INTRODUCTION
Host specificity is one of the most important characteristics of a parasite species. Although host specificity can vary to some degree among different populations of the same parasite species across its geographical range, it still represents a true character of a parasite species, being less variable within parasite species than among parasite species (Krasnov et al. 2004a). This suggests that the level of host specificity is subjected to natural selection rather than merely reflecting the local restrictions caused by a variety of ecological, morphological, chemical and/or genetic factors (Fox and Morrow, 1981).
From an evolutionary perspective, selection for higher or lower levels of host specificity (or, in a broader sense, ecological specialization) is affected by a variety of both parasite (=forager)-related and host (=resource)-related factors (Fox and Morrow, 1981; Futuyama and Moreno, 1988; Fry, 1996; Desdevises et al. 2002). In particular, the models of Ward (1992) suggested that species tend to specialize on predictable resources, i.e. resources that are relatively stable in both space and time. This likely minimizes extinction rate for an optimal forager.
The main resource for a parasitic species is its host, which provides a parasite with food, habitat and mating grounds. Consequently, specialization in parasites is expected to be associated with the level of predictability of its host resources (Ward, 1992). Persistence of a host individual in time, in turn, is associated with its size. In general, larger host species live longer and, thus, represent a more predictable resource for a parasite (Peters, 1983). In addition, larger hosts offer more niches for parasites. As a result, larger host species are expected to harbour mainly parasite species with higher host specificity, whereas small-bodied hosts should be exploited mainly by generalist parasites. Indeed, it has been shown that larger fish species harbour more species of specialist monogenean ectoparasites compared with smaller fish species (Sasal et al. 1999; Simkova et al. 2001).
Here, we address this issue in fleas, which are common ectoparasites of higher vertebrates. The overwhelming majority of fleas parasitize mammals (more than 94% of species; Vatschenok, 1988), whereas their association with birds is much weaker. Fleas usually alternate between periods when they occur on the body of their hosts and periods when they occur in their hosts' burrows or nests. In most cases, the pre-imaginal development is entirely off-host. The degree of association between a particular flea species and a particular host species varies, with flea species ranging from highly host-specific to host-opportunistic (Marshall, 1981).
We searched for an association between the level of flea specialization and the predictability of the host resource, by relating the level of host specificity of fleas with the mean body mass of their hosts, using published data on host occurrences of fleas from 2 large, distinct geographical regions. Among mammals, a positive correlation between body mass and life-span has been repeatedly demonstrated (Peters, 1983; Brown, 1995). Consequently, we expected that the degree of host specificity of a flea species would be positively correlated with mean body mass of its hosts. We defined host specificity as the number, but also the identity, of host species that are exploited by a parasite species. In other words, rather than just taking the mere number of host species used by a flea as a measure of host specificity, we also applied a measure of host specificity that takes into account the taxonomic or phylogenetic affinities of the various host species (Poulin and Mouillot, 2003). This measure places the emphasis on the taxonomic distance between host species used by a flea rather than on their number, providing a different and complementary perspective on host specificity, one that truly focuses on the specialization of the flea for its host habitat.
MATERIALS AND METHODS
Data on the host range of fleas were obtained from published regional monographs on fleas from the entire spectrum of mammalian and avian hosts of South Africa (Segerman, 1995) and the northern part of North America (Canada, Alaska and Greenland; Holland, 1985; hereafter referred to as North America). Only mammalian hosts and their fleas were used in the analyses. These regions were selected because (a) both of them are not isolated but instead represent parts of larger contiguous biogeographical regions and (b) their mammalian faunas are mostly composed of different representatives of the same mammalian orders. We cross-checked the species lists with the catalogues of Lewis and Lewis (1990a) and Medvedev et al. (2005) to resolve cases of synonymy. Bats (Chiroptera) represent an exception to the general rule of a positive association between body size and life-span: bats live relatively long despite their small size (Jurgens and Protero, 1987; Wilkinson and South, 2002). In general, bat life-span is about 3·5 times longer than that of other mammals of comparable body sizes (Wilkinson and South, 2002). Consequently, bats and their specific fleas (family Ischnopsyllidae) were excluded from the analysis.
