INTRODUCTION
The first outstanding contribution to the knowledge of Polychaeta from Cuba was made by Hartman (Reference Hartman1942), in which 18 new species were described from collections of the ‘Atlantis’ cruise around the island (1938–1939). Kiselieva (Reference Kiselieva1968, Reference Kiselieva1971) recorded 68 species, 16 new to Cuba, from the Cuban–Soviet expedition (1964–1965). Rullier (Reference Rullier1974) reported 106 species collected in sponges from the Batabanó Gulf, and described 1 new genus and 5 new species. Suárez & Fraga (Reference Suárez and Fraga1978) and Suárez (Reference Suárez1981) listed the errant and sedentary polychaetes recorded for the island, and several studies on the systematics, distribution and ecology of polychaetes from Cuba were published during the 1980s (Ibarzábal, Reference Ibarzábal1985, Reference Ibarzábal1986, Reference Ibarzábal1988).
The family Syllidae from Cuba and neighbouring areas were studied by San Martín (Reference San Martín1990, Reference San Martín1991a, Reference San Martínb, Reference San Martín1992) and San Martín & Estapé (Reference San Martín and Estapé1993). A total of 18 new species of Syllidae were described in those papers. Despite these contributions the fauna of polychaetes is still not well known in extensive areas of Cuba, and this is especially true for syllids.
In 1987, the Guanahacabibes Peninsula was declared a Biosphere Reserve by UNESCO. This region covers 1500 km2 and it is located in the westernmost part of Cuba, in the province of Pinar del Rio. In 2001, the National Park was declared within the area of the Reserve. The Guanahacabibes Biosphere Reserve comprises about 39,000 ha, of which 16,000 is a marine area. So far, no study has been carried out about the polychaetes from the Guanahacabibes Biosphere Reserve. This paper contributes with a preliminary list of syllids from algal and sandy bottoms samples taken in this area in 2006 and 2007, one of which is here described as new. The descriptions and the iconography of two species of Myrianida only identified at genus level are also included because they show unusual features that should be of interest in future studies.
MATERIALS AND METHODS
The Guanahacabibes Peninsula suffers a higher incidence of hurricanes. The main current in this area flows in a northerly direction, with a small countercurrent in the south of the Peninsula, due to the high pressures. The submerged platform of the north coast is bordered by a long fringing coral reef which extends from Los Morros to Cayo Buenavista, and the coastline is almost entirely occupied by mangroves. Coral reefs at the south coast are located just opposite the Corrientes Cape, but most of the coastline is formed by sandy beaches alternated with emerged fossil reef platforms.
Eight samples of algae and sand were collected in one of the southern beaches (Playa Antonio) during two field trips in July 2006 and July 2007 (Table 1). After washing the samples, polychaetes were fixed in 10% formaldehyde–seawater solution or 100% ethanol, and preserved in 70% ethanol. Syllids were studied under a Nikon Optiphot microscope equipped with differential interference contrast system (Nomarsky), ocular micrometer and camera lucida drawing tube. Length and width (at the level of the proventricle, excluding parapodia) of specimens were measured with the ocular micrometer. Those specimens selected for scanning electron microscopy (SEM) were firstly cleaned with a chitinous fibre, and then critical point dried (Quorum Technologies cpd 7501 dryer) and sputter-coated with gold (sc510 Biorad). They were examined and photographed with a FEI Quanta 200 SEM with integrated system Oxford Instruments Analytical—INCA at SME (Servicio de Microscopía Electrónica), Museo Nacional de Ciencias Naturales de Madrid (MNCN). All the studied specimens and the comparative material are deposited at the MNCN.
Table 1. Coordinates, substrate, depth and data of the studied samples.

RESULTS
Fourteen species of Syllidae belonging to nine genera have been identified from six samples (Table 2). One species is described as new: Sphaerosyllis sandrae sp. nov. Two more species, Myrianida sp. 1 and Myrianida sp. 2 are only identified at the generic level and discussed below because they have some interesting features. They could be new species, but there are not enough specimens to ensure that. Syllides sp. and Parexogone sp. could not be identified at the specific level due to the lack of diagnostic features.
Myrianida Milne-Edwards, Reference Milne-Edwards1845: 180. Nygren, Reference Nygren2004: 115–162
Autolytus Grube, Reference Grube1850: 310. San Martín, Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Jerrano and Templado2003: 466–468.
