Introduction
Global warming, nutritional variability and increase of metabolic disorders are factors affecting both human and animal reproduction (Sirotkin, Reference Sirotkin2010), environmental heat stress is one of the major factors affecting animals, particularly lactating cows (Dunlap & Vincent, Reference Dunlap and Vincent1971; Badinga et al., Reference Badinga, Collier, Thatcher and Wilcox1985; Pavani et al., Reference Pavani, Carvalhais, Faheem, Chaveiro, Reis and Moreira da Silva2015b). Summer heat factors, such as high temperature humidity indexes (THI), are major problems for the dairy industry and contribute to economic losses among about 60% of the world's cattle population (Edwards et al., Reference Edwards, Bogart, Rispoli, Saxton and Schrick2009). High THI may not only decrease milk production but also affects the reproductive performance of lactating cows. It has been postulated that an increase in THI (≥72) affects the body temperature of dairy cows (Armstrong, Reference Armstrong1994), which leads to hypothermia and impairs the cellular function of various tissues and parts of the reproductive system (Wolfenson et al., Reference Wolfenson, Roth and Meidan2000). Heat-induced hypothermia studies, both in vivo and in vitro, have shown that rectal temperature may reach or exceed 41°C (Ealy et al., Reference Ealy, Drost and Hansen1993), which impairs hormonal secretion, oocyte fertilization and embryo development (Wolfenson et al., Reference Wolfenson, Roth and Meidan2000; Rivera & Hansen, Reference Rivera and Hansen2001; Sartori et al., Reference Sartori, Sartor-Bergfelt, Mertens, Guenther, Parrish and Wiltbank2002), resulting in a decrease in pregnancy rate of approximately 25% for every 1°C increase in rectal temperature (Pavani et al., Reference Pavani, Carvalhais, Faheem, Chaveiro, Reis and Moreira da Silva2015b).
Several experiments have demonstrated the direct effect of heat stress on fresh and maturated bovine oocytes. Research conducted by Payton et al. (Reference Payton, Romar, Coy, Saxton, Lawrence and Edwards2004) reported that heat shock at 41°C for 6 h or 12 h reduced embryo development to the 8-cell through to the 16-cell stages and blastocyst stage. Research conducted by Lima (Reference Lima2012) showed that keeping cultures of bovine embryos oocytes at 41°C for 14 h did not have an effect on cleavage rate, but reduced embryo development. The intensity of stress plays a prominent role both in terms of temperature and exposure time. Moderate heat shock (40–41°C) for 12 h during IVM reduced the proportion of cleaved embryos at day 3 and blastocysts at day 8 (Roth & Hansen, Reference Roth and Hansen2004; Paula-Lopes et al., Reference Paula-Lopes, Milazzotto, Assumpcao and Visintin2008). In research conducted by Ju et al. (Reference Ju, Parks and Yang1999), heat shock at 40.5°C and 41.5°C for 30–40 min during IVM showed no effect on embryo development, while heat shock at 43°C for 45–60 min reduced blastocysts and increased blastocyst rates. So far, no experiments have been performed on the effect of kinetic heat stress on bovine oocytes, apart from these studies on the effects of heat shock on oocytes and embryos and a limited number of seasonal studies on the effects of maternal heat stress (Roth et al., Reference Roth, Arav, Bor, Zeron, Braw-Tal and Wolfenson2001, Reference Roth, Inbar and Arav2008; Gendelman et al., Reference Gendelman, Aroyo, Yavin and Roth2010; Gendelman & Roth, Reference Gendelman and Roth2012) as maternal hypothermia requires two to three estrus cycles to normalize competent oocytes. Research conducted by Payton et al. (Reference Payton, Rispoli, Saxton and Edwards2011) shows that heat stress may induce alterations in the transcriptional levels of genes involved in cell growth, cell cycle and programmed cell death. Also, Gendelman & Roth (Reference Gendelman and Roth2012) have shown that specific developmental genes have less mRNA expression patterns in the summer than in the winter. It has been proven that the Cx43 gene is involved in embryonic development and maternal zygotic transition (Houghton, Reference Houghton2005), while the CDH1 gene controls the embryonic compaction process (Vestweber & Kemler, Reference Vestweber and Kemler1984; Riethmacher et al., Reference Riethmacher, Brinkmann and Birchmeier1995). Furthermore, the DNMT1 gene is known to affect mammalian preimplantation development, which represents a critical stage for the establishment of the epigenome (Golding & Westhusin, Reference Golding and Westhusin2003). Studying DNMT1 in oocytes and embryos may provide a better understanding of the epigenetic rearrangements occurring in early stage embryos in which first cell divisions impact chromatin configuration during cell differentiation (Giraldo et al., Reference Giraldo, DeCourcy, Ball, Hylan and Ayares2013). Gene expression changes are an integral part of cellular response to heat shock. From the literature review conducted by Sonna et al. (Reference Sonna, Fujita, Gaffin and Craig2002), genes encoding heat shock proteins (HSPs) affect a substantial number of genes that are not directly associated with HSPs. So far, the HSPA14 gene is not well understood, so analyzing the HSPA14 gene may provide more functional details about HSPs. The aim of the current study is to check the hourly impact of kinetic heat stress on the maturation of bovine oocytes by varying temperature conditions in order to comprehend the extent of the effect of heat shock on bovine oocyte maturation. Additionally, gene expression changes in targeted genes (Cx43, CDH1, DNMT1 and HSPA14) in different developmental stages (2-cell, 4-cell, morula and blastocyst) of embryos developed from oocytes under prolonged heat shock as well as oocytes collected during hot and cold seasons were studied.
Materials and methods
Experimental design
In the first part of the experiment, the kinetic effect of heat shock in nuclear maturation was studied. For such purposes, oocytes (n = 1124) were collected from winter months (December, January, February and March) and divided in three groups: a control group (n = 125), which was kept at 38.5°C for 24 h of in vitro oocyte maturation (IVM), the first heat shock group, which was kept at temperature HS1 (n = 500) and the second heat shock group, which was kept at temperature HS2 (n = 499). In both of the experimental heat shock groups, oocytes were divided into four different subgroups and exposed to different durations of heat exposure (6, 12, 18 or 24 h). In all of the time duration subgroups, oocytes were returned to the control temperature after heat exposure. From each heat stress group, all hourly samples were subjected to meiotic assessment and each hourly sample experiment was repeated four times, for HS1 (6 h (n = 127), 12 h (n = 122), 18 h (n = 124), 24 h (n = 127)), HS2 (6 h (n = 125), 12 h (n = 126), 18 h (n = 120), 24 h (n = 128)). Apart from this experiment, researchers compared the nuclear maturation rate at 24 h for HS1 and HS2 during the summer and winter months.
