Introduction
Patients with major depressive disorder commonly suffer from anxiety, nervousness, and various somatic symptoms, such as autonomic and gastrointestinal symptoms, related to these conditions.Reference Fava, Rush and Alpert 1 The higher level of anxiety accompanying major depressive disorder has been associated with greater severity of depression, greater illness chronicity, poorer cognitive function, lower quality of life and well-being, and suicidality. 1–3 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial reported that patients with anxious depression were significantly more likely to be older, unemployed, less educated, more severely depressed, and have suicidal ideations.Reference Fava, Alpert and Carmin 3
Increasing evidence has suggested the adverse effect of anxiety comorbidity and greater levels of anxiety in the treatment response to antidepressant medications.Reference Fava, Rush and Alpert 1 , Reference Braund, Palmer, Williams and Harris 4–Reference Wiethoff, Bauer and Baghai6 Fava et alReference Fava, Rush and Alpert 1 defined anxious depression as a score ≥ 7 on the anxiety/somatization factor of the 17-item Hamilton Depression Rating Scale (HAMD). They demonstrated that patients with anxious depression were less likely to respond to antidepressant drugs than those with nonanxious depression. The international study to predict optimized treatment in depression demonstrated that situational anxiety and somatic anxiety predicted poorer treatment outcomes from the administration of escitalopram, sertraline, or venlafaxine.Reference Braund, Palmer, Williams and Harris 4 , Reference Braund, Palmer, Williams and Harris 5
In this decade, low-dose ketamine infusion has been proved to exhibit a rapid antidepressant effect for treatment-resistant depression (TRD).Reference Murrough, Iosifescu and Chang 7 , Reference Su, Chen and Li 8 However, only a few studies have assessed the potential effect of baseline anxiety and anxious depression in the treatment response to low-dose ketamine infusion among patients with TRD.Reference Salloum, Fava and Freeman9–Reference Ionescu, Luckenbaugh, Niciu, Richards and Zarate11 In a multisite, double-blind, placebo-controlled trial of 99 patients with TRD who were randomized to either a low-dose ketamine or midazolam placebo infusion, Salloum et alReference Salloum, Fava and Freeman 9 demonstrated that treatment response to ketamine infusion did not differ between patients with anxious and nonanxious TRD (ANX-TRD and NANX-TRD, respectively). In an open-label study of 26 patients with TRD receiving a single infusion of ketamine, Ionescu et alReference Ionescu, Luckenbaugh and Niciu 10 discovered that patients with ANX-TRD responded better to ketamine than those with NANX-TRD. However, small sample size, open-label study design, and enrollment of only Caucasian patients were major limitations of the aforementioned studies. The potential role of anxious depression in the treatment response to ketamine infusion has remained unknown for Asian patients with TRD.
In this study, we reanalyzed data from our adjunctive ketamine study on Taiwanese patients with TRD.Reference Su, Chen and Li 8 We focused on the antidepressant effect of low-dose ketamine infusion for patients with ANX-TRD and NANX-TRD. Because anxiety has been demonstrated to be a significant predictor of poor treatment response in TRD when traditional antidepressants are used, we hypothesized that patients with ANX-TRD were less likely to respond to low-dose ketamine infusion than those with NANX-TRD.
Methods
Inclusion criteria and study procedure
The detailed study design, patient enrollment and clinical results of our study have been published.Reference Su, Chen and Li 8 , Reference Li, Chen and Lin 12 Seventy-one patients aged between 21 and 64 years with TRD were randomized to three groups. Each group respectively received a single 40-minute intravenous saline infusion mixed combined with either 0 (a normal saline infusion), 0.2, and or 0.5 mg/kg of ketamine. Patients with TRD who had major medical or neurological diseases or a history of alcohol or substance use disorders were excluded in current study. Patients were assessed using the HAMD prior to initiation of infusions and at 40, 80, 120, and 240 minutes postinfusion and sequentially on days 2, 3, 4, 5, 6, 7, and 14 after ketamine or placebo infusion.Reference Hamilton 13 , Reference Montgomery, Rani, McAuley, Roy and Montgomery 14 This study was performed in accordance with the Declaration of Helsinki and was approved by the Taipei Veterans General Hospital Institutional Review Board. Informed consent was provided by all the participants. Clinical trials registration: UMIN Clinical Trials Registry (UMIN-CTR): Registration number: UMIN000016985.
