUSSION
The results of our experiments provide evidence of the ability of sub-adult and adult symbionts to complete long-distance migrations and of their important role in colonization of hosts, and confirmed results obtained earlier (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012). However, it is not clear yet what ‘transport corridor’, the surface of substrate or the water column, symbionts employed for migration from host donor to host recipient. Results of the experiment with crinoids exposed in the water column inside cages (set 2) indicate that at least some groups of symbionts, viz. polychaetes, galatheids, and shrimps are able to reach a host recipient by drifting or swimming in the water column. The movement in the water has not been reported before as a method of dispersion for symbionts, but near-bottom dispersion with currents is a known phenomenon for different groups of benthic invertebrates, especially for crustaceans (e.g. Virnstein & Curran, Reference Virnstein and Curran1986).
The ability of symbionts to crawl on the substrate from host to host was demonstrated earlier for the crab Trapezia ferruginea Latreille, 1828 (Castro, Reference Castro1978). Crabs were absent among Himerometra robustipinna symbionts treated in our studies. So, to check this ability in symbionts belonging to other taxonomic groups we compared infestation characteristics of symbiotic species in the set 1 and set 2 experiments. The highest indexes of host infestation among studied symbionts were observed in polychaete Paradyte crinoidicola. Prevalence was 100% in the control and close to that (83–100%) in set 1, and substantially lower in set 2 experiments (43%, Table 2). Respectively, the abundance was significantly higher in set 1 than in the set 2 depopulated crinoids with comparable exposure (7–14 days exposure). These observations suggest that colonization of hosts located on the bottom is occurring more intensively than of hosts suspended in the water column, or in other words, that the substrate or near-bottom water layer are more favourable ‘corridors’ for symbiont migrations.
So as to understand whether the substrate has an influence on the symbionts' migration ability we compared infestation indexes of crinoids exposed on ‘islands’ surrounded by sandy substrate (our data set 1), and on ‘islands’ surrounded by pebbles with fragments of dead corals (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012; exposure in both cases was 7 days). Prevalence in our experiments was slightly higher (92.0 and 64.3%, respectively) while abundance and species richness were significantly lower than in crinoids from the ‘island’ surrounded by pebbles (1.6 vs 5.7 specimens per host, P ≤ 0.01, and 1.3 vs 3.7 species per host, P ≤ 0.05, respectively, post-hoc Tukey's unequal N test) suggesting that ground consisting of pebbles and providing shelters for symbionts is a more favourable substrate for symbiont migrations than sand.
Comparison of experimental results allows the division of crinoid symbionts into two conventional groups according to the dispersal ability of their post-settled stages: (1) species able to complete long-distance migrations, and (2) species unable to migrate or having limited dispersal ability. The first group includes the polychaete Paradyte crinoidicola, galatheid Allogalathaea elegans, shrimps (five species), and probably the gastropod Annulobalcis vinarius, mentioned among species with a high colonization rate previously (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012) and accidentally found in both sets of our experiments. The second group includes two species, the ophiuroid Gymnolophus obscura and the myzostomid Hypomyzostoma crosslandi. Both species were present in the control and absent in experimental sets. Gymnolophus obscura was recorded on depopulated crinoids in their dense aggregations (Dgebuadze et al., Reference Dgebuadze, Mehova and Britayev2012), and evidently is able to migrate for short distances. Myzostomids were not found among crinoid colonizers in these and previous experiments with recolonization. That is in accordance with observations on the locomotion of another myzostomid species, Myzostoma cirriferum Leuckart, 1836, which is limited to the surface of their host crinoid (Lanterbecq et al., Reference Lanterbecq, Bleidorn, Michel and Eeckhaut2008), and characterizes them as species not able to migrate even for short distances.
Special attention has to be given to the shrimp Periclimenes diversipes which intensively colonized crinoids suspended in the water column within cages (set 2). This species is recorded in association with several scleractinian species (Bruce, Reference Bruce1972), but has never been found on crinoids. Nevertheless, the prevalence (71%), abundance (9 specimens per host) and total number of this shrimp (63 specimens) were much higher than other shrimp species. One may suggest two possible reasons of intensive colonization of crinoids by this species in cages: (1) the position of cages suspended 1.0–1.5 m above the bottom, and exposed to an appropriate current with drifting shrimps, and (2) interspecific competition does not allow them to colonize crinoids already infested by other symbionts. However, further studies are necessary to check these hypotheses.
ACKNOWLEDGEMENTS
We would like to thank the Russian-Vietnam Tropical Center of A.N. Severtsov Institute of Ecology and Evolution of Russian Academy of Sciences (SIEE) for the opportunity to undertake this investigation, Dr I.N. Marin for field assistance and help with identification of shrimps, and Y.V. Deart for valuable help in statistics treatment. The advice of two anonymous reviewers allowed substantial improvement to our manuscript.
FINANCIAL SUPPORT
The experimental part was supported by the Russian Foundation for Basic Research (grant number 14-04-32153). The publication preparation was supported by the Russian Scientific Foundation (grant number 14-14-01179).