In the second part of the experiment, oocytes (n = 706) were aspirated for 1 year and then divided into two groups (1 and 2), based on when they were collected. The cold months (December, January, February and March) had temperatures varying from 6–12°C and the warm months (June, July, August and September) had temperatures ranging from 23–25°C.
In group 1, oocytes (n = 249) were maturated for 24 h and then evaluated for meiotic maturation, while in group 2, oocytes (n = 457) were maturated for 24 h and then fertilized in vitro. Cleavage rate and early embryonic development (2-cell and 4-cell stages) were determined microscopically (×100 magnification) 3 days after fertilization, while later embryonic development from morula and blastocyst stages were evaluated 8 days after fertilization. On day 9, embryos were separated based on their developmental stage (2-cell, 4-cell, morula or blastocyst), washed three times with diethylpyrocarbonate (DEPC) water and stored at −80°C for further analysis.
In the third part of the study, oocytes from the winter months (n = 891), were aspirated and maturated for 24 h at three different temperatures: group 1 was kept at the control temperature (38.5°C; n = 285), group 2 was exposed to HS1 (39.5°C; n = 324) and group 3 was exposed to HS2 (40.5°C; n = 282). After maturation, oocytes were selected based on meiotic maturation and 160 oocytes from group 1, 167 oocytes from group 2 and 160 oocytes from group 3 were used for IVF as described by Faheem et al. (Reference Faheem, Carvalhais, Chaveiro and Moreira da Silva2011). Results were evaluated 9 days after fertilization as described in the first part of the experiment design, and the embryos were washed three times with DEPC water for gene expression analysis.
Collection of oocytes, maturation, nuclear staining and fertilization
The chemicals and reagents used in the experiment were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ovaries were harvested once a week based on the seasons for 1 year from a local abattoir, trimmed of adhering tissue and transported to the laboratory in Dulbecco's phosphate-buffered saline (DPBS) at a temperature ranging from 34–37°C within 2 h post-slaughtering. All ovaries were rinsed once with 70% alcohol and followed by a wash with fresh DPBS upon arrival at laboratory. IVM, nuclear staining and IVF were performed as previously described by Pavani et al. (Reference Pavani, Carvalhais, Faheem, Chaveiro, Reis and Moreira da Silva2015b). Briefly, cumulus–oocytes complexes (COCs) were collected by aspiration from antral follicles (2–8 mm in diameter) with an 18-gauge needle. Good quality COCs based on their morphological appearance (covered by at least four layers of compacted cumulus cells and evenly granulated ooplasm), were washed twice in Tissue Culture Medium 199 (TCM-199), supplemented with 2% fetal bovine serum (FBS), 0.3 mg/ml glutamine and 50 μg/ml gentamycin and oocytes collected from the winter months of the first experiment were subjected to nuclear maturation analysis by splitting the matured oocytes at HS1 and HS2, based on exposure times (6, 12, 18 or 24 h). Then oocytes were denuded by vortexing and fixed in 3:1 methanol:glacial acetic acid solution for 24 h at room temperature by air sealing up a Petri dish with parafilm. After fixing, each denuded oocytes was dyed with 1% orcein and meiosis stages were recorded. For the second experiment, oocytes collected in cold and warm seasons were cultured for 24 h and they were divided into two groups based on cumulus expansion. One group was subjected to IVF [fertilization techniques were performed according to Faheem et al. (Reference Faheem, Carvalhais, Chaveiro and Moreira da Silva2011)]; the other group was subjected to nuclear staining, as stated above. Apart from this, oocytes collected in cold seasons were also subjected to heat stress, maturated at HS1 and HS2 for 24 h and subjected to dyeing and IVF as mentioned above.
Total RNA extraction
After embryonic development, all embryos were collected and divided separately according to their developmental stages (2-cell, 4-cell, morula and blastocyst), washed three times extensively to avoid the presence of any co-culture cells with DEPC-treated water and stored as pools of 15 embryos at −80°C. Bovine embryos were divided equally into three groups: the control, HS1 and HS2. Total RNA was extracted from all the embryos developed during the study, after being stabilized in Trizol, as per manufacturer instructions (Life Technologies, Inc., Carlsbad, CA, USA). Using the modifications to extract from bovine oocytes and embryos described by Pavani et al. (Reference Pavani, Baron, Faheem, Chaveiro and Moreira da Silva2015a), samples were dissolved in 30 µl of RNase-free water and quantified using the average of triplicate spectrophotometric readings at 260 nm in a CARY 60 UV-Vis spectrophotometer (Agilent Technologies). Purity of total RNA was confirmed by a reading of 260/280 nm.
Single-stranded cDNA synthesis
An amount of 3 µg of total RNA was reverse transcribed using an oligo dT12–18 (1.0 µl or 500 ng) primer. The reaction mix contained 1× reaction buffer [250 mM Tris–HCl (pH 8.3), 250 mM KCl, 20 mM MgCl2, 50 mM DTT], 20 U of RiboLock™ RNase Inhibitor, 20 mM of dNTP mix and 200 U of RevertAid™ H Minus M-MuLV reverse transcriptase (Fermentas Life Science), (20 µl final volume). The cDNAs were synthesized at 42°C for 60 min following the inactivation of transcriptase by heating at 70°C for 5 min. To remove templates of mRNAs, samples were treated with 1 U of RNase H enzyme at 37°C for 30 min.
Specific primer design
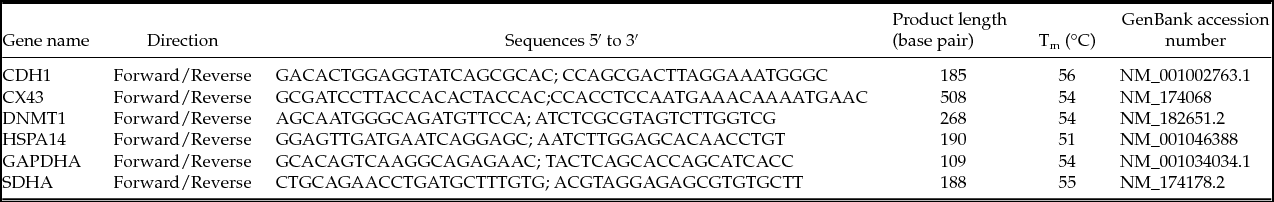
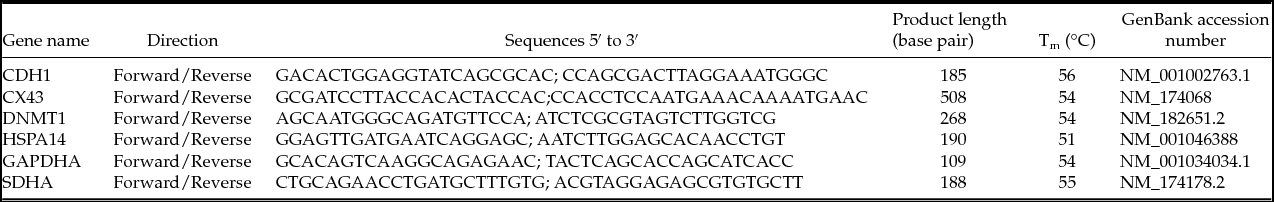
In order to perform the relative quantification of gene expression in real-time and to construct standard curves, specific primers for four chosen genes and two reference genes were designed using Prime3 software (Rozen & Skaletsky, Reference Rozen and Skaletsky2000) and synthesized by Invitrogen (Life Technologies Corporation Europe) from GenBank sequences (www.ncbi.nlm.nih.gov) as shown in Table 1.