Anxious depression definition
Anxious TRD (ANX-TRD) was defined as major depressive disorder with a total score of 7 or more on the HAMD Anxiety-Somatization factor (HAMD-AS). Six items from the original 17-item HAMD makes up HAMD-AS: psychic anxiety, somatic anxiety, somatic symptoms-gastrointestinal, somatic symptoms-general, hypochondriasis, and insight.Reference Cleary and Guy 15 HAMD-AS subscale has been used in STAR*D and was proven reliable in assessing anxious depression.Reference Fava, Rush and Alpert 1
Statistical analysis
Continuous and nominal variables were analyzed through one-way analysis of variance and Fisher’s chi-square tests, respectively, to assess the differences in the demographic and clinical data between the two groups (ANX-TRD vs NANX-TRD). Three generalized estimating equation (GEE) models were performed to investigate the roles of treatment group and depression type in treatment outcome. First, GEE model with the autoregressive method for correlations of repeated measures for the same individual over time was used to examine the effects of ketamine on reduction of depressive symptoms during the treatment period with the group (0.5 or 0.2 mg/kg ketamine or placebo) and depression type (ANX-TRD vs NANX-TRD) as a between-patient factor and time (baseline, 40 and 240 minutes, days 2-7, and day 14) as a within-patient factor as well as all possible interactions. Second, stratified by depression type, GEE models with the autoregressive method for correlations of repeated measures for the same individual over time was used to examine the effects of ketamine on depression symptoms during the treatment period with the group as a between-patient factor, time as a within-patient factor, and baseline depression score as a between-patient predictor as well as all possible interactions. Furthermore, stratified by treatment group, GEE models with the autoregressive method for correlations of repeated measures for the same individual over time was used to examine the effects of ketamine on depression symptoms during the treatment period with the depression type as a between-patient factor, time as a within-patient factor, and baseline depression score as a between-patient predictor as well as all possible interactions. Two-tailed P < .05 was considered statistically significant. All data processing and statistical analyses were performed using SPSS, version 17 (SPSS Inc.).
Results
In all, 42 patients with ANX-TRD and 29 with NANX-TRD were enrolled in our study and were randomized to three treatment groups (Table 1). Patients with ANX-TRD had the older age (P < .001), lower education (P = .016), and higher baseline scores of HAMD (P < .001) and HAMD-AS (P < .001) compared with those with NANX-TRD (Table 1).
Table 1. Demographic and Clinical Data Among Patients with TRD.

Abbreviations: ANX, anxious; HAMD, Hamilton Depression Scale; HAMD-AS, Hamilton Depression Rating Scale Anxiety-Somatization factor; NANX, nonanxious; SD, standard deviation; TRD, treatment-resistant depression.
Table 2 showed that only treatment group (P = .003), but not depression type (P = .393), predicted the reduction of depression symptoms during the follow-up period (Table 2). Stratified by depression type, GEE model found that among patients with NANX-TRD, both 0.5 and 0.2 mg/kg ketamine infusion significantly reduced the depressive symptoms during the follow-up period compared with placebo infusion (group effect: P < .001) (Figure 1). But, the depression reduction did not differ between three groups among patients with ANX-TRD (group effect: P = .436) (Figure 1). Furthermore, stratified by treatment group, patients with NANX-TRD were more likely to have the greater reduction in depressive symptoms after a single 0.5 mg/kg ketamine infusion than those with ANX-TRD (group effect: P < .001) (Figure 2). However, the depression reduction did not differ between patients with ANX-TRD and with NANX-TRD after 0.2 mg/kg ketamine (group effect: P = .688) or placebo (group effect: P = .098) infusion (Figure 2). Supplementary Information, Table S1 showed the role of depression type in the treatment response, which was defined by response (⩾50% reduction of mood ratings) at any two daily HAMD measures during the period of 24 to 96 hours (days 2–5),Reference Su, Chen and Li 8 to ketamine infusion (Table S1).