Table 2. List of species from samples.

TYPE SPECIES
Myrianida fasciata Milne-Edwards, Reference Milne-Edwards1845.
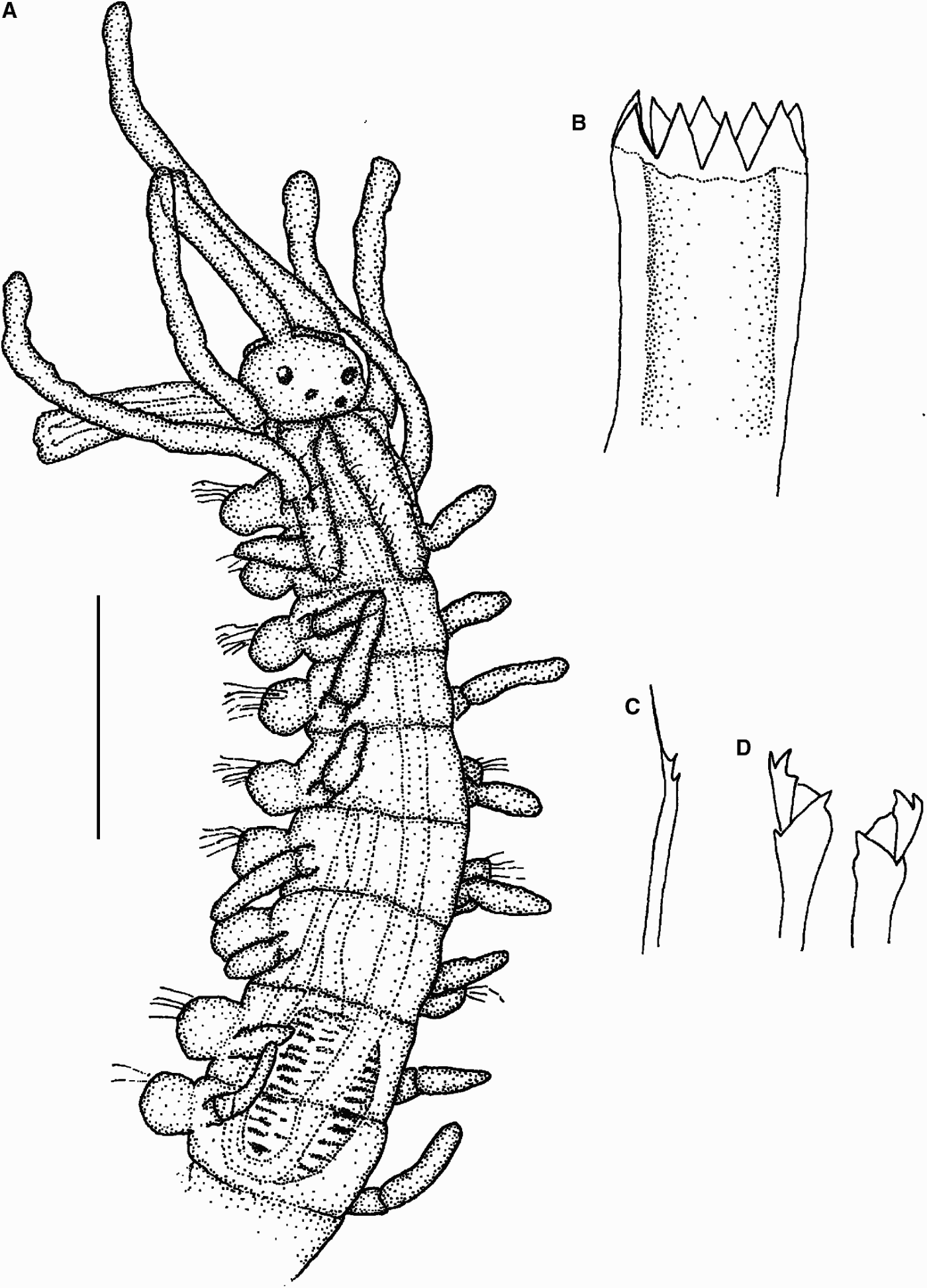
Fig. 1. Myrianida sp. 1. (A) Anterior part, dorsal view; (B) trepan; (C) simple bayonet chaeta; (D) compound chaetae. Scale bars: A, 0.18 mm; B–D, 40 µm.