For each flea species, 2 measures of host specificity were used: (1) the number of mammalian species on which the flea species was found, and (2) the specificity index, STD, and its variance VarSTD (Poulin and Mouillot, 2003). The number of hosts examined often covaries with the number of parasite individuals and species found in a survey (Morand and Poulin, 1998). As a result, unequal between-host study effort may result in confounding variation in estimates of flea host specificity. However, the sources used did not provide data on the number of host individuals examined and the number of recorded fleas as is usually the case with regional monographs. Consequently, it was not possible to account for the potential bias related to unequal sampling effort. In addition, parasites characteristic for a prey species can often be transferred to a predator species during predator/prey interaction (Marshall, 1981). As a result, a false parasite–host association can be recorded. To avoid this, we excluded flea species characteristic of rodents and insectivores from the lists of fleas recorded on carnivores based on information on the typical hosts of a flea species from the respective sources. For example, a total of 5 flea species (Echidnophaga gallinacea, Ctenocephalides connatus, Ctenocephalides felis, Synosternus caffer and Xenopsylla erilli) were recorded on the banded mongoose Mungo mungo in South Africa (Segerman, 1995). However, we only considered the former 3 flea species as ‘true’ parasites of this host because the normal hosts of S. caffer and X. erilli are springhare (Pedetes capensis) and the Cape ground squirrel (Xerus inaurus), respectively (Segerman, 1995).
The index STD measures the average taxonomic distinctness of all host species used by a parasite species. When these host species are placed within a taxonomic hierarchy, the average taxonomic distinctness is simply the mean number of steps up the hierarchy that must be taken to reach a taxon common to 2 host species, computed across all possible pairs of host species (see Poulin and Mouillot, 2003 for details). For any given host species pair, the number of steps corresponds to half the path length connecting two species in the taxonomic tree, with equal step lengths of one being postulated between each level in the taxonomic hierarchy. The greater the taxonomic distinctness between host species, the higher the number of steps needed, and the higher the value of the index STD: thus it is actually inversely proportional to specificity. A high index value means that on average the hosts of a flea species are not closely related. Using the taxonomic classification of Wilson and Reeder (1993), all mammal species included here were fitted into a taxonomic structure with 5 hierarchical levels above species, i.e. genus, subfamily, family, order, and class (Mammalia). The maximum value that the index STD can take (when all host species belong to different orders) is thus 5, and its lowest value (when all host species are congeners) is 1. However, since the index cannot be computed for parasites exploiting a single host species, we assigned a STD value of 0 to these flea species, to reflect their strict host specificity. The variance in STD, VarSTD, provides information on any asymmetries in the taxonomic distribution of host species (Poulin and Mouillot, 2003); it can only be computed when a parasite exploits 3 or more host species (it always equals zero with 2 host species). To calculate STD and VarSTD, DM and RP have developed a computer programme using Borland C++ Builder 6.0 (available at http://www.otago.ac.nz/zoology/downloads/poulin/TaxoBiodiv1.2). All 3 measures (number of host species, STD and VarSTD) are, thus, inversely indicative of host specificity. Estimates of STD and VarSTD were affected by the number of exploited hosts (r2=0·1–0·43, F=6·4–72·7 for South Africa and r2=0·1–0·53, F=14·8–155·2 for North America; P<0·002 for all). Consequently, in subsequent analyses we substituted the original values of STD and VarSTD by residuals of the regression of log-transformed values of STD and VarSTD against the log-transformed number of exploited hosts.
Our approach was 2-fold. First, for each flea species, we calculated the mean body mass of all exploited host species. Data on mean mammalian body mass were obtained from Silva and Downing (1995). To test for the correlation between flea host specificity and host body mass, we regressed log-transformed measures of host specificity (after controlling for host number for STD and VarSTD) against log-transformed values of mean host body mass across flea species, separately for South Africa and North America. This approach determined whether specific fleas do indeed exploit larger host species than generalist fleas; but another way of addressing the same issue is to determine whether large-bodied host species harbour more host-specific fleas than small-bodied ones. Second, therefore, we calculated the mean host specificity (mean number of host species, mean STD and mean VarSTD) across all flea species recorded on a host, for each host species. Then, we regressed log-transformed measures of mean flea host specificity (after controlling for host number for STD and VarSTD) against log-transformed values of body mass, across all host species, separately for each geographical region. The analyses were carried out using both conventional regression and the method of phylogenetically independent contrasts (Felsenstein, 1985).