Table 2. Effect of Anxious Depression Status and Treatment Group on Trajectory of Depressive Symptoms.

Abbreviations: ANX, anxious; df: degree of freedom; HAMD, Hamilton Depression Scale; NANX: nonanxious; TRD, treatment-resistant depression.
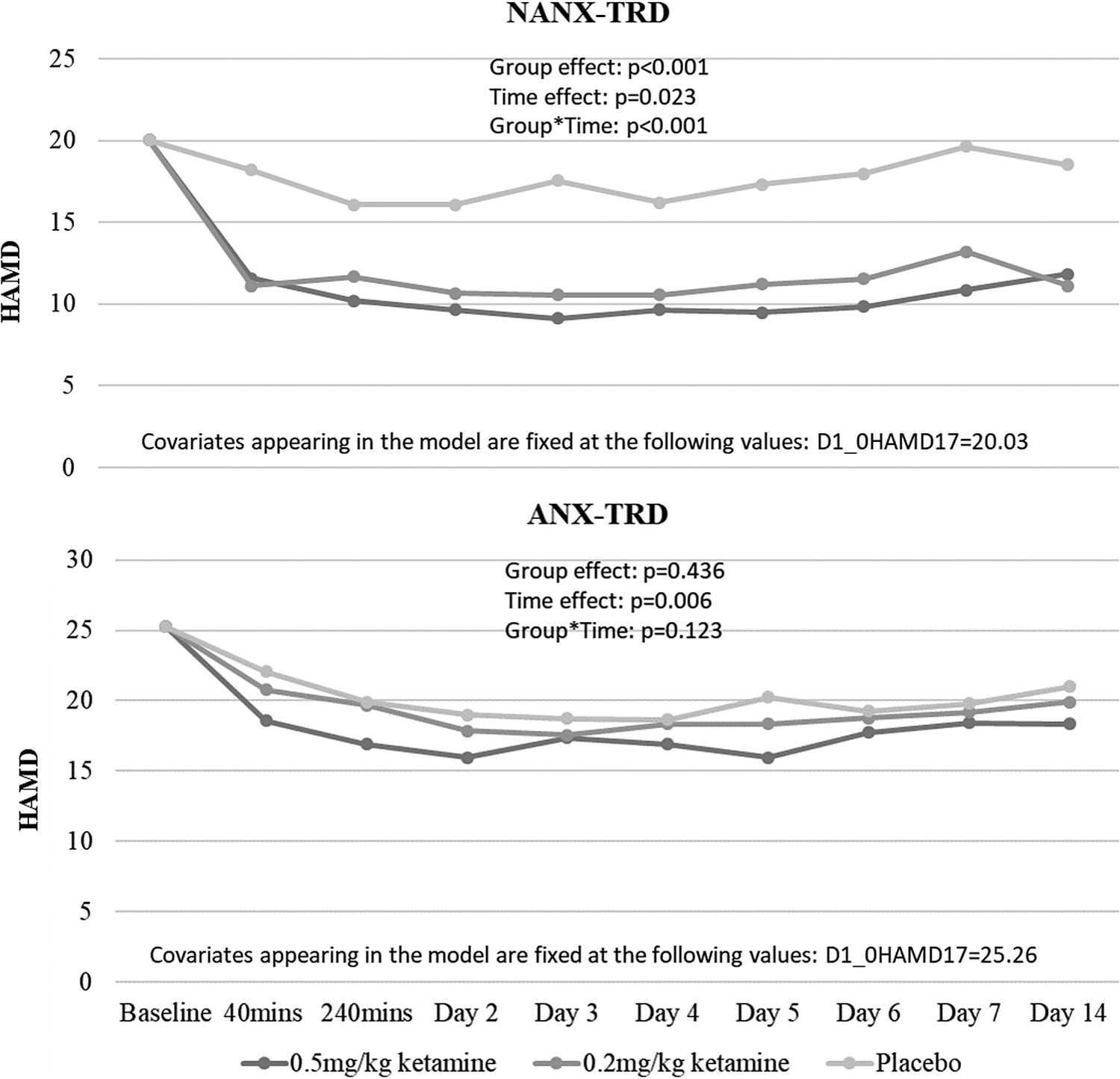
Figure 1. Clinical trajectory of patients with TRD after ketamine/placebo infusion, stratified by depression type. ANX, anxious; HAMD, Hamilton Depression Scale; NANX, nonanxious; TRD, treatment-resistant depression.