Table 1 Oligonucleotide primers used for quantitative real-time polymerase chain reactions (qRT-PCR)
Standard curves
To determine the polymerase chain reaction (PCR) efficiency and calibration curves, fivefold serial dilutions of cDNA were made and amplified using primers for both desired and reference genes. The Ct versus Log10cDNA dilution was plotted to determine the slope of the line. PCR efficiency was then calculated by the equation m = −(1/log E), where m is the slope of the line and E is the efficiency.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Real-time PCRs were performed using the Thermo Scientific Absolute Blue QPCR SYBR Green Low ROX Mix (Thermo Scientific ABgene® UK). The amplification was run in an ABI Prism 7500 Fast system (Applied Biosystems) in 96 microwell plates. All samples, including the external standards and non-template control, were run in triplicate. The reaction conditions had been established through a series of preliminary optimization experiments, including calibration curves. Each 25 µ reaction contained 1× Blue QPCR SYBR Low ROX (which includes Thermo-Start™ DNA polymerase and 3 mM of MgCl2 in addition to Blue dye and ROX dye), 1 µM of each forward and reverse primers, water and template cDNA. Template cDNA corresponded to 2-cell, 4-cell, morula and blastocyst samples from cold month, warm month, control, and HS1- and HS2-treated groups. A non-template control was included. The amplification program included a preincubation step at holding stage 50°C for 2 min and 95°C for 10 min, followed by 40 three-step amplification cycles consisting of 15 s denaturation at 95°C, 1 min at 54°C and 30 s at 95°C. A melting curve for the verification of amplification product specificity was recorded at the final dissociation stage to confirm that there was no contamination from primer dimers. The quantification was carried out using the comparative cycle threshold (Ct) method, with the results expressed in relation to endogenous reference genes and a control group. In this experiment, the reference genes were GAPDH and SDHA, which presented little variation in mRNA levels during the development of bovine blastocysts. The studied genes were Cx43, HSPA14, CDH1 and DNMT1.
Data analysis
Oocytes and embryonic development data were analyzed by one way analysis of variance (ANOVA) and expressed as mean ± standard error of the mean (SEM) calculated from the surviving oocytes for each group. Comparisons were considered to be significantly different if P < 0.05. All analyses were performed using the program SPSS Statistics 17.0 (SPSS Inc., Chicago, Illinois, USA).
Amplicon sizes and the specificity of products generated were confirmed by dissociation curves after running amplification cycles. The relative expression ratio of a target gene was determined from the real-time PCR efficiency (E). For each gene, cDNA dilution curves were generated and used to calculate the individual real-time PCR efficiencies by E = 10(–1/slope) – 1. Relative gene expression analysis was performed using the Relative Expression Software Tool (REST©) with the Pair Wise Fixed Reallocation Randomization Test© method (Pfaffl et al., Reference Pfaffl, Horgan and Dempfle2002; Faheem et al., Reference Faheem, Baron, Carvalhais, Chaveiro, Pavani and Moreira da Silva2014) with the calculated E values introduced. The relative expression values (Ct) are presented as mean ± SEM. For comparisons between genes, data from fresh embryos (control group) at the same embryonic stage were used as a calibrator group for relative gene expression analysis. The mRNAs levels of the Cx43, CDH1, DNMT1 and HSPA14 genes were compared between in vitro-produced bovine embryos at 2-cell, 4-cell, morula and blastocyst stages using GAPDH and SDHA as reference genes for normalization.
Results
Assay 1: Kinetic effect of heat shock on nuclear oocyte's maturation
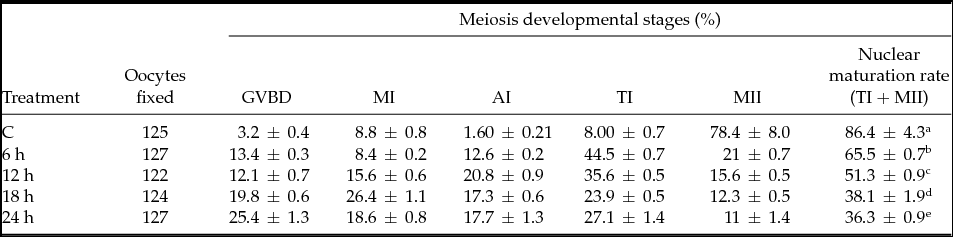
The effect of heat shock on nuclear oocyte maturation rate (NMR) for every 6 h of heat stress at HS1 and HS2 showed a significant standard difference (P < 0.05) with a decline in nuclear maturation rate (Tables 2 and 3). The results in Table 2 show that the meiotic development rate of oocytes experienced a decline at 6 h (when compared with the control) and at 18 h (when compared with 12 h). In the case of the kinetic effect at HS2 (Table 3), the decline in NMR rate was constant and no drastic decline was observed (P < 0.05). When comparing the control temperature groups and the hourly heat-shocked samples, it was apparent that most of the oocytes stopped while progressing to telophase I (TI) and metaphase II (MII). The majority of oocytes experienced arrest in the early stages of oocyte development (germinal vesicle breakdown (GVBD), metaphase I (MI) and anaphase I (AI)). The kinetic effect of heat shock treatment provides a better understanding of how oocytes can maintain NMR in moderate temperatures (HS1) for 6 h and 12 h. After this point, it was observed that exposure time had a greater impact.
