Introduction
In North Africa Saharan regions, date palm plantations are the most important horticultural crops (Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). These artificial environments are well adapted to Saharan conditions and constitute an effective barrier against sand encroachment (Mihi et al. Reference Mihi, Tarai and Chenchouni2017, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). In Biskra, there are two types of date palm plantations. Traditional plantations are characterized by (i) short distances between trees (generally 4 m), (ii) very high, large trees, (iii) olive, citrus, and pomegranate trees planted between the date palm trees, and (iv) traditional irrigation. Modern plantations are marked by (i) long distances between trees (up to 10 m), (ii) relatively small trees, and (iii) drip irrigation system. These arboreal stands host many wintering birds (e.g. European Robin Erithacus rubecula and Common Chaffinch Fringilla coelebs ), migratory birds (e.g. European Pied Flycatcher Ficedula hypoleuca and Eurasian Wryneck Jynx torquilla), and breeding species (e.g. Laughing Dove Spilopelia senegalensis, Common Kestrel Falco tinnunculus, and Blackbird Turdus merula). In Algeria, the number of date palm trees in the Biskra region alone exceeds four million (D.S.A. 2019, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). This number is markedly more important than that recorded in Morocco (4.4 million) (Sedra Reference Sedra2003) and Tunisia (3.9 million) (Kassah Reference Kassah2002).
The Eurasian Turtle-dove Streptopelia turtur (hereafter Turtle Dove) is a sub-Saharan migratory bird that has an extensive breeding distribution range in the Western Palearctic from temperate regions to North Africa (Cramp Reference Cramp1985). This Columbidae species is typically associated with scrub (Browne et al. Reference Browne, Aebischer and Crick2005), woodland edges (Reino et al. Reference Reino, Beja, Osborne, Morgado, Fabiao and Rotenberry2009, Hanane Reference Hanane2018, Chiatante et al. Reference Chiatante, Porro and Meriggi2020), and also man-made environments, such as olive orchards (Hanane and Baâmal Reference Hanane and Baâmal2011, Dias et al. Reference Dias, Moreira, Beja, Carvalho, Gordinho, Reino, Oliveira and Rego2013, Hanane et al. 2018), orange orchards (Brahmia et al. Reference Brahmia, Zeraoula, Bensouilah, Bouslama and Houhamdi2015, Hanane Reference Hanane2016a, Reference Hanane2016b), and date palm plantations (Absi et al. Reference Absi, Belhamra, Farhi and Halis2015). The Turtle Dove population experienced a drastic decline (78%) in 1980–2013 (Dunn et al. Reference Dunn, Grice and Morris2017, PECBMS 2019) and has been classified as ‘Vulnerable’ throughout Europe (‘Near Threatened’ within the EU27 countries) following a recent assessment (BirdLife International 2015, 2017). Several factors have contributed to this alarming situation including (i) nest habitat degradation (Browne et al. Reference Browne, Aebischer, Yfantis and Marchant2004), (ii) changes in food availability in breeding (Browne and Aebischer Reference Browne and Aebischer2003) and wintering grounds (Eraud et al. Reference Eraud, Boutin, Riviere, Brun, Barbraud and Lormé2009), (iii) hunting (Boutin and Lutz Reference Boutin and Lutz2007), (iv) removal of hedgerows (Chiatante et al. Reference Chiatante, Porro and Meriggi2020), and (v) variation in ecological conditions throughout the migration route (Browne and Aebischer Reference Browne and Aebischer2001, Eraud et al. Reference Eraud, Boutin, Riviere, Brun, Barbraud and Lormé2009). Over the last decade, many studies on Turtle Doves focused on (i) breeding biology (Rocha and Hidalgo Reference Rocha and Hidalgo2002, Browne et al. Reference Browne, Aebischer, Yfantis and Marchant2004, Reference Browne, Aebischer and Crick2005, Hanane and Baâmal Reference Hanane and Baâmal2011, Kafi et al. Reference Kafi, Hanane, Bensouilah, Zeraoula, Brahmia and Houhamdi2015, Hanane Reference Hanane2016a, Reference Hanane2016b), (ii) breeding habitat use (Browne and Aebischer Reference Browne and Aebischer2004, Browne et al. Reference Browne, Aebischer, Yfantis and Marchant2004, Reference Browne, Aebischer and Crick2005, Bakaloudis et al. Reference Bakaloudis, Vlachos, Chatzinikos, Bontzorlos and Papakosta2009, Dunn and Morris Reference Dunn and Morris2012, De Buruaga Reference De Buruaga, Onrubia, Fernandez-Garcia, Campos, Canales and Unamuno2012, Dias et al. Reference Dias, Moreira, Beja, Carvalho, Gordinho, Reino, Oliveira and Rego2013, Yahiaoui et al. Reference Yahiaoui, Arab, Belhamra, Browne, Boutin and Moali2014, Dunn et al. Reference Dunn, Grice and Morris2017, Hanane Reference Hanane2018, Reference Hanane2019), (iii) foraging habitat use (Browne and Aebisher Reference Browne and Aebischer2003, Dunn et al. Reference Dunn, Morris and Grice2015, Rocha and Quillfeldt Reference Rocha and Quillfeldt2015, Gutiérrez-Galán and Alonso Reference Gutiérrez-Galán and Alonso2016), and (v) migration (Eraud et al. Reference Eraud, Rivière, Lormée, Fox, Ducamp and Boutin2013, Lormée et al. Reference Lormée, Boutin, Pinaud, Bidault and Eraud2016, Marx et al. Reference Marx, Korner-Nievergelt and Quillfeldt2016).
Knowledge on the size of North African Turtle Dove populations is practically non-existent (but see Hanane et al. Reference Hanane, Besnard and Aafi2012). A transboundary study recently conducted in Morocco and Spain has, however, suggested that the species is distinctly more common in Morocco than in Spain (Tellería et al. Reference Tellería, Carbonell, Fandos, Tena, Onrubia, Qninba, Aguirre, Hernandez-Téllez, Martin and Ramizez2020). Even though this species is moderately well studied in the south-western Palaearctic, to our knowledge, there is no study focused on disentangling factors affecting nest habitat selection in Saharan agro-ecosystems which may be very different from other agro-ecosystems across its range.
Here, we investigated the effects of a series of variables,measured at occupied nest trees and an equivalent number of random unoccupied nest trees, on the probability of Turtle Dove nest presence. Specifically, we aimed to disentangle the roles of (i) tree characteristics, (ii) microhabitat features, and (iii) human presence. We used variation partitioning with an unbiased statistical estimator to ensure the correct interpretation of the results (Legendre Reference Legendre2008). Such an approach aims at identifying which of these three potential predictors is the most relevant in nest habitat selection by Turtle Doves in date palm plantations. A marked sensitivity towards an individual predictor will determine the nature and the scope of management measures to be undertaken (Farhi et al. Reference Farhi, Hanane, Mezerdi, Kahl and Khemis2019).