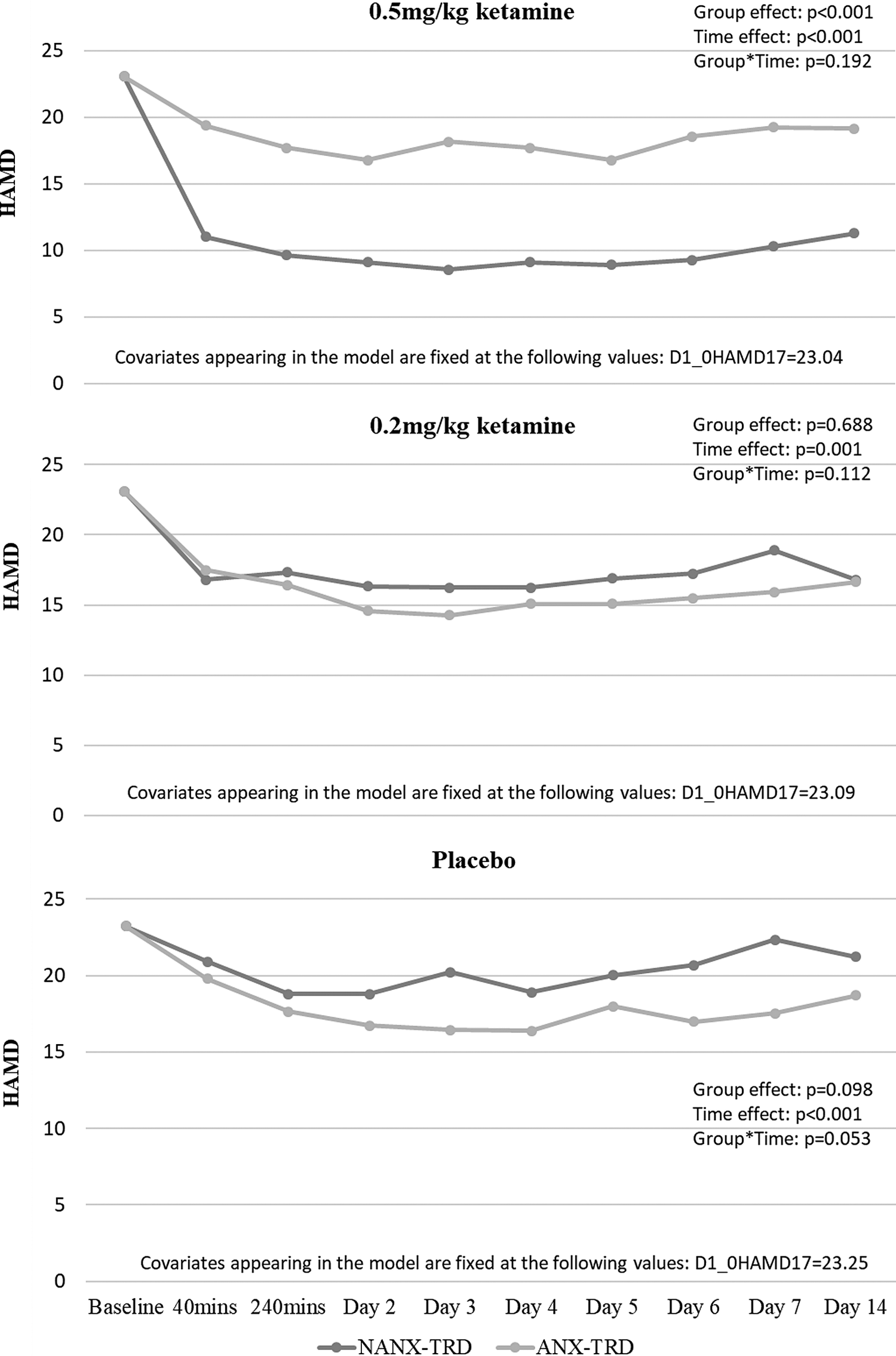
Figure 2. Clinical trajectory of patients with TRD (ANX vs NANX) after ketamine/placebo infusion, stratified by treatment group. ANX, anxious; HAMD, Hamilton Depression Scale; NANX, nonanxious; TRD, treatment-resistant depression.