Table 2 Kinetic effect of heat shock on nuclear maturation rate at 39.5°C
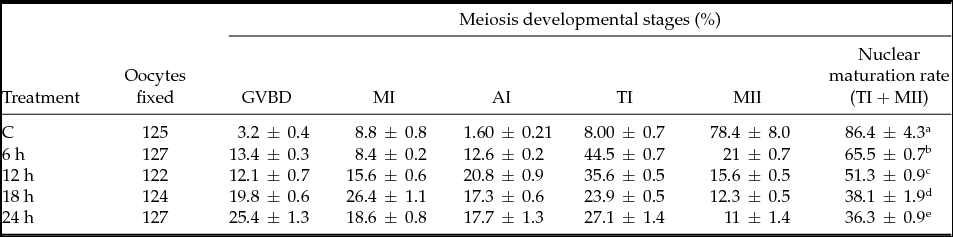
C = Control (38.5°C), heat shock for 6 h at 39.5°C and placed back to control, similarly for 12 h, 18 h, 24 h. GVBD = germinal vesicle breakdown; MІ = metaphase I; AІ = anaphase I; TI = telophase I; MІІ = metaphase ІІ. Data represent mean ± standard error of the mean (SEM). a,b,c,d,eRepresent statistical mean differences (P < 0.05).
Table 3 Kinetic effect of heat shock on nuclear maturation rate at 40.5°C
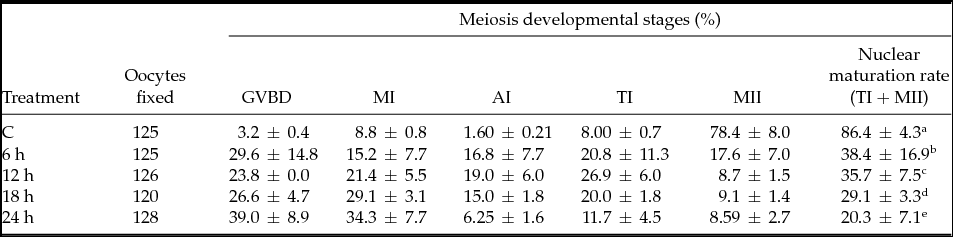
Heat shock for 6 h at 40.5°C and placed back to control, similarly for 12 h, 18 h, 24 h. GVBD = germinal vesicle breakdown; MІ = metaphase I; AІ = anaphase I; TI = telophase I; MІІ = metaphase ІІ. Data represent mean ± standard error of the mean (SEM). a,b,c,d,eRepresent statistical mean differences (P < 0.05).
Assay 2: In vitro effect on gene expression during warm and cold months
Cold versus warm months had a pronounced effect and was observed in the meiotic maturation rate of oocytes as well as in embryonic developmental ability. On average, the meiotic maturation of oocytes was 78.4 ± 8.0% versus 44.3 ± 8.1% (P < 0.001) for cold and warm months, respectively. Similarly, cleavage and embryonic development rates after IVF were higher (P < 0.05) during the cold period (78.0 ± 4.5; 53.8 ± 5.8) as compared with the hot period (48.2 ± 4.2; 36.3 ± 3.3) for cleavage and embryonic development, respectively.
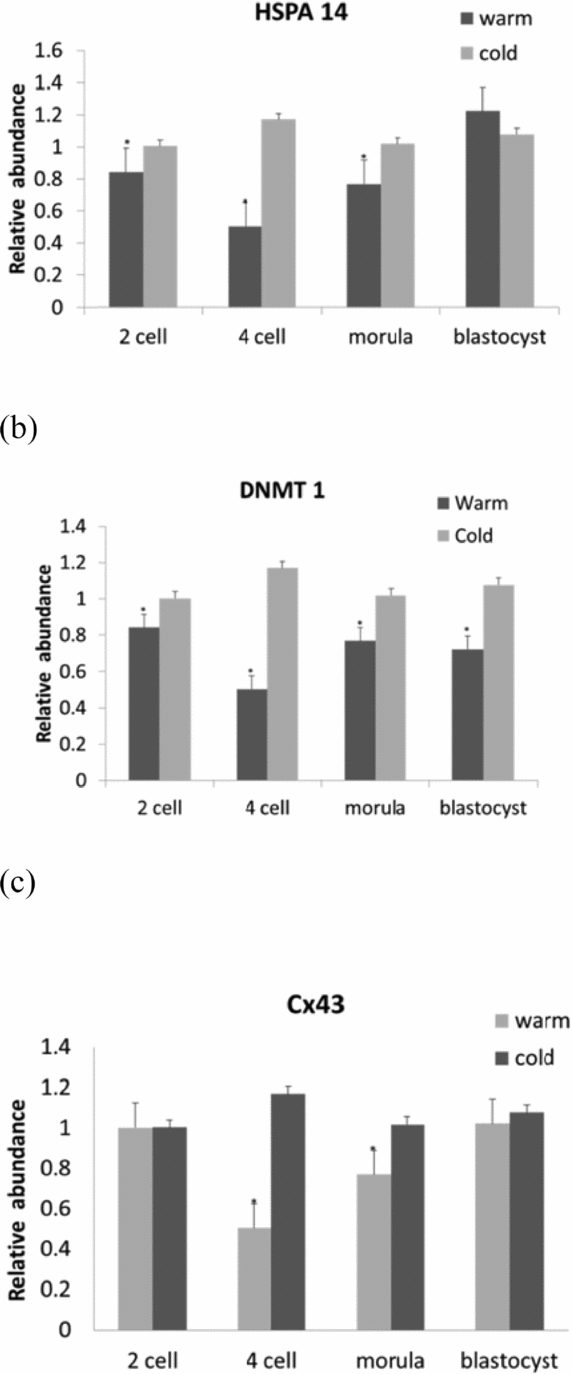
The examination of gene quantification at different developmental stages (2-cell, 4-cell, morula, blastocyst) during warm as compared with cold seasons showed a significant (P < 0.05) down-regulation in DNMT1 and HSPA14 at every stage of embryo development with respect to reference genes GAPDH and SDHA. In the blastocyst stage, the HSPA14 gene remained unexpressed (Fig. 1 a, b). For Cx43, a down-regulation was observed in the 4-cell and morula stages (Fig. 1 c). As for the CDH1 gene, no regulation was observed when compared with the data collected for the cold months (P < 0.05).