The results of this investigation will help inform on the management and conservation of this vulnerable species. Given the known ecology of the Turtle Dove, we hypothesized that the selection of date palm trees for nesting would depend both on tree characteristics (Hanane Reference Hanane2018) (for instance, dense tall trees with different age) and human presence (Hanane and Baâmal Reference Hanane and Baâmal2011, De Buruaga et al. Reference De Buruaga, Onrubia, Fernandez-Garcia, Campos, Canales and Unamuno2012, Dias et al. Reference Dias, Moreira, Beja, Carvalho, Gordinho, Reino, Oliveira and Rego2013). Considering the relative importance of these factors, we expected that the selection of nest trees would depend on both of them, i.e. with a preference for given tree characteristics, while avoiding excessive human disturbance.
Methods
Study area
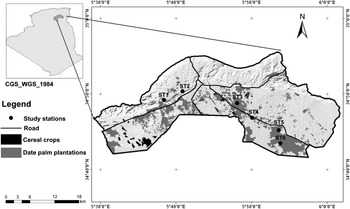
Our study was conducted in the Saharan region of Biskra, Algeria (34°48.00N; 5°44.00"W; Figure 1). The region (702.2 km2) has a Saharan climate, with an annual average rainfall of 149.7 mm, with a maximum recorded during the winter season (December–January) (ONM 2016, Farhi et al. Reference Farhi, Hanane, Mezerdi, Kahl and Khemis2019, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). Temperatures vary widely, being more moderate during winter (6°C) and hot in summer often reaching 44°C (Farhi et al. Reference Farhi, Hanane, Mezerdi, Kahl and Khemis2019, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). The mean relative humidity is 35% (min = 25.6% in July; max = 59.1% in December) (Farhi et al. Reference Farhi, Hanane, Mezerdi, Kahl and Khemis2019, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). Altitude ranges from 125 to 300 m above sea level.
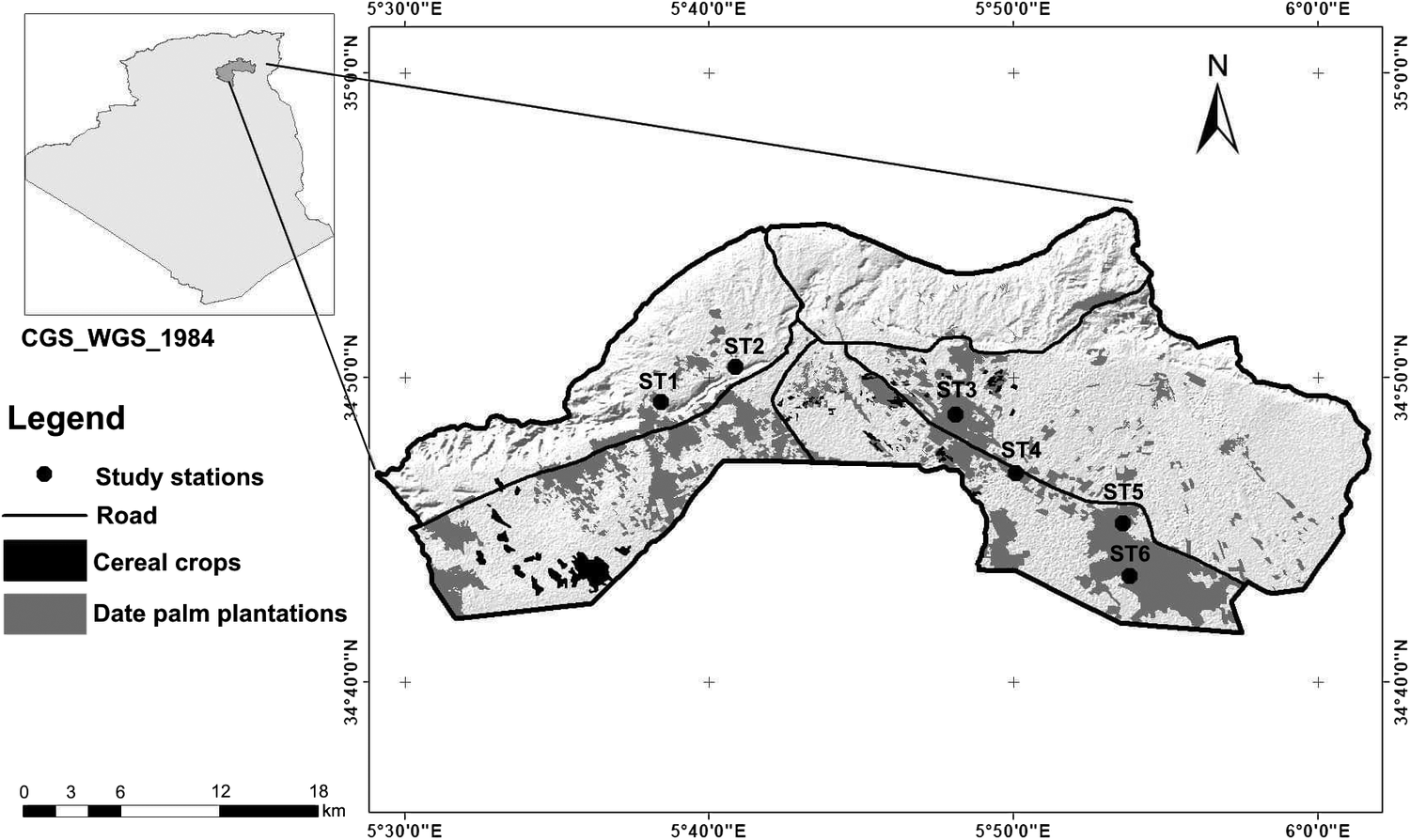
Figure 1. Map showing the position of the six study stations in the region of Biskra.
Without being very representative, i.e. occupying just 2% of the study area (14.1km2), fruit trees (olive Olea europaea, pomegranate Punica granatum, and fig Ficus carica) are present in some localities of Biskra. With an area of 63.2 km2, date palm Phoenix dactylifera plantations occupy 9% of the study area. Modern plantations, generally characterised by large date palm trees at the begening of their production phase (mean age = 18 ±2.35 years), are the most dominant, covering 75% (48.5 km2), while 25% (16.2 km2) are traditional plantations. Cereals, composed of wheat (Triticum turgidum and T. aestibum) and barley Hordeum vulgare cover 14.1 km2 (2%). The remaining landscape composition is constituted by pasture (50%; 351.1 km2), urban areas (17%; 119.4 km2), bare ground (8%; 56.2 km2), forests (0.3%; 2.11 km2), and other (9%; 63.2 km2).
The distance between the city of Biskra and the nearest palm grove varies between a minimum of 1.95 km, and a maximum of 100 km. In most palm orchards, there is only one or no house (Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). According to Diab (Reference Diab2015), the most common annual plant species present in date plantations are the lesser crystal ice plant Mesembryanthemum nodiflorum, milk thistle Silybium marianum, field sowthistle Sonchus arvensis, and smooth sowthistle Sonchus olearaceus (Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020).