Discussion
Our findings support the hypothesis that patients with ANX-TRD are less likely to respond to a single low-dose ketamine infusion than those with NANX-TRD. Among patients with NANX-TRD, low-dose ketamine infusion was significantly superior to placebo in the reduction of depressive symptoms. However, among patients with ANX-TRD, ketamine infusion was not superior to placebo; nonetheless, approximately 30% of patients still responded to ketamine infusion, a higher proportion than the 13% who responded to placebo. This finding is supported by a vast literature describing the difficulty of treating this population.
As mentioned, anxious depression is related to a higher level of depression severity, longer duration of illness, greater functional impairment, and poorer response to antidepressant medications.Reference Fava, Rush and Alpert1–Reference Fava, Alpert and Carmin3 STAR*D demonstrated that baseline clinical characteristics can be used to identify subgroups that exhibit responses ranging from a low likelihood of response (18%) to citalopram (comorbid generalized anxiety disorder, <16 years of education) to a high likelihood (68%) of response (no comorbid posttraumatic stress disorder; PTSD).Reference Jakubovski and Bloch 16 Fava et alReference Fava, Rush and Alpert 1 discovered that patients with anxious depression fared significantly worse in both switching (to bupropion, sertraline, or venlafaxine) and augmentation (with bupropion or buspirone) options. In addition to pharmacological treatment, anxious depression may also be associated with a poorer response to repetitive transcranial magnetic stimulation (rTMS).Reference Clarke, Clarke, Gill, Paterson, Hahn and Galletly 17 Clarke et al reported that only approximately 40% of patients with ANX-TRD responded to rTMS, which was much lower than the average response rate (up to 60%) of rTMS treatment.Reference Clarke, Clarke, Gill, Paterson, Hahn and Galletly 17 , Reference Su, Huang and Wei 18 It is noteworthy that previous studies have demonstrated that low-dose ketamine may be equally effective for Caucasian patients with ANX-TRD and NANX-TRD. This result is discordant with our findings that Taiwanese patients with ANX-TRD are less likely to respond to ketamine infusion than those with NANX-TRD. Furthermore, the exact mechanism that causes the differential response to ketamine vs other conventional antidepressants in anxious depression has remained unclear.Reference Salloum, Fava and Freeman 9
The therapeutic efficacy of low-dose ketamine infusion in anxiety disorders and anxious distress accompanying depression has remained unclear.Reference Glue, Medlicott and Harland 19 , Reference Taylor, Landeros-Weisenberger and Coughlin 20 Glue et alReference Glue, Medlicott and Harland 19 discovered preliminary positive results of ketamine infusion in refractory generalized anxiety disorder and/or social anxiety disorder with a dose–response pattern for anxiolytic effects. Feder et alReference Feder, Parides and Murrough 21 reported that compared with midazolam, ketamine infusion—when assessed 24 hours after infusion—was associated with significant and rapid reduction in PTSD comorbid depressive symptoms. However, Taylor et alReference Taylor, Landeros-Weisenberger and Coughlin 20 compared the effects of ketamine and placebo infusions among patients with social anxiety disorder and found that self-reported anxiety on a visual analog scale did not differ between the ketamine and placebo groups. Whether anxiety distress and severe anxiety symptoms may reduce the antidepressant effect of ketamine warrants further investigation. In addition, our previous study observed lower serum levels of ketamine and norketamine among Taiwanese patients with TRD. This may compromise the antidepressant efficacy among patients having higher levels of depression severity (ie, ANX-TRD).Reference Su, Chen and Li 8 Taking the findings from our and other previous studies together, we hypothesize that a higher dose, such as 1.0 mg/kg, of ketamine infusion may be necessary for Taiwanese patients with ANX-TRD, and the standard dose (0.5 mg/kg) or a lower dose (0.2 mg/kg) may be beneficial for those with NANX-TRD.