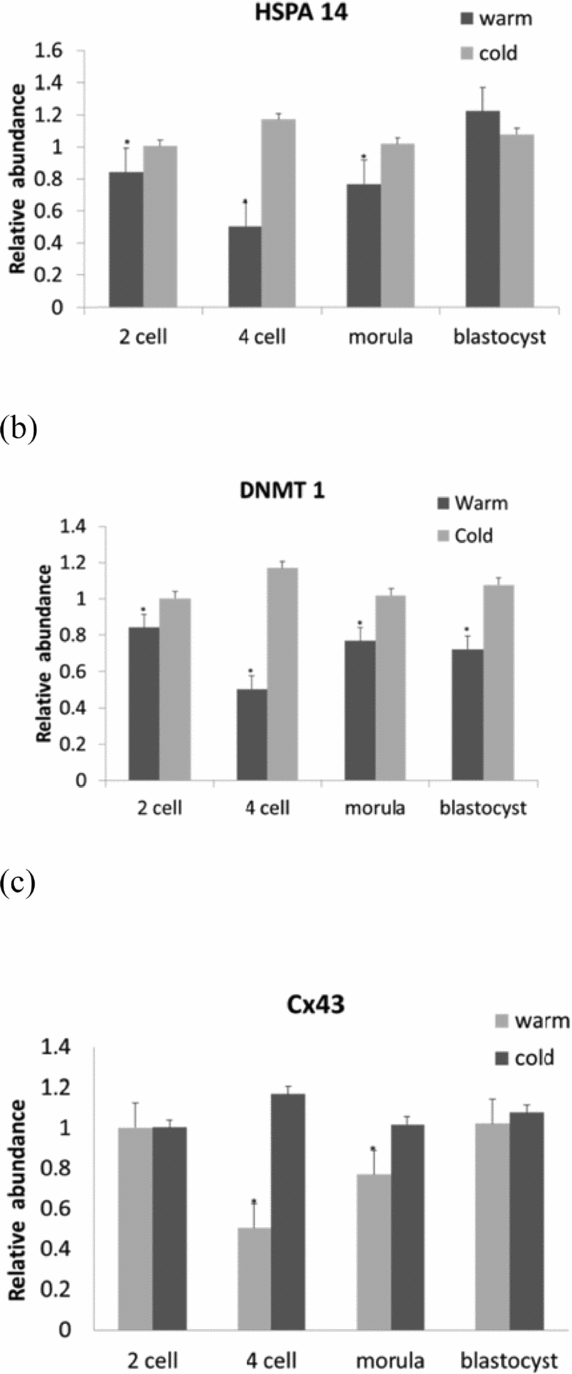
Figure 1 Gene expression transcripts of in vitro bovine embryos at different developmental stages in the warm months with reference to the cold months: (a) HSAP14; (b) DNMT 1; (c) Cx43. Columns with (*) represent significant differences (P < 0.05) within the gene. Bars indicate standard error of the mean.
Assay 3: Heat shock effect of in vitro developed oocytes and embryos in gene expression
Oocytes subjected to heat shock treatment (HS1, HS2) experienced a substantial decline in nuclear maturation rate: 78.4 ± 8.0; 21.7 ± 3.1 and 8.9 ± 2.0, for control, HS1 and HS2 temperatures, respectively. A similar tendency was observed after IVF at different developmental stages: for cleavage rate, results ranged from 70.7 ± 2 in the control to 35.4 ± 1 and 15.5 ± 2 for HS1 and HS2, respectively. Embryonic development demonstrated 48.0 ± 1% in the control and 20.5 ± 1% and 9.5 ± 2%, for HS1 and HS2 (P < 0.05).
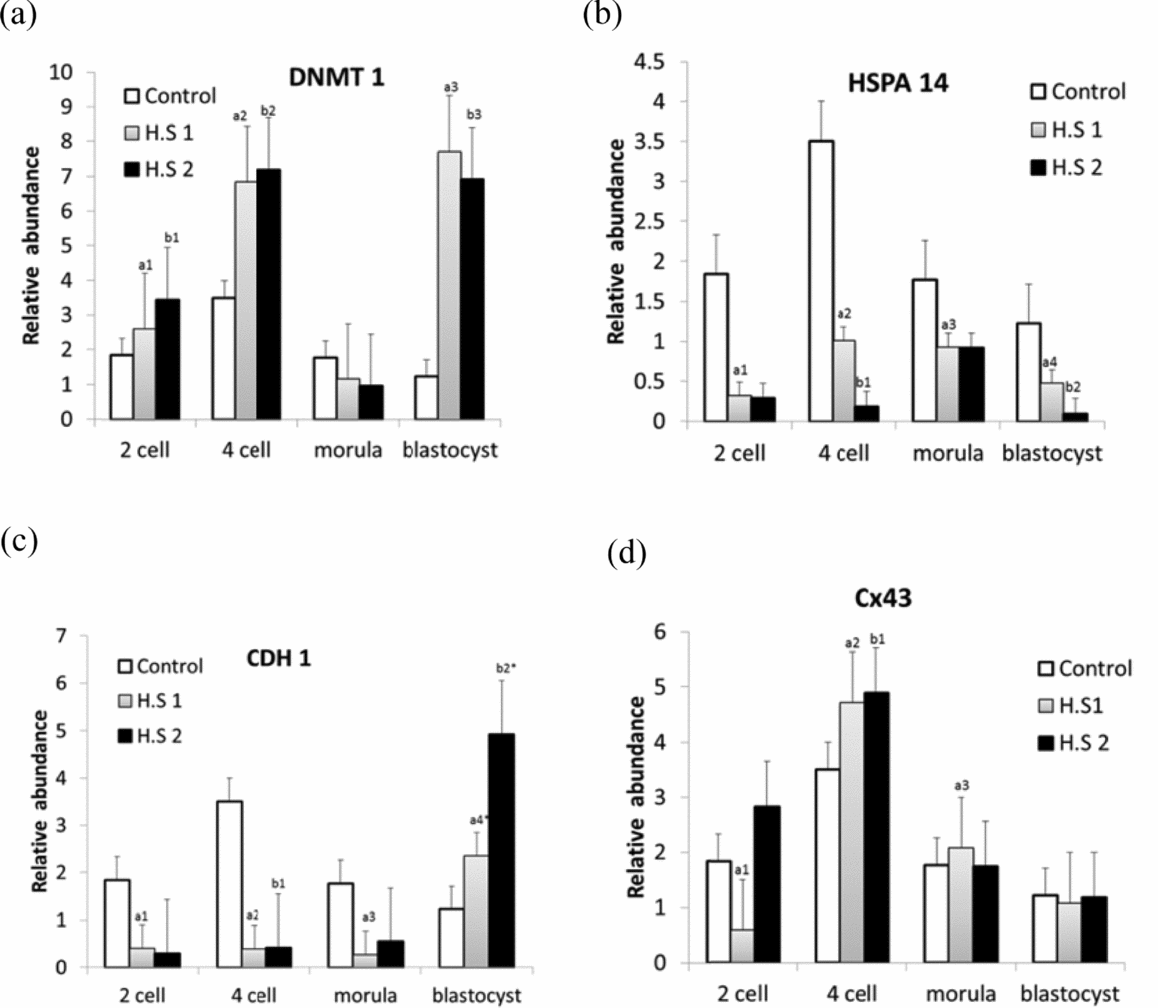
Gene expression analysis gave a new insight to better understanding the effects of heat stress on embryo development by probing the targeted genes. After examining gene expression in different developmental stages (Fig. 2), it was observed that prolonged heat stress altered gene regulation. DNMT 1 was up-regulated (P < 0.05) in all developmental stages except morula in both HS1 and HS2 groups (Fig. 2 a), while HSPA14 was down-regulated (P < 0.05) in all stages of embryo development in HS1 and HS2 (Fig. 2 b). A down-regulation was observed for CDH1 in all the stages of embryo development in both groups (HS1, HS2), except in the blastocyst stage, where an up-regulation was evident in both groups when compared with the control group (Fig. 2 c). With regard to Cx43, altered gene expression patterns were observed in all development stages of embryos in both HS samples; a down-regulation was observed in the 2-cell stage in HS1 and an up-regulation was seen in the 4-cell stage in HS2 and in the morula stage in HS1 (P < 0.05) (Fig. 2 d).