In this North African Saharan region, the Turtle Dove is very common within and around palm groves. In these plantations, Turtle Dove take advantage of the daily availability of cereals (mostly barley) provided to livestock, close to stables. Outside these plantations, cereals also contribute to the food requirements of Turtle Doves.
Data collection
Fieldwork was carried out in Biskra’s date palm plantations during the 2019 breeding season (beginning of March-end of August). Six farm stations were randomly chosen by drawing six numbers from the list of date palm farms at Biskra (n = 21,000) (D.S.A. 2019, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020). Four sample plots, each corresponding to the area occupied by 49 palm trees (seven rows and seven columns), were selected ramdomly choosing coordinates x (length) and y (width). The study area, therefore, comprised six farms x four study plots per farm x 49 palm trees per plot (mean = 1,854.00 m2 ± 90.00; min-max = 1,764–2,304 m2), resulting in a total of 1,176 surveyed trees.
In each week we searched for new nests in all date palm trees present at the 24 sampling plots. To do this, we performed movements back and forth at the four lines of trees present at each in each of them. The location of each nest tree was marked on a map of the study plot. A nest was considered active when eggs, nestlings, or incubating adults were present. To assess nest habitat selection within the date palm plantations, the same number of non-nest trees as nest trees (n = 92) was selected randomly following the steps described by Bakaloudis et al. (Reference Bakaloudis, Vlachos, Papageorgiou and Holloway2001), Hanane et al. (Reference Hanane, Besnard and Aafi2012) and Hanane (Reference Hanane2018). Each random date palm tree was situated 100–700 m from the nest trees. Three steps were followed to establish each random date palm tree. First, the date palm stand centered on the nest tree was divided into four quadrants (1 = north-east, 2 = south-east, 3 = south-west, and 4 = north-west), and one of these was randomly selected. Second, two randomly numbers between 0 and 400 were selected to calculate the distance in metres to the random point along the north-south axis and the east–west axis. The intersection of lines extending from these points perpendicular to the axes identified the location of the centre of the random plot. Finally, the closest date palm tree to this central point was selected and defined as the random nest tree. In no case did a randomly selected tree following this procedure contain a Turtle Dove nest.
Explanatory variables
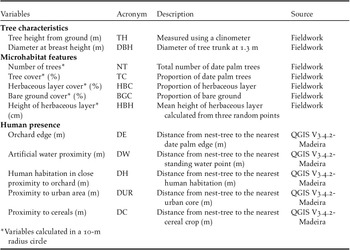
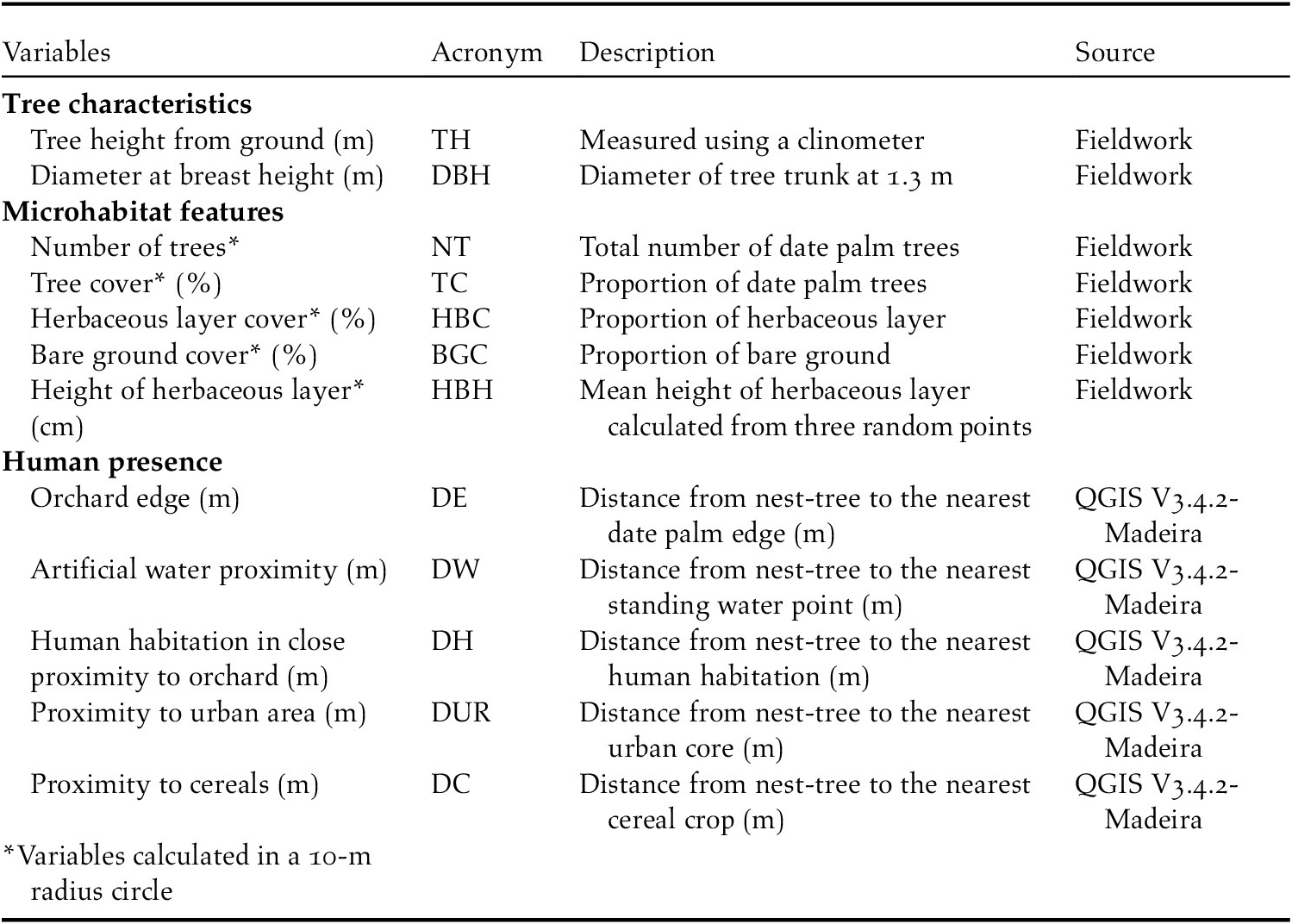
We selected 12 environmental variables related to tree characteristics, microhabitat, and human presence (Table 1) because they can potentially influence the selection of nesting habitat by Turtle Dove (Hanane Reference Hanane2017, Reference Hanane2018). Variables of tree characteristics and microhabitat were measured in the field by the same person (N. Saâd) (Table 1). Microhabitat variables were characterised within circle plots of 10-m radius (0.03 ha) (Danielsen et al. Reference Danielsen, Jensen and Burgess2014, Kumordzi et al. Reference Kumordzi, de Bello, Freschet, Le Bagousse-Pinguet, Lepš and Wardle2015). Five vegetation variables were measured around each sample point (Table 1). We visually estimated the percentage cover of date palm trees, herbaceous plants, and bare ground (Alaya-Ltifi and Selmi Reference Alaya-Ltifi and Selmi2014, Hanane et al. Reference Hanane, Cherkaoui, Magri and Yassin2019). All vegetation parameter estimations were conducted by the same observer (N. Saâd) to avoid observer-related biases in vegetation sampling (Prodon and Lebreton Reference Prodon and Lebreton1981). The height of date palm trees was determined using a clinometer. Variables related to human presence were all performed using QGIS.