The method of independent contrasts was used to control for the possible effects of either flea or mammal phylogenies (Felsenstein, 1985; Harvey and Pagel, 1991). The phylogenetic tree for fleas was based on a detailed morphological taxonomy (see details in Krasnov et al. 2004a). The phylogenetic tree for mammals was based on various sources (see details in Morand and Poulin, 1998; Matthee and Robinson, 1999; Krasnov et al. 2004b,c; Flynn et al. 2005). The initial branch length for both trees was set to 1·0. To compute independent contrasts, we used the PDAP:PDTREE module (Garland et al. 1993; Midford et al. 2005) implemented in Mesquite Modular System for Evolutionary Analysis (Maddison and Maddison, 2005). Procedures for the analyses followed Garland et al. (1992, 1993) and Pagel (1992).
Because we examined the correlations of 3 measurements of host specificity with a single independent variable, we avoided an inflated Type I error by performing Bonferroni adjustments of the α-level. Significance is only recorded at the adjusted level.
RESULTS
There were 85 flea species exploiting 105 mammalian host species in South Africa, and 131 fleas exploiting 137 mammalian host species in North America. Six flea species only occurred in both regions (Xenopsylla cheopis, Pulex irritans, Ctenocephalides canis, Ctenocephalides felis, Leptopsylla segnis and Nosopsyllus fasciatus). The body mass of host species ranged from 0·006 kg (Mus minutoides) to 160 kg (Panthera leo) in South Africa and from 0·003 kg (Sorex hoyi) to 200 kg (Ursus arctos) in North America.
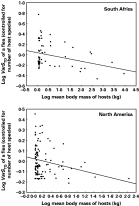
In both regions, flea host specificity in terms of either the number of exploited host species or their taxonomic distinctness (STD) did not correlate with mean body mass of the hosts (Table 1). On the contrary, flea host specificity measured as the taxonomic asymmetry of the host spectrum (VarSTD) decreased significantly with an increase in the mean body mass of exploited hosts (Table 1, Fig. 1). However, no correlation was found between any of the host specificity measures and mean body mass of hosts in either of the two regions when the data were controlled for the confounding effect of phylogeny (Table 2).

Fig. 1. Relationship between the mean body mass of host species exploited by a flea and the taxonomic asymmetry (VarSTD) of these hosts, across flea species from two different geographical regions.


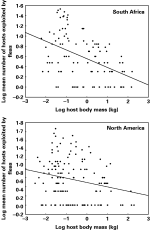
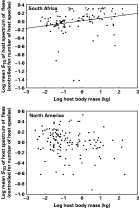
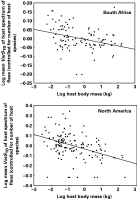
When we instead determined whether large-bodied host species harbour more host-specific fleas than small-bodied ones, i.e. when we considered the mean number of hosts exploited by the fleas of a host's assemblage, it was negatively correlated with this host's body mass (r2=0·20, F=26·7 for South Africa and r2=0·05, F=6·8 for North America; P<0·01 for both) (Fig. 2). The mean taxonomic diversity of the host spectrum of fleas exploiting a host species correlated positively with body mass of this host in South Africa (r2=0·06, F=6·2, P<0·01), but no relationship between these two parameters was found for the North American data (r2=0·03, F=3·4, P=0·07) (Fig. 3). In both regions, the body mass of a host was negatively correlated with the mean taxonomic asymmetry of the host spectrum of the fleas that this host harboured (r2=0·11, F=13·0 for South Africa and r2=0·17, F=28·2 for North America; P<0·001 for both; Fig. 4).

Fig. 2. Relationship between body mass of a host and mean number of hosts exploited by the fleas harboured by this host, across host species from two different geographical regions.

Fig. 3. Relationship between body mass of a host and the mean taxonomic diversity (STD) of the host spectrum exploited by the fleas harboured by this host, across host species from two different geographical regions.

Fig. 4. Relationship between body mass of a host and the mean taxonomic asymmetry (VarSTD) of the host spectrum exploited by the fleas harboured by this host, across host species from two different geographical regions.
Relationships between host body mass and measurements of host specificity of fleas harboured by a host were only partly supported by the method of independent contrasts. In South African hosts, contrasts in host body mass were not correlated with contrasts in either mean number or mean STD of hosts exploited by the fleas composing an assemblage (r=−0·11 and r=0·007, respectively, P>0·2 for both). However, contrasts in host body mass correlated negatively with contrasts in mean VarSTD of hosts exploited by a host's fleas (r=−0·24, P<0·01). In North America, contrasts in host body mass were negatively correlated with contrasts in the mean number of hosts used by a host's fleas (r=−0·21, P<0·01), but no relationship was found between host body mass and either mean STD or mean VarSTD of hosts exploited by a host's fleas (r=0·12 and r=−0·01, respectively, P>0·1 for both).