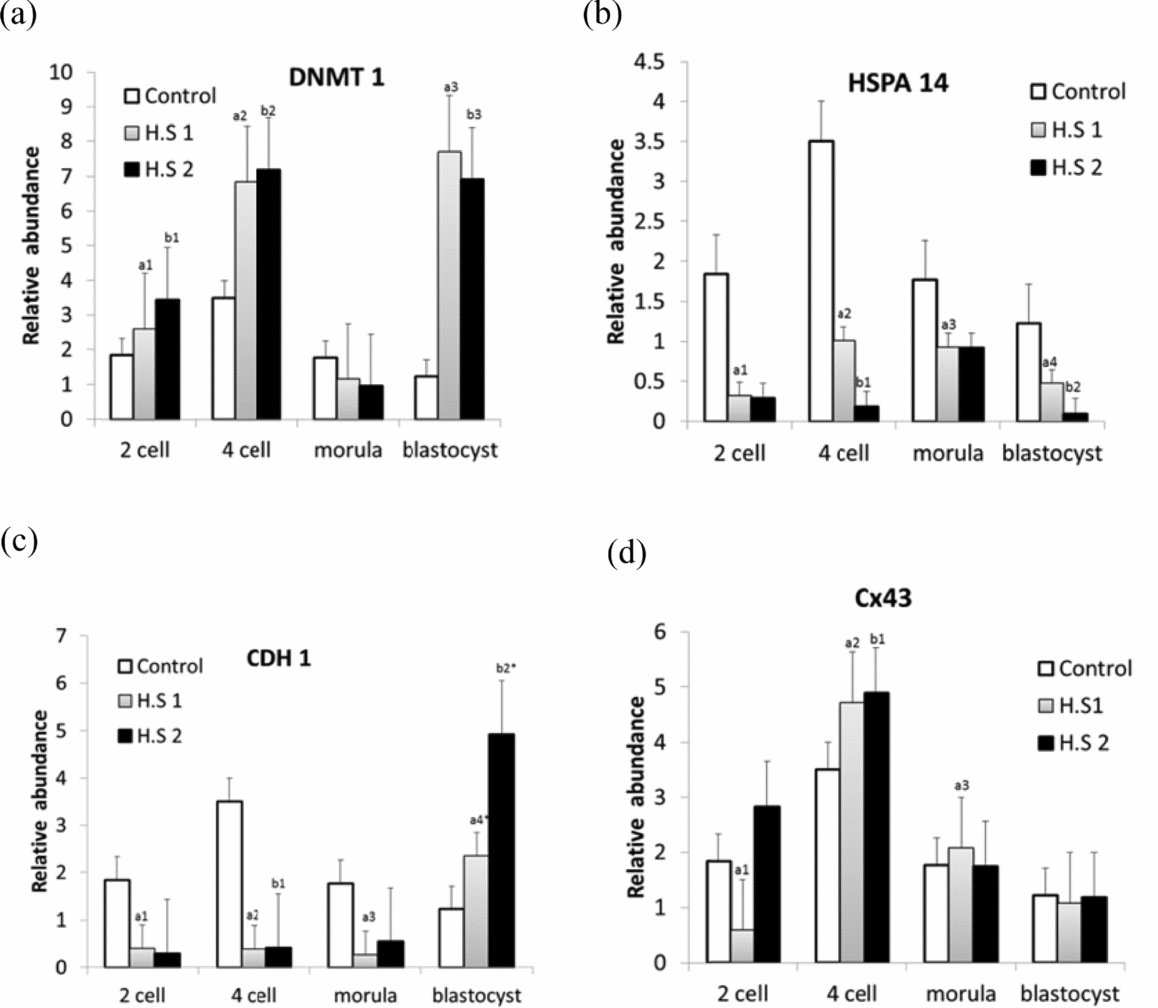
Figure 2 Relative quantification of the genes DNMT1 (a), HSPA14 (b), CDH1 (c) and CX43 (d) in bovine in vitro fertilized embryos at different development stages, developed from heat-shocked oocytes at 38.5°C (Control), 39.5°C (HS1) and 40.5°C (HS2). (a) Columns with a1,a2,a3,b1,b2,b3 show different superscripts expressing a similar significant difference within a gene and indicate heat shock effect within embryonic developmental stages, P < 0.05. (b) Columns with a1,a2,a3,a4,b1,b2 show different superscripts expressing similar significant differences within a gene and indicate heat shock effect within embryonic developmental stages, P < 0.05. (c) Columns with a1,a2,a3,b1 show different superscripts expressing similar significant difference within a gene and indicate heat shock effect within embryonic developmental stages, P < 0.05. Columns with a4*,b2* superscript expressing different values with respective to others superscripts (P < 0.05). (d) Columns with a1,b1,b2 show different superscripts expressing similar significant difference within a gene and indicate heat shock effect within embryonic developmental stages, P < 0.05. Column with a2* superscript is different with respective to other superscripts (P < 0.05).
Discussion
Fertility is a multi-factorial feature and its deterioration has been shown to be related with a network of genetic and environmental factors. In the present study, both environmental and genetic factors were considered in order to understand their effect on fertility. Previous studies investigated heat stress factors in bovine oocytes and embryo developmental competence by varying temperature and culture conditions, which provided evidence that inducing heat stress conditions in oocytes leads to the alteration of nuclear structures, including microtubules and microfilaments (Tseng et al., Reference Tseng, Chen, Chou, Yeh and Ju2004; Paula-Lopes et al., Reference Paula-Lopes, Milazzotto, Assumpcao and Visintin2008). The impact of kinetic heat stress on bovine oocytes has been studied as the intensity of stress depends both on temperature and exposure time and, in the real world setting, cows are exposed to the sun for short or long durations, depending on climatic conditions. Thus, the present study provides a better understanding of how bovine oocytes are affected by heat stress as results showed that developmental competence is affected after 6 h of heat shock at moderate temperature (HS1) and a larger number of oocytes experience arrest in early meiotic development after 12 h of heat shock. This effect, which occurs specifically at 6 h and 12 h, could be due to the impact of heat shock occurring at initial stage of nuclear maturation (6 h), which leads to the meiotic arrest of oocytes at the GVBD stage. Conversely, in the control, the nuclear membrane disappeared and entered GVBD, possibly within a 5 h -6 h time span. In non-stressed conditions at 12 h, oocytes pass though GVBD to MI (Gordon, Reference Gordon2003). Nevertheless, when heat shock is provided up to 12 h, the meiotic development of oocytes would fail to progress to further stages. The oocyte development rate remains low when oocytes are exposed to high temperatures (HS2), regardless of exposure time. This result shows that oocytes can sustain their developmental rate when exposed to moderate temperature heat shock for specified durations of time.