Table 1. Explanatory variables used to characterize European Turtle Dove nest habitat selection in the Biskra’s date palm plantations, Algeria
Statistical analyses
Each group of explanatory variables (those related to tree characteristics, microhabitat, and human presence; Table 1) was considered separately when building models. This approach is widely adopted in similar studies (Balbontin Reference Balbontin2005, Jedlikowski et al. Reference Jedlikowski, Chibowski, Karasek and Brambilla2016, Assandri et al. Reference Assandri, Giacomazzo, Brambilla, Griggio and Pedrini2017, Hanane Reference Hanane2018, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020).
As a first step, we checked for possible correlations among variables using Pearson’s correlation coefficient. Moreover, we evaluated collinearity among predictor variables using variance inflation factors (VIFs), which ranged from 1.06 to 2.98, thus indicating a negligible effect of multicollinearity on estimates. Therefore, all variables were considered for modelling.
To test the effect of tree characteristics, microhabitat, and human presence on nest habitat selection by Turtle Dove in date palm plantations, Generalized Linear Mixed Models (GLMM) with a binomial error (logistic regression) was performed using the ‘lme4’ package in R (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). Study stations and plot identities were included as random factors in the model to account for potential non-independence of multiple observations at the same station, as well as the same point within a station. We tested the effect of each group of tree characteristics, microhabitat, and human presence on nest habitat selection by Turtle Dove separately. For each set of predictors, an all-inclusive design (all possible combination models) was developed using multimodel inference (Burnham and Anderson Reference Burnham and Anderson2002). Models were then ordered by increasing Akaike Information Criterion corrected for small sample sizes using AICc, and all models with ΔAICc lower than 2 were considered