Our study has several limitations: first, it was an add-on ketamine study because the medications used by patients with TRD were not discontinued during ketamine treatment. Thus, the observed antidepressant effects of ketamine could have resulted from a combination of ketamine and a patient’s simultaneously consumed medication. However, patient medications were not changed during the ketamine treatment. Therefore, our findings can be justifiably explained as stemming from the add-on effects of low-dose ketamine infusion; an add-on study design is ethically appropriate in patients with severe depression and may provide more naturalistic data. Second, the sample size was still small; however, our study is—as far as we know—the largest randomized, placebo-controlled trial to date insofar as it involved 42 patients with ANX-TRD and 29 with NANX-TRD. In addition, the identification of lower serum levels of ketamine and norketamine in Taiwanese patients with TRD is another important clinical issue. Further study with a larger sample size and a higher dose of ketamine (0.75 or 1.0 mg/kg) may be required to clarify the potential antidepressant effect of ketamine infusion in patients with ANX-TRD. Third, patients with ANX-TRD were much older than those with NANX-TRD. In the GEE model with adjustment of age, we found that age was not related to the trajectory change of depression symptoms (P = .872). However, further studies with the age-matched sample of ANX-TRD and NANX-TRD may be required to validate our findings. Fourth, previous studies suggested that concomitant benzodiazepine use may attenuate the antidepressant response of low-dose ketamine infusion.Reference Frye, Blier and Tye 22 In our study, up to 80% of patients with TRD were concomitantly treated with benzodiazepines. But, the benzodiazepine use did not differ between patients with ANX-TRD and those with NANX-TRD. Whether benzodiazepines may attenuate the antidepressant response of ketamine in Asian patients with TRD would need further investigation. Fifth, normal saline was used as the placebo in current study. Active placebo, such as midazolam, would be considered in the future research. Finally, we only assessed the role of ANX- vs NANX-TRD in a single infusion of low-dose ketamine. Further studies would be necessary to clarify the role of anxious depression in the multiple infusions of low-dose ketamine.
In conclusion, low-dose ketamine infusion was effective for Taiwanese patients with NANX-TRD but was not so effective for those with ANX-TRD. Higher level of anxiety severity accompanying depression was related to greater depression severity. This may confound and reduce the antidepressant effect of ketamine infusion. The exact mechanism behind the differential response to ketamine in ANX-TRD and NANX-TRD warrants further investigation.
Acknowledgments
The study was sponsored by grants from Ministry of Science and Technology, Taiwan (101-2314-B-010-060, 102-2314-B-010-060, 107-2314-B-075-063-MY3), Taipei Veterans General Hospital (V106B-020, V107B-010, V107C-181), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22), and the Kun-Po Soo Medical Foundation. None of the aforementioned funding organizations had any role in the study design, data collection, analysis, interpretation of result, writing of the report, and the ultimate decision to submit the paper for publication. And thank all research assistants, physicians, pharmacist, and nursing staffs at D020 Unit of Taipei Veterans General Hospital for their assistance during the study process, without whom this work could have been possible. We thank Mr I-Fan Hu for his support and friendship.
Funding
The study was sponsored by grants from Ministry of Science and Technology, Taiwan (101-2314-B-010-060, 102-2314-B-010-060, 107-2314-B-075-063-MY3), Taipei Veterans General Hospital (V106B-020, V107B-010, V107C-181), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22), and the Kun-Po Soo Medical Foundation. Funding source had no role in any process of study.
Disclosures
The authors do not have any conflicts of interest to disclose.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1092852920001194.