MATERIAL EXAMINED
Playa Antonio, Guanahacabibes Peninsula, Cuba, (M2 sample), 1 incomplete spec.
DESCRIPTION
Body 0.12 mm wide, with 29 chaetigers. Prostomium small, oval, semicircular, with two pairs of eyes similar in size, in flattened trapezoid arrangement. Long antennae, especially central one (0.27 mm long), inserted in front of anterior eyes. Lateral antennae on anterior edge of the prostomium. Palps short, slightly visible dorsally. Nuchal epaulettes extend until posterior part of chaetiger 2. Peristomial dorsal cirri slightly longer than lateral antennae, peristomial ventral cirri much shorter. Dorsal cirri throughout much shorter than central and lateral antennae, except for those on chaetiger 1 (Figure 1A). Compound chaetae as heterogomph bidentate falcigers, with proximal tooth 10 µm long, longer and thicker than distal one (Figure 1D). Dorsal simple chaeta as thin bayonet chaeta, present from chaetiger 1 (Figure 1C). Pharynx long and thin, with two sinuations, the first one in chaetiger 9 and the second at the level of chaetigers 5–6 (Figure 1A). Trepan with 9 equal sized triangular teeth (Figure 1B). Proventricle short, barrel shaped, extending for 1.5 segments, with about 13 rows of muscle cells (Figure 1A).
REMARKS
This species is very similar to Myrianida convoluta (Cognetti, 1953), but this latter species has the central antenna clearly inserted between the pair of posterior eyes and a very thin pharynx with numerous sinuations near the proventricle (it is not possible to see this feature in our specimen because the pharynx is everted). Our species has no small glands present across each segment and the compound chaetae have no serration (see Cognetti, Reference Cognetti1957; Nygren, Reference Nygren2004). In the absence of more specimens, we cannot identify the specimen at species level with certainty.

Fig. 2. Myrianida sp. 2. (A) Anterior part, dorsal view; (B) dorsal cirrus with long cirrostyle; (C) dorsal cirrus with short cirrostyle; (D) single bayonet chaeta; (E) anterior compound chaetae; (F) posterior compound chaetae. Scale bars: A, 0.18 mm; B–C, 0.1 mm; D–F, 40 µm.
MATERIAL EXAMINED
Playa Antonio, Guanahacabibes Peninsula, Cuba (M2 sample), 1 spec.
DESCRIPTION
Body 2.5 mm long, 0.2 mm wide, with 39 chaetigers. Prostomium circular, with two pairs of large eyes similar in size, in flattened trapezoid arrangement. Long antennae, all about same length (0.33 mm), central antenna inserted slightly ahead to anterior eyes. Palps short, fused along their entire length. Nuchal epaulettes reaching posterior margin of chaetiger 2 (Figure 2A). Peristomial dorsal cirri about same length as antennae, peristomial ventral cirri as long as peristomial dorsal cirri. Two types of dorsal cirri (Figure 2B, C) throughout about half length of peristomial dorsal cirri, except for those on chaetiger 1, which are about as long as antennae. Compound chaetae as heterogomph bidentate falcigers, with proximal tooth 14 µm length, longer and thicker than distal tooth, anterior chaetae slightly longer than posterior chaetae (Figure 2E, F). Dorsal simple chaeta as thin bayonet chaeta, present from midbody chaetigers (Figure 2D). Pharynx long and thin, forming a sinuation near proventricle (Figure 2A). Anterior part of pharynx and trepan not visible. Proventricle short, barrel shaped, present on segments 10–13, with 23 rows of muscle cells. Pygidium with a pair of anal cirri slightly longer than dorsal cirri.
REMARKS
This species is very similar to Myrianida inermis Saint-Joseph, 1887, but according to the descriptions by Gidholm (Reference Gidholm1967), San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Jerrano and Templado2003) and Nygren (Reference Nygren2004), M. inermis is longer and has a greater number of segments, its nuchal epaulettes reach chaetiger 5, the blades of its chaetae are longer and thinner than those of our specimen, and its pharynx is longer and more convoluted, with a poorly differentiated trepan. Trepan and part of the pharynx are not visible in our specimen. Therefore, we cannot identify it as Myrianida inermis with certainty.