as equally good (Burnham and Anderson Reference Burnham and Anderson2002). Variance explained was calculated using the methods of Nakagawa and Schielzeth (Reference Nakagawa and Schielzeth2013). Marginal R2 which describes the variance explained by fixed effects, and conditional R2 which describes variance explained by the full model, were also calculated
To ensure that observations were independent of each other and to be able to subsequently address spatial autocorrelation in data before analysing them, we implemented, for each set of variables, the Moran’s index of the residuals of the best models based on AICc. For subsequent variation partitioning (VP) analyses, we retained only the variables with confidence intervals of parameter estimates not encompassing zero. VP was applied to evaluate the specific contribution of each of the three sets of predictors and their joint fractions in the selection of nest-trees. We applied VP to the final and parsimonious models, i.e., simplified models resulting from model selection (Peres-Neto and Legendre Reference Peres-Neto and Legendre2010, Assandri et al. Reference Assandri, Boglianib, Pedrinia and Brambilla2019). We tested for the significance of the unique fractions of tree characteristics, microhabitat, and human presence using the function ‘rda’ from the ‘vegan’ package (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, O’Hara, Simpson and Wagner2015). However, it was not possible to test the significance of the shared variation (Truchy et al. Reference Truchy, Göthe, Angeler, Ecke, Sponseller, Bundschuh, Johnson and McKie2019). A Kruskal-Wallis test was used to examine differences in tree height between the six study stations (Sokal and Rohlf Reference Sokal and Rohlf1981).
All statistical analyses were performed in R-3.0.2 software (R Development Core Team 2013). We used the package ‘car’ (Fox and Weisberg Reference Fox and Weisberg2011) to calculate Variance Inflation Factor (VIF), and the package ‘MuMIn’ to calculate AICc (Bartoń Reference Bartoń2015), and the marginal and conditional R2 