DISCUSSION
The results of this study demonstrate a weak but consistent association between the level of flea host specificity and host body mass. However, this association was not always supported by the method of independent contrasts and was somewhat differently expressed in the two geographical regions.
The association between flea host specificity and host body mass was revealed mainly when host specificity was considered in terms of the number of exploited host species and their taxonomic asymmetry. The latter parameter describes how much taxonomic heterogeneity there is among a group of host species (Warwick and Clarke, 2001; Poulin and Mouillot, 2003). A high variance usually means that one main branch in the taxonomic tree of host species contributes proportionally more species to the list than other branches. For example, Oropsylla labis and Corypsylla kohlsi exploit 4 hosts each. However, all hosts of O. labis belong to the same subfamily (Sciurinae) and 3 of them are congenerics (Spermophilus columbianus, Spermophilus franklinii and Spermophilus richardsonii). On the contrary, hosts of C. kohlsi belong to 2 different orders (Insectivora and Rodentia). They are represented by 3 congeneric insectivores (Sorex obscurus, Sorex pacificus and Sorex trowbridgii) and 1 rodent (Microtus townsendii). As a result, VarSTD values for these species differ sharply (0·25 versus 4). The relationships between host body mass and flea host specificity showed a similar pattern when considered from both the host and flea perspectives. In general, small-bodied hosts harboured mainly host-opportunistic fleas (with a high number and/or low taxonomic ‘evenness’ of exploited hosts), whereas host-specific fleas tend to use larger hosts. This finding supports the observations of Sasal et al. (1999), Simkova et al. (2001) and Desdevises et al. (2002) for fish-monogenean systems and suggests some role for the predictability of resources in the evolution of flea host specificity. Indeed, large hosts live longer than smaller hosts and, thus, represent more permanent sources of food and living space for fleas. As a result, specialization can be favoured in fleas exploiting larger and, thus, more predictable hosts. This supports the hypothesis about the link between specialization and predictability of the resource (Ward, 1992). However, host predictability as a resource for fleas can be manifested in other ways than host body size. Indeed, some small colonial hosts (ground squirrels, prairie dogs, whistling rats, meerkats) can represent highly predictable resources because their colonies may persist in a location for a long time, i.e. longer than the life-span of an individual host. In addition, the relatively high frequency of body-to-body contact in small colonial hosts increases the predictability of the resource in terms of transmission probability compared with many large hosts which are solitary. Nevertheless, the majority of small mammals are solitary rather than colonial. Consequently, the higher predictability of small colonial hosts as a resource for fleas could possibly weaken the relationship between host body size and flea host specificity found in this study, but it is unlikely to reverse the direction of this relationship.
Another measure of the resource predictability, host abundance, has been suggested to influence the evolution of host specificity in parasites (Norton and Carpenter, 1998). However, this appeared not to be the case for fleas and mammalian hosts because larger mammals are usually less abundant (i.e. lower population density) than smaller mammals (Peters, 1983). The explanation can be that the effect of relative host abundance on parasite specificity should depend on parasite dispersal abilities (Reed and Hafner, 1997; Desdevises et al. 2002). If these abilities are relatively high, then the relative effect of host abundance on specificity should likely be low. Indeed, fleas can transfer from host to host by free movement or during between-host contacts. Individual fleas can disperse rather long distances (Bates, 1962; Marshall, 1981; Kuznetzov et al. 1999). Direct contact between different host species has also been shown to ensure host-to-host flea transfer (Krasnov and Khokhlova, 2001). On the other hand, the probability of host-to-host transfer decreases with a decrease in host density. Therefore, the probability of colonization of a new host is likely lower for a flea that parasitizes larger hosts than for a flea that parasitizes smaller hosts. The relatively lower degree of host specificity in fleas exploiting large mammals in comparison with fleas exploiting small mammals can be a result of this difference in colonization probability.
Larger hosts may include a greater variety of niches (Kuris et al. 1980). Different fleas prefer different host body areas when they occur on relatively large hosts (Muller et al. 2001; Hsu et al. 2002). For example, Echidnophaga myrmecobii preferred to settle on the head and body of the rabbit Oryctolagus cuniculus, whereas Echidnophaga perilis occurred mainly on the fore- and hind-feet (Shepherd and Edmonds, 1979). Although different parts of a host body provide the same basic resource for fleas (i.e. host blood), the pattern of acquisition of this resource by fleas can differ among these body parts. Skin thickness, capillary depth and defensibility differ among body parts of a mammal, and these differences are likely more pronounced in larger than in smaller species. As a result, flea specialization can be facilitated in larger hosts (Kuris et al. 1980). The negative correlation between host body mass and mean number of hosts exploited by a host's fleas supports this explanation.