Our previous studies (Pavani et al., Reference Pavani, Carvalhais, Faheem, Chaveiro, Reis and Moreira da Silva2015b) clearly show that the reproductive performance of Holstein cows is lower in the summer months (June, July, August and September) than in the winter months (December, January, February and March), both in in vitro and in vivo. Induced heat stress for 24 h had a significant effect on oocyte development stages as well as on embryos after raising the temperatures by 1°C during oocyte maturation. The genetic component of this study was performed as an extension of our previous study, Pavani et al. (Reference Pavani, Carvalhais, Faheem, Chaveiro, Reis and Moreira da Silva2015b), which provided new insight into understanding maternal heat stress and heat shock effects on embryonic development. Seasonal studies conducted by Gendelman et al. (Reference Gendelman, Aroyo, Yavin and Roth2010) showed that delayed cleavage was more common in the hot season than in the cold season. Accordingly, delayed cleavage effects were more commonly observed in the summer months than in the winter months. In similar studies, gene expression of GAPDH, GDF9 and POU5F1 was higher in early cleaved embryos than in late cleaved embryos. In contrast, our studies showed no gene expression in reference to the GAPDH with respect to maternal heat stress and heat shock. Research conducted by Monica et al. (Reference Monica, Dorella, Enrica, Romina, Giovanni, Angela and Franca2004) also contradicts Gendelman et al. (Reference Gendelman, Aroyo, Yavin and Roth2010) and supports our findings. Monica et al. (Reference Monica, Dorella, Enrica, Romina, Giovanni, Angela and Franca2004) showed that the expression of different genes in relation to GAPDH on mesangial cells at different culture conditions (including high glucose and phorbol 12-myristate 13-acetate stimulation) showed no significant modulation in the GAPDH gene. Similar results were obtained by Dode et al. (Reference Dode, Dufort, Massicotte and Sirard2006), who reported a constant expression of GAPDH in both early and late cleaved bovine embryos.
DNMT1 plays a prominent role in early embryonic growth and development (Hiendleder et al., Reference Hiendleder, Mund and Reichenbach2004) as well as in the maintenance of the methylation imprints in post-implantation embryos. The metabolism of this enzyme during the preimplantation stage of embryo development is still unclear. During cleavage, the genome is demethylated as the non-allelic methylation marks of the imprinted genes escape demethylation and reach successful sustainability (Hirasawa et al., Reference Hirasawa, Chiba and Kaneda2008). The decline of the DNMT1 concentration observed throughout the preimplantation stages suggests that this variant has an origin in maternal stress. High levels of DNMT1 enzyme in oocytes possibly represents a maternal shock that could be used for the maintenance and/or de novo methylation in later cell cycles (Cirio et al., Reference Cirio, Ratnam and Ding2008; Giraldo et al., Reference Giraldo, DeCourcy, Ball, Hylan and Ayares2013). In the current study, a constant down-regulation of the DNMT1 gene was observed in every stage of embryo development in the warm months. Similar results were obtained in a study performed on mice by Suzuki & Bird (Reference Suzuki and Bird2008). Delayed embryonic development rate during the summer period can possibly be explained due to the low expression of the DNMT1 gene. It has been hypothesized that epigenetic modification may be responsible for programmed cell death. Transient depletion of DNMT1 in frog embryos induces DNA hypomethylation, which produces altered phenotypes and causes apoptosis (Stancheva et al., Reference Stancheva, El-Maarri, Walter, Niveleau and Meehan2002).
Heat shock proteins are highly conserved across most organisms. Therefore, it is conceivable to hypothesize that they play a vital role in the survival and development of cells. These proteins, as molecular chaperones, are involved in the maintenance of the intra-cellular homeostasis, primarily by controlling the process of protein folding (Beaman, Reference Beaman2012). Concerning HSPA14, this gene has two isoforms possessing 4 or 14 exons. Research conducted by Zhang et al. (Reference Zhang, Peñagaricano, Driver, Chen and Khatib2011) demonstrated that high expression levels of HSPA14 recorded in degenerated embryos provide evidence that this protein could aid in embryo survival and avoid apoptosis (Betts & King, Reference Betts and King2001; Levy et al., Reference Levy, Cordonier, Czyba and Goerin2001). Higher and low expression of HSPs indicates induced stress factors; the transcript level reflects both cell response and stress intensity (Neuer et al., Reference Neuer, Spandorfer, Giraldo, Dieterle, Rosenwaks and Witkin2000). Furthermore, studies developed by Gad et al. (Reference Gad, Besenfelder, Rings, Ghanem, Salilew-Wondim, Hossain, Tesfaye, Lonergan, Becker, Cinar, Schellander, Havlicek and Hölker2011) showed high expression of HSPA14 in blastocysts obtained from heifers ovulating under heat stress conditions. So far, the HSPA14 gene was less understood, so in the current study we focused on its expression. During the summer months, low expression of HSPA14 was observed in all the developmental stages expect in the blastocyst stage. This down-regulation of the HSPA14 gene can be due to the fact that oocytes harvested from summer months experienced slowed embryo development from the 2-cell to morula stage.
Results published by Gómez et al. (Reference Gómez, Caamaño, Bermejo-Alvarez, Díez, Muñoz, Martín, Carrocera and Gutiérrez-Adán2009) showed how connexin 43 (GJA1) and CDH1 (E-cadherin) are related on the intercellular level in embryo development. Boni et al. (Reference Boni, Tosti, Roviello and Dale1999) explained the differences in the in vitro and parthenotes produced in bovine embryos, as intercellular structures were altered in parthenotes. The genes involved in the alteration of these structures were Cx43 and CDH1, and their expression was down-regulated. In the current study, we observed down-regulation in 4-cell and morula stages of embryos of the Cx43 gene, whereas CDH1 remained unexpressed in all embryonic development stages. Lower expression of Cx43 reflects the low quality of embryos developed from oocytes with low developmental competence (Rizos et al., Reference Rizos, Gutierrez-Adan, Perez-Garnelo, De La Fuente, Boland and Lonergan2003; Nemcova et al., Reference Nemcova, Machatkova, Hanzalova, Horakova and Kanka2006). The altered expression of targeted genes in the first assay possibly provides evidence that maternal stress contributed to a delayed cleavage rate and a low developmental rate among embryos during summer months with respect to cold months.