via the function ‘rsquared.glmm’. The package ‘spdep’ was used to calculate Moran’s I autocorrelation index (Paradis et al. Reference Paradis, Claude and Strimmer2004). Model diagnostics were conducted in the R package ‘DHARMa (Hartig et al. Reference Hartig2020) (see Figures S1–S3 in the online supplementary material for details of model diagnostics). Furthermore, to plot the relationship between predictive probability of date palm tree selection and explanatory variables included in the best models, the ‘visreg’ package (Breheny and Burchett Reference Breheny and Burchett2013) was used. Means are quoted ± standard errors.
Results
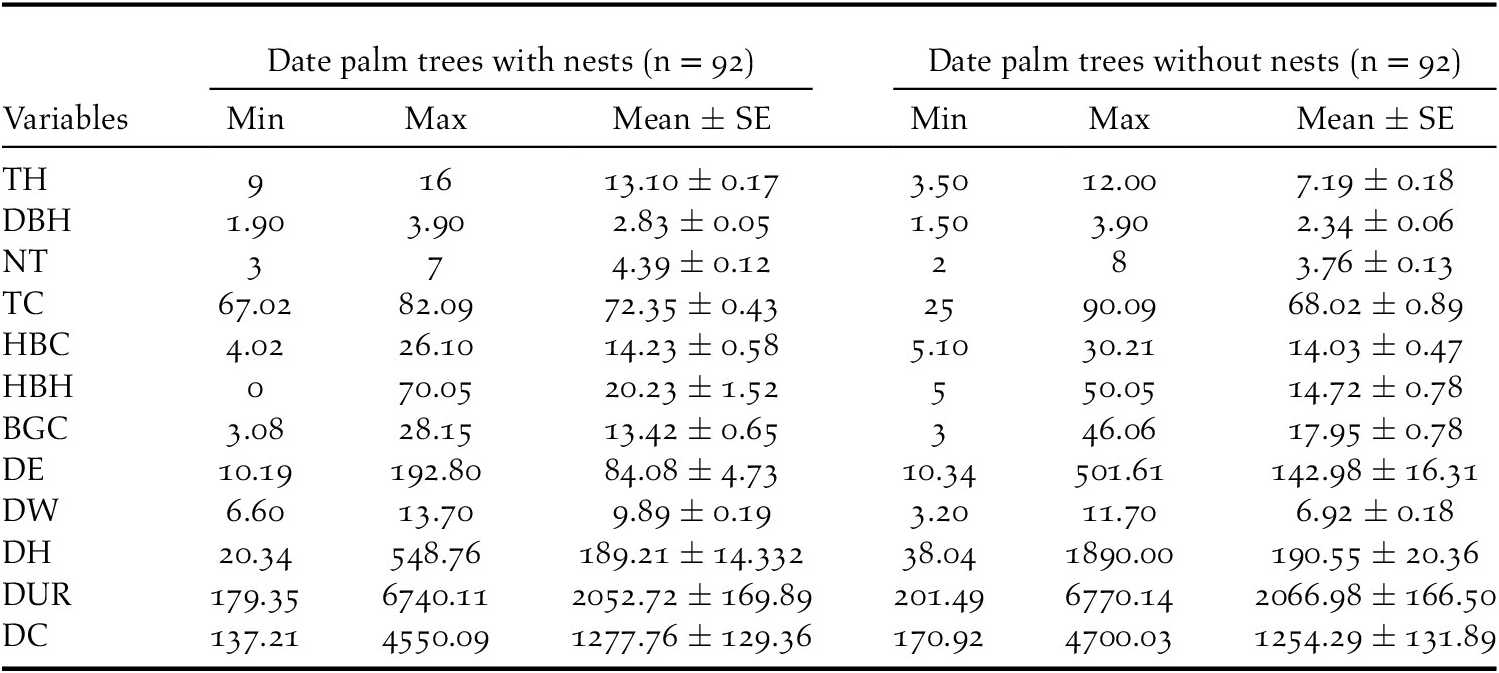
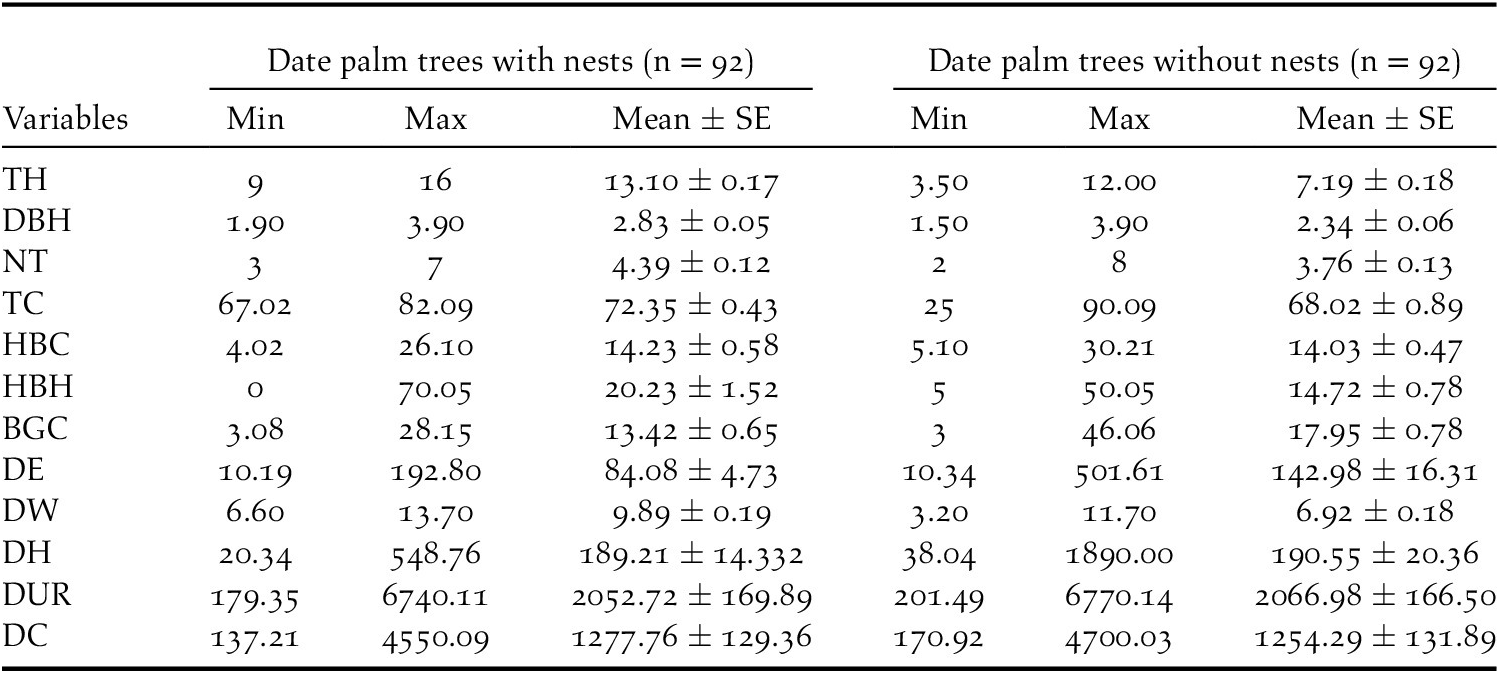
In the 2019 breeding season, 92 Turtle Dove nests were found in the 24 date palm study plots. The average characteristics of nest trees and non-nest trees are summarised in Table 2. In the Biskra region, no variation in tree height (mean = 10.15 ± 0.25; min-max = 3.5–16.0 m) was recorded across the six study stations (Kruskal-Wallis chi-squared = 2.3237, df = 5, P = 0.80), confirming a homogeinety in height of palm stands.
Table 2. Descriptive statistics for variables measured at European Turtle Dove nest and non nest trees in the Biskra’s date palm plantations, Algeria
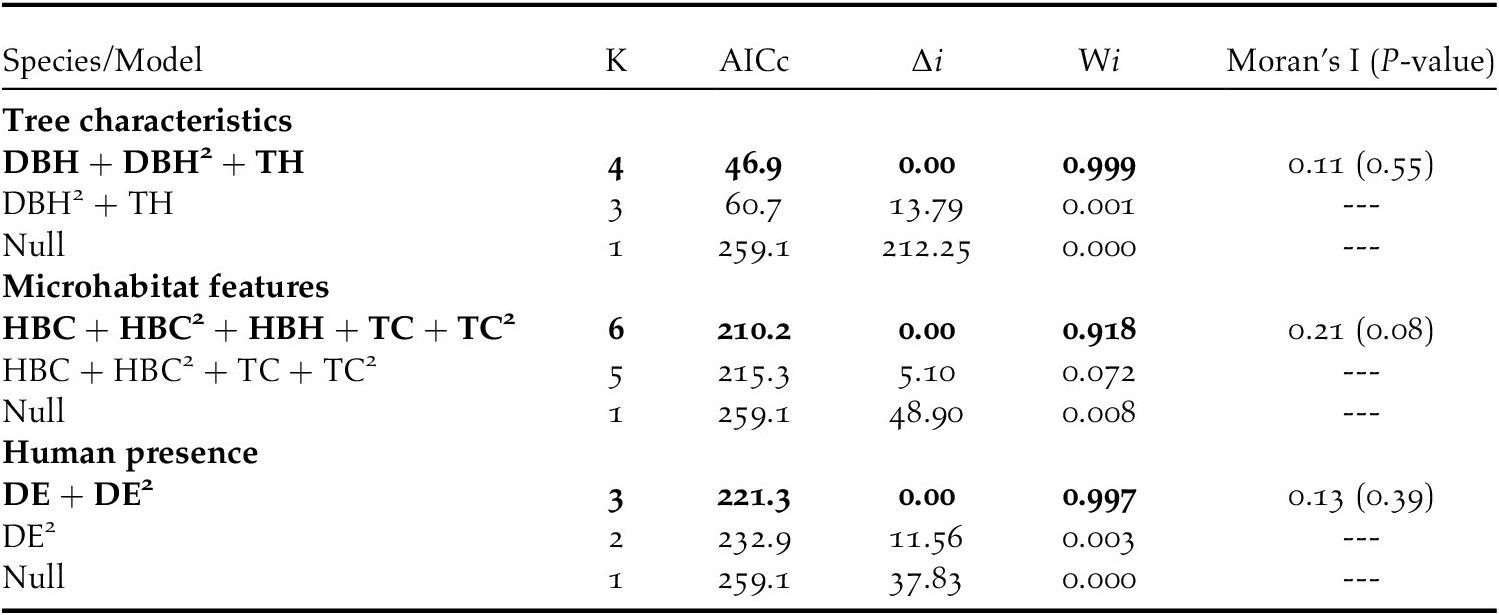
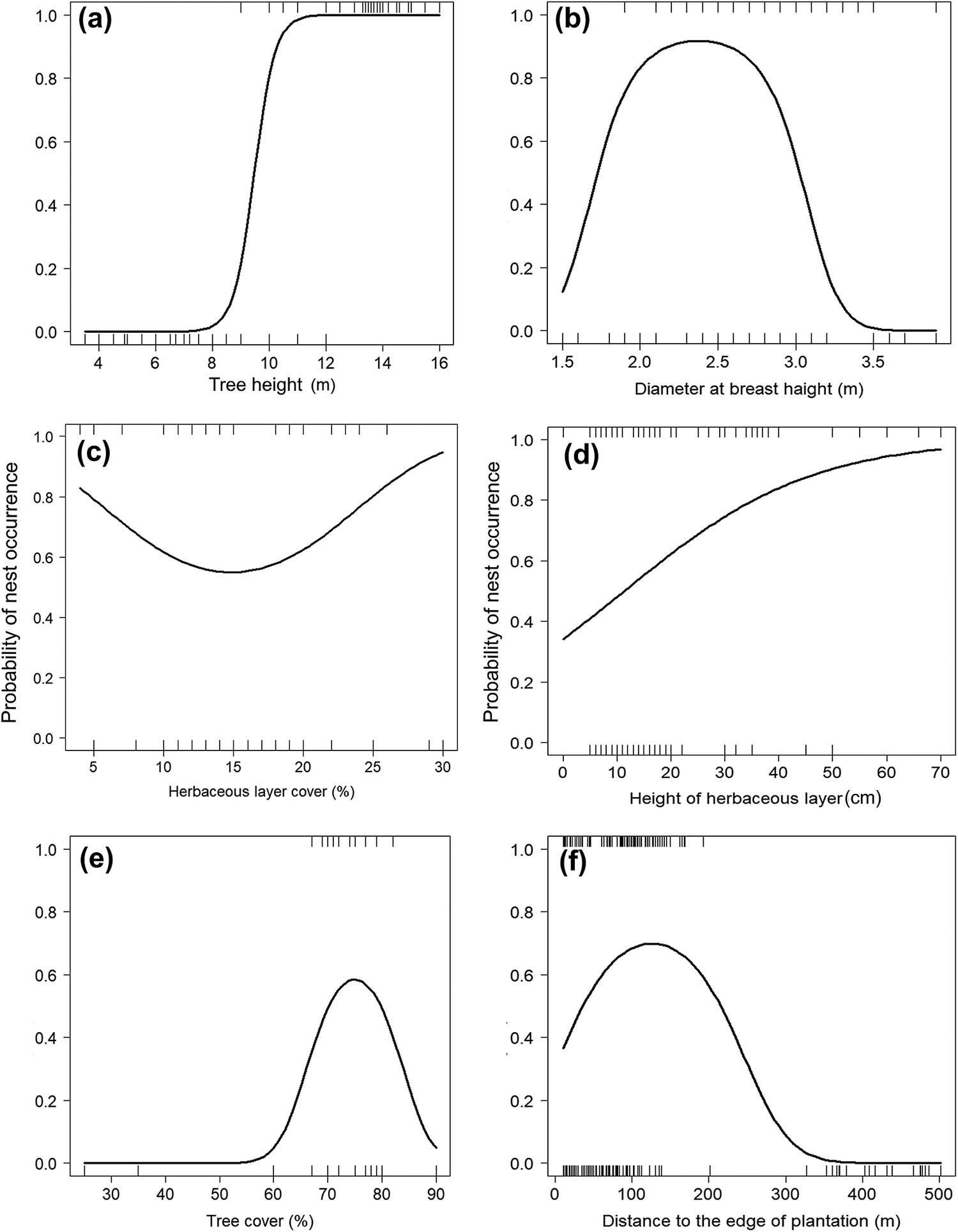
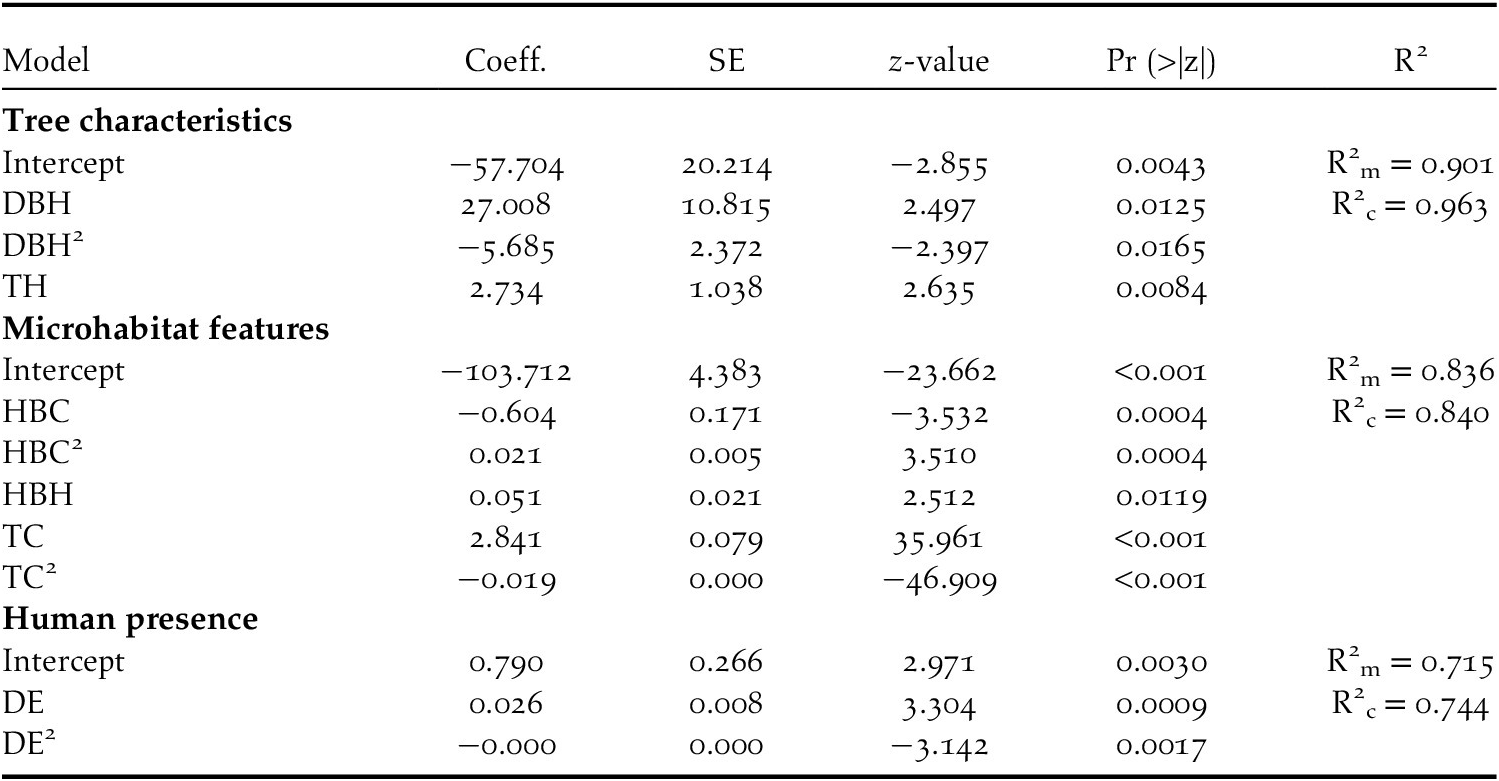
The top-ranking model assessing the influence of tree characteristics indicated that Turtle Doves specifically choose date palm trees exceeding 10 m in height (Table 3, Figure 2a) and with a DBH range of 1.5–2.5 m. Beyond this range, the occupation probability of a date palm tree by a Turtle Dove nest decreases markedly (Tables 3 and 4, Figure 2b). The variances explained by marginal and conditional R2 were both above 0.90 (Table 4), demonstrating the importance of tree characteristics in selecting nest habitat within date palm plantations. At this scale, no evidence of spatial autocorrelation in the best model’s residuals was highlighted (Table 3).