The negative but triangular distribution of points in the relationship between body mass and flea host specificity implies that smaller hosts can harbour both host-specific and host-opportunistic fleas, whereas larger hosts harbour mainly host-specific fleas. For example, the mean number of hosts exploited by 6 fleas recorded on Malacotrix typica (body mass about 100 g) is 25·2 and by 8 fleas recorded on Parotomys littledalei (body mass about 130 g) is 11·3, whereas that of 2 fleas recorded on Potamochoerus porcus (body mass about 54 kg) is 11·5. This, as well as the relative weakness of the association between host body size and flea host specificity, suggests that resource predictability alone cannot explain the patterns and pathways of the evolution of host specificity in fleas. Other factors must be involved as well. For example, a trade-off between the ability to use many host species and the average fitness achieved in these hosts is expected (Poulin, 1998). This is because close adaptation to one host species may be likely achieved at the expense of adaptations to other host species due to the presumably high cost of parasite adaptations against multiple host defence mechanisms. However, this appeared not to be the case for fleas, as host opportunistic flea species were shown to be also the ones that attain higher local abundances on their hosts (Krasnov et al. 2004c). On the contrary, fleas using either many host species or taxonomically diverse host species achieve not only greater average abundance, but also a broader geographical range than the more host-specific fleas (Krasnov et al. 2005). The reason for this can be that the advantages of having many host species and therefore a broader geographical range prevails over the physiological costs of adaptations against the hosts' antiparasitic defences. Another factor affecting the evolution of host specificity in fleas is related to the variation in the off-host environment (Krasnov et al. 2004a). In general, environmental conditions, measured as departures from average conditions across the geographical range of a flea, influence all measures of flea host specificity even if the host specificity of flea species has been shown to be a true species character more-or-less repeatable across populations of a flea species (Krasnov et al. 2004a). The effect of environmental variables on the degree of local host specificity of a flea is likely related to the microclimatic preferences of the pre-imaginal stages. For example, the flea Xenopsylla ramesis parasitizes several host species (mainly gerbils) throughout the Middle East (Lewis and Lewis, 1990b). However, in some areas Meriones crassus is dropped out of the host spectrum of this flea (Krasnov et al. 1997). The reason for this is the unsuitability of microclimatic and substrate conditions in M. crassus burrows for the successful survival of eggs, larvae and newly-emerged imago of X. ramesis (Krasnov et al. 2001).
Furthermore, the local availability of taxonomically-related hosts can affect, to some extent, the level of host specificity of a given flea species. Among parasites of fish, for instance, parasites exploiting hosts belonging to species-rich taxa have been found to be less host specific than parasites exploiting hosts belonging to species-poor taxa (Poulin, 1992; Barker et al. 1994; Sasal et al. 1998).
Finally, the different pattern in the specificity-host body size relationship between South Africa and North America calls for some explanation. Indeed, in most cases when the relationship was found, it was stronger for South African than for North American flea-mammal associations. This difference may be associated with the differences between these two regions during the Cenozoic when the main evolutionary development of flea-mammal associations occurred (Medvedev, 2005). Glaciations with repeated advances and retreat of ice-sheets were characteristic for North America and not for South Africa, especially during the Quarternary. Glaciation-interglaciation cycles may have lead to the repetitive break-ups and restorations of associations between flea and host species in North America, whereas these associations in South Africa were likely more stable on a geological time-scale. The ‘historical instability’ of the relationships between flea and host species in North America could have resulted in a relatively weak association between flea and host traits.
In conclusion, the results of this study demonstrated that predictability of resources measured as host body size matters to the evolution of parasite host specificity. A stable and predictable host resource will select for greater specialization in parasites. However, the predictability of host resources alone cannot explain all patterns of host specificity among flea species.
We thank two anonymous referees for their helpful comments on an earlier version of the manuscript. This study was partly supported by the Israel Science Foundation (Grant no. 249/04 to B.R.K.). This is publication no. 199 of the Ramon Science Center and no. 509 of the Mitrani Department of Desert Ecology.