Sphaerosyllis Claparède, Reference Claparède1863: 45. San Martín, Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Jerrano and Templado2003: 187, 188; 2005: 86, 87.
TYPE SPECIES
Sphaerosyllis hystrix Claparède, Reference Claparède1863.

Fig. 3. Sphaerosyllis sandrae sp. nov. (A) Anterior part, dorsal view; (B) anterior dorsal simple chaeta; (C) anterior compound chaetae; (D) mid-body dorsal simple chaeta; (E) mid-body compound chaetae; (F) posterior dorsal simple chaeta; (G) posterior compound chaetae; (H) posterior ventral simple chaeta; (I) acicula. Scale bars: A, 0.1 mm; B–I, 40 µm.

Fig. 4. SEM micrographs of Sphaerosyllis sandrae, sp. nov. (A) Anterior and mid-body part, dorsal view; (B) anterior part, dorsal view; (C) mid-body part with papillae, dorsal view; (D) anterior compound chaetae; (E) anterior simple chaeta; (F) mid-body simple and compound chaetae; (G) anterior chaetiger; (H) posterior chaetiger.
MATERIAL EXAMINED
Holotype (MNCN 16.01.11384), 12 paratypes (MNCN 16.01.11385), Playa Antonio, Guanahacabibes Peninsula, Cuba (S2 sample, 21°54′01.82″N 84°39′42.18″W, very coarse calcareous sand (1–2 mm), 8 m depth).
COMPARATIVE MATERIAL EXAMINED
Sphaerosyllis glandulata Perkins, Reference Perkins1981 (MNCN 16.01.6160), Sphaerosyllis piriferopsis Perkins, Reference Perkins1981 (MNCN 16.01.6376, 1 spec.) and Sphaerosyllis taylori Perkins, Reference Perkins1981 (MNCN 16.01.6378, 1 spec. and 16.01.6380, 1 spec.).
DESCRIPTION
Holotype 1.4 mm long, 0.22 wide, with 22 chaetigers. Larger paratype 1.7 mm long, 0.1 mm wide with 23 chaetigers. Prostomium wider than long, with two pairs of eyes in similar size. Antennae pyriform, central antenna inserted slightly anteriorly to anterior pair of eyes. Lateral antennae are inserted at anterior margin of prostomium. Palps fused at base, about as wide as prostomium. Peristomium slightly shorter than anterior segments, covering posterior part of prostomium. Pair of peristomial cirri directed forwards, similar to antennae in size and morphology. Dorsal cirri throughout with bulbous bases and short tips, similar to peristomial cirri; dorsal cirri absent on chaetiger 2. Parapodial glands containing hyaline material, always on dorsal side, beginning in chaetiger 4 provide each with a small papillae as long as all other papillae (Figure 3A). Compound chaetae heterogomph falcigers, posterior chaetae with tips of shafts shorter and thicker than anterior chaetae. Anterior and midbody chaetae with long and spinulated blades, posterior chaetae with smooth blades (Figures 3C, E, G & 4D, F–G). Dorsal simple chaetae present on all parapodia, serrated on edge near tips (Figures 3B, D, F & 4E–G). Ventral simple chaetae on posterior parapodia, smooth, unidentate (Figure 3H). Solitary aciculae, relatively slender, sharply curved anteriorly near tips at right angle (Figure 3I). Pharynx short occupying three segments. Mid-dorsal tooth large in anterior end of pharynx. Proventricle short, barrel shaped, extending through 2 segments, with about 16–18 muscular rows (Figure 3A). Few, scattered short papillae distributed on dorsum, more abundant laterally (Figure 4A–C). Pygidium short with pair of anal cirri similar in shape to dorsal cirri.