Regarding heat-induced stress factors, for every 1°C increase from the control temperature there was a substantial decline in oocyte and embryonic development rates (Pavani et al., Reference Pavani, Carvalhais, Faheem, Chaveiro, Reis and Moreira da Silva2015b). This phenomenon is supported by a second study. DNA methylation in gene expression can play a critical role. Errors in methylation could lead to several abnormalities and diseases such as cancer, lupus, muscular dystrophy and also complications that arise during embryonic development due to abnormalities in X chromosome methylation and gene imprinting (Robertson, Reference Robertson2005). In the current study, we observed up-regulation of DNMT1 at every stage of embryonic development, except in the morula stage in both heat-induced stress samples (HS1 and HS2). Similarly high expression levels were observed in bovine SCNT (somatic cell nuclear transfer) when compared with in vitro fertilized embryos in satellite 1 sequence (Sibley et al., Reference Sibley, Coan, Ferguson-Smith, Dean, Hughes, Smith, Reik, Burton, Fowden and Constancia2004; Cho et al., Reference Cho, Kang and Lee2014). In research conducted by Su et al. (Reference Su, Wang, Xing, Liu and Zhang2014), it was shown that aberrant DNMT1 genes in the placenta of cloned calves may have caused developmental issues, and ultimately resulted in their death. Therefore, the altered expression of the DNMT1 gene could possibly be due to heat shock of bovine oocytes at maturation level, which apparently affects the embryo development rate.
Conversely, a significant down-regulation of the HSPA14 gene was observed in all stages of embryonic development. This up-regulation of DNMT1 and down-regulation of HSPA14 illustrates a possible correlation between these genes, as this phenomenon was observed early in the maternal heat stress sample in the first experiment. In line with the findings of this study, Tilman et al. (Reference Tilman, Arnoult, Lenglez, Van Beneden, Loriot, De Smet and Decottignies2012) reported that two distinct pathways are responsible for the activation of HSPs in cancer cell or pathogenic cells; one relying on heat shock pathway activation through HSF1 (Heat Shock Factor 1), and the other induced by overexpression of DNMT1 (Eymery et al., Reference Eymery, Souchier, Vourc'h and Jolly2010). Activation of the heat shock pathway in response to the high expression of DNMT1 can also lead to genome-wide DNA damage (Palii et al., Reference Palii, Van Emburgh, Sankpal, Brown and Robertson2008), which could be the reason behind slow embryo development and embryo arrest in early stages.
As far as the CDH1 gene is concerned, down-regulation was observed in every stage of embryonic development except in the blastocyst stage in both heat shock treatment samples. This result can be explained in relation to HSPA14. In research conducted by Ahlskog et al. (Reference Ahlskog, Björk, Elsing, Aspelin, Kallio, Roos-Mattjus and Sistonen2010) a direct association between HSF2 (Heat Shock Factor 2) and the APC/C (Anaphase-promoting Complex/Cyclosome) co-activators Cdc20 and CDH1 was observed. HSF2 exhibited a longer half-life and less heat-shock-induced ubiquitination, when APC/C co-activations or subunits were silenced by specific siRNAs. Similarly, the down-regulation of HSF2 upon heat shock treatment was rescued in cells in which the APC/C co-activators in Cdc20 and CDH1 were depleted. These findings support the results of the present study, in which a constant down-regulation HSPA14 was related to CDH1. One possible reason for the up-regulation of CDH1 observed at the blastocyst stage can be explained along with DNMT1 up-regulation, as the high expression pattern of CDH1 and DNMT1 is observed mostly in cancerous cells or otherwise afflicted samples (heat-stressed, pathogenic or degenerated samples) (Palii et al., Reference Palii, Van Emburgh, Sankpal, Brown and Robertson2008; Ahlskog et al., Reference Ahlskog, Björk, Elsing, Aspelin, Kallio, Roos-Mattjus and Sistonen2010). Future research could provide a better understanding of how prolonged heat stress could alter the development of the embryo, leading to embryo damage.
Normally, low expression of Cx43 in different developmental stages of embryos has been associated with low embryo quality and reduced survival capacity (Rizos et al., Reference Rizos, Gutierrez-Adan, Perez-Garnelo, De La Fuente, Boland and Lonergan2003; Lonergan et al., Reference Lonergan, Rizos, Gutierrez-Adan, Moreira, Pintado, De la Fuente and Boland2003). In the present study, even after induced heat stress, both treatment samples demonstrated a constant up-regulation, which was observed after the 4-cell stage of embryonic development, which provides evidence that the cell survival rate was higher after the 4-cell stage. CDH1 intervenes in cell-to-cell adhesion as it is associated with Cx43 in aiding in cell survival (Gómez et al., Reference Gómez, Caamaño, Bermejo-Alvarez, Díez, Muñoz, Martín, Carrocera and Gutiérrez-Adán2009). At the morula stage in both heat shock treatment samples, no gene regulation was observed. This finding is supported by research conducted by Edwards & Hansen (Reference Edwards and Hansen1997), which showed that instead of blastocyst stage development, the morula from heat-stressed ova cultured at 41°C had high stability. In this context, the consequences of exposing maturing ova to heat stress go beyond obvious effects that reduce development. The few embryos had developed thermoability from heat stress, minimizing exposure to stressors during maturation.
Conclusion
Each experiment in this study has achieved results that add to the knowledge of bovine reproduction. The effect of kinetic heat shock on nuclear maturation showed that oocyte developmental competence is based on heat shock temperature and exposure time. The more oocytes are exposed to heat stress, the lower the development rate. Also, the genetic studies support the conclusion that maternal heat stress slows embryo development during the summer months, possibly due to the altered expression of DNMT1, HSPA14 and Cx43 in these conditions. Data on in vitro heat stress show that heat stress reduces the embryo development rate and increases embryo arrest during early stages, which may be due to the constant, high expression of the DNMT1 gene and variations in the expression of HSPA14, Cx43 and CDH1. Results clearly demonstrated that heat stress affects bovine oocytes and influences maturation and cleavage and contributes to embryonic arrest, and possibly apoptosis, in the early stages of development.
Acknowledgments
Dr Marwa Faheem is fully acknowledged for her supervision in reproductive techniques and Ms Meredith Cannella is fully acknowledged for her English correction and documentation of the manuscript.
Financial support
Project supported by the Azorean Agency for Science and Technology, Grant BD M3.1.2/F/044/2011. CITA-A is also fully acknowledged.
Conflict of interest
None of the authors has any conflict of interest to declare.