Table 3. Best model combinaisons explaining nest habitat selection by European Turtle Doves in Biskra’s date palm plantations, Algeria. Models are ranked according to Akaike’s information criterion corrected for small sample size (AICc) and only models within an interval of ΔAICc <2 are shown. The difference in AICc from the best supported model (ΔAICc), Akaike’s weights (wi), and P-value of Moran-test are also shown. See methods for details.
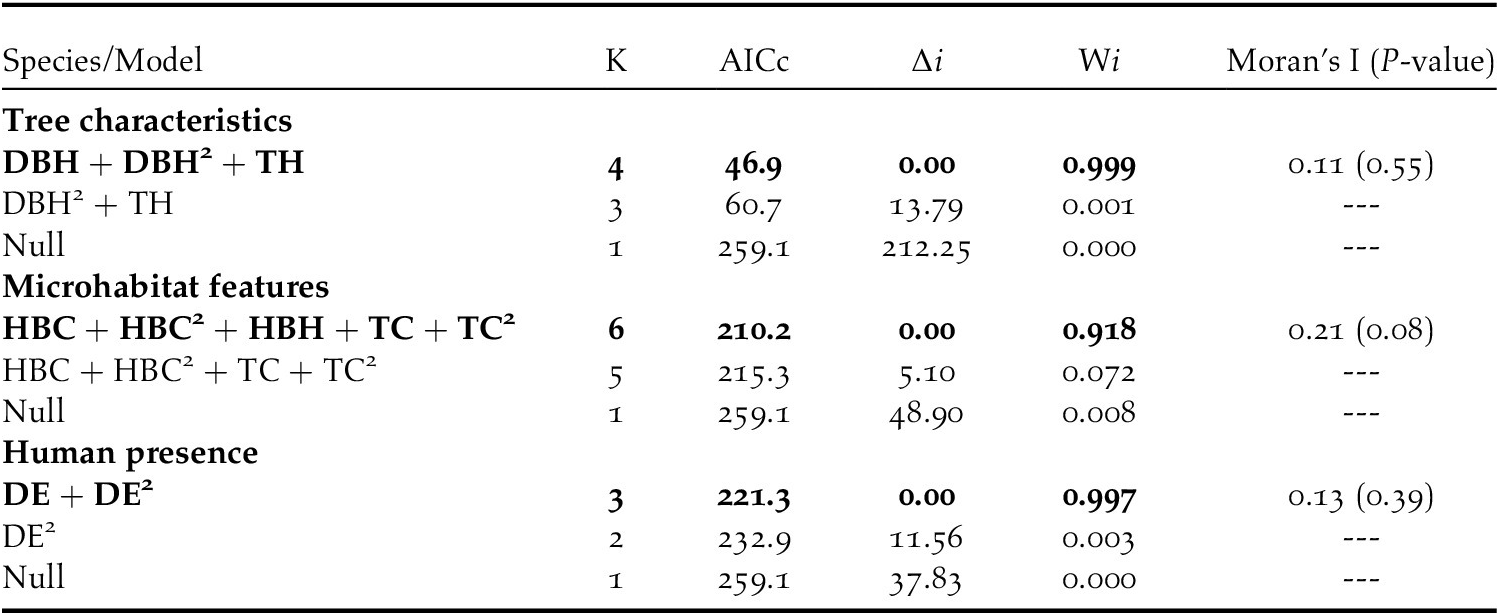
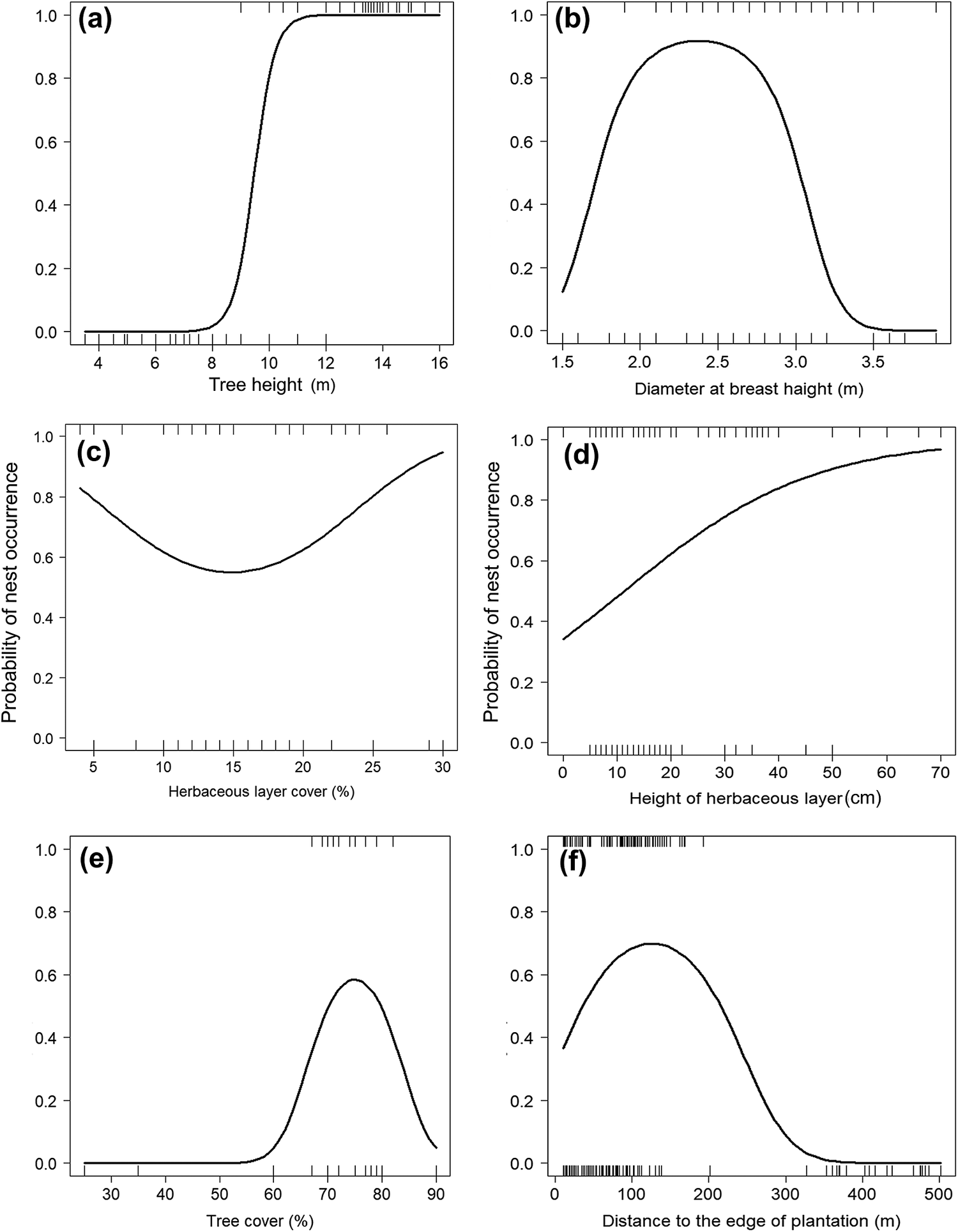
Figure 2. Turtle Dove nest occurrence probability according to tree height (a), diameter at breast height (b), herbaceous layer cover (c), height of herbaceous layer (d), tree cover (e), and distance to the edge of plantation (f) in the Biskra’s date palm plantations, Algeria.