REMARKS
The most similar Caribbean species are Sphaerosyllis glandulata Perkins, Reference Perkins1981, Sphaerosyllis magnidentata Perkins, Reference Perkins1981, Sphaerosyllis piriferopsis Perkins, Reference Perkins1981 and Sphaerosyllis taylori Perkins, Reference Perkins1981. Sphaerosyllis glandulata has much more slender chaetae than the new species, and parapodial glands containing granular material. Sphaerosyllis magnidentata also has parapodial glands with granular material and the central antenna inserted at the level of lateral ones. Sphaerosyllis piriferopsis lacks parapodial glands, and S. taylori has parapodial glands containing needle-like rods. This latter species also has chaetae very different to S. sandrae sp. nov. (see Perkins, Reference Perkins1981; Russell, Reference Russell1991; Ruíz-Ramírez & Salazar-Vallejo, Reference Ruíz-Ramírez and Salazar-Vallejo2001).
The Iberian species Sphaerosyllis thomasi, San Martín, Reference San Martín1984, Sphaerosyllis hystrix Claparède, Reference Claparède1863 and Sphaerosyllis parabulbosa San Martín & López, Reference San Martín and López2002 are the most similar species to S. sandrae sp. nov. Sphaerosyllis thomasi has much shorter chaetae and anterior parapodia with two types of aciculae; S. hystrix has a slender pharynx, and S. parabulbosa has the peristomial and dorsal cirri and antennae significantly smaller and bidentate posterior compound chaetae. These three species have parapodial glands with fibrillar material each provided with large dorsal papillae (see San Martín, Reference San Martín1984, 2003; San Martín & López, Reference San Martín and López2002).
With regard to Pacific species, Sphaerosyllis georgeharrisoni San Martín, Reference San Martín2005 and Sphaerosyllis hirsuta Ehlers, 1897, have antennae of similar morphology and position, and also similar chaetae. However, S. georgeharrisoni possesses large parapodial glands of two types (anterior glands contain granular material and posterior ones contain hyaline material), which begin on the chaetiger 1, and posterior falcigers thinner than those of the new species. The parapodial glands of S. hirsuta begin on the chaetiger 4, but containing granular material and are significantly smaller than those of S. sandrae sp. nov. Sphaerosyllis hirsuta is also much larger and has very different peristomial and dorsal cirri, with granular internal glands opening outside through a duct, and many papillae distributed on the dorsal surface (see San Martín, Reference San Martín2005). Sphaerosyllis californiensis Hartman, 1966 is also a much larger species, with falcigers with blades much shorter and the acicula is not curved at right angle (see Hartman, Reference Hartman1969). Sphaerosyllis bifurcata (Hartmann-Schröder, Reference Hartmann-Schröder1979) has similar dorsal cirri and chaetae, except for ventral simple chaetae on posterior chaetigers which are sigmoid and distally hooked; the parapodial glands are smaller than those of our species, with granular material, and begin on chaetiger 3. Sphaerosyllis bifurcata has also numerous and long papillae covering dorsal midbody surface. Sphaerosyllis bardukaciculata San Martín, Reference San Martín2005 has dorsal cirri much shorter and anal cirri much larger than those of our species; the parapodial glands contain granular material and begin on chaetigers 4–5, and the falcigers have blades much shorter than those of S. sandrae sp. nov. Sphaerosyllis densopapillata Hartmann-Schröder, Reference Hartmann-Schröder1979, has very similar peristomial and dorsal cirri and similar compound chaetae, but its dorsal surface is provided with numerous, rounded papillae, present from peristomium to pygidium; the parapodial glands have also granular material. Sphaerosyllis capensis Day, 1953 has large parapodial glands, with fibrillar material, each one provided with a distinct large dorsal papillae; peristomial and dorsal cirri and chaetae are similar to those of S. sandrae sp. nov. Sphaerosyllis lateropapillata Hartmann-Schröder, 1986 has also similar peristomial and dorsal cirri and few papillae more abundant on lateral edges, but parapodial glands are only visible on midbody segments of some specimens and contain granular material (see Hartmann-Schröder, Reference Hartmann-Schröder1979; San Martín, Reference San Martín2005).
DISTRIBUTION
Only known from the type locality: Playa Antonio, Guanahacabibes Peninsula, Cuba.
ETYMOLOGY
The species is named in honour of the sister of the first author, Sandra Álvarez Campos.
ACKNOWLEDGEMENTS
We thank the staff of the invertebrate collection at MNCN for loaning comparative material of Sphaerosyllis glandulata, Sphaerosyllis piriferopsis and Sphaerosyllis taylori, and Drs María Teresa Aguado and João Nogueira for useful suggestions and comments on the manuscript.