Table 4. Parameters and standard errors (SE) of the best GLMM model for European Turtle Dove nest habitat selection in Biskra’s date palm plantations, Algeria. See Table 1 for variable acronyms
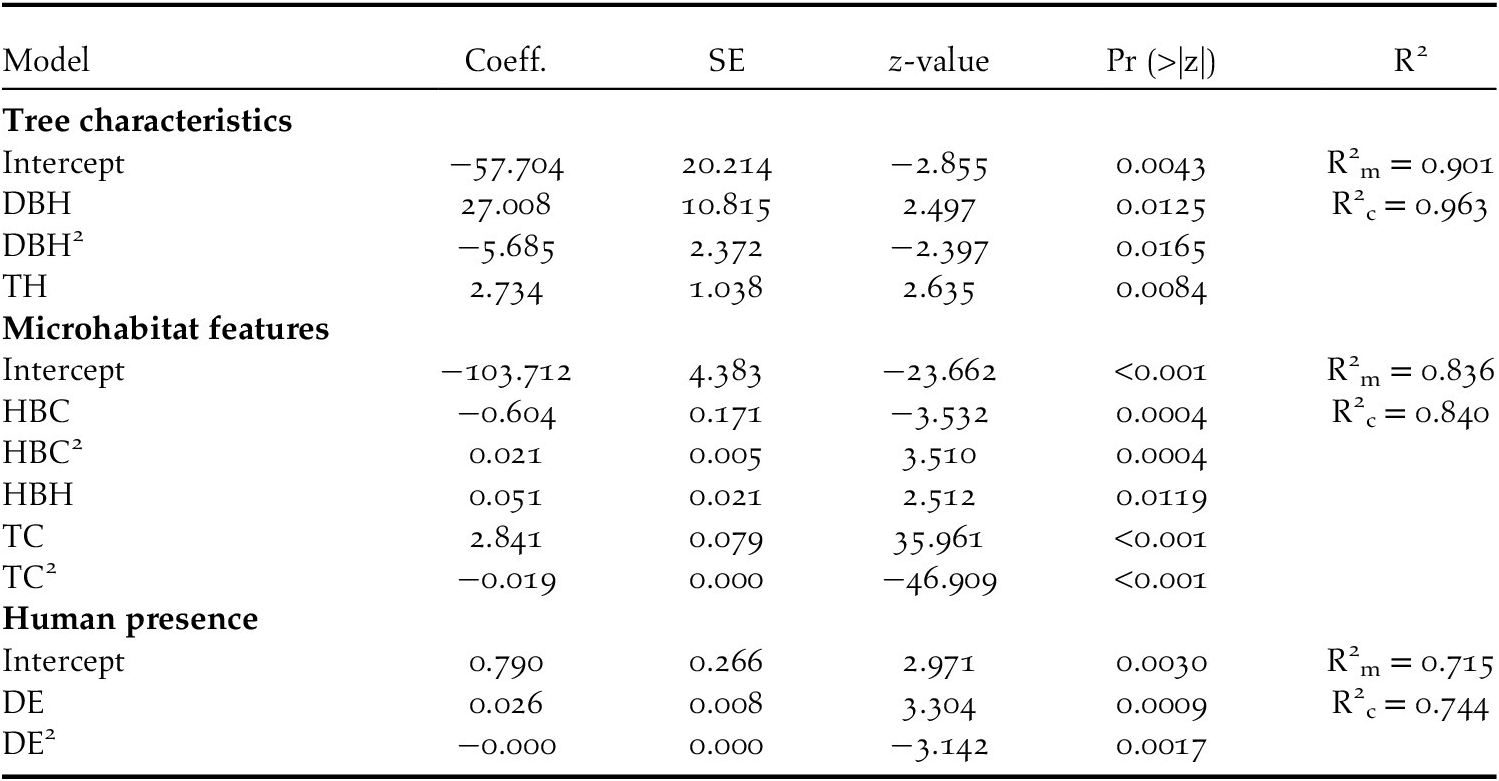
At the microhabitat scale, the Turtle Dove selects areas having a date palm tree cover ranging between 60% and 75% (Tables 3 and 4, Figure 2e). Below and beyond this range, the probability of nest occurrence decreased markedly (Figure 2e). The Turtle Dove also selects nest habitat characterised by a medium-height (<1 m) herbaceous layer (Tables 3 and 4, Figure 2d). This probability also decreased with herbaceous layer cover to about 15% and then increased after this value (Tables 3 and 4; Figure 2c). Nonetheless, even with a low herbaceous layer cover (i.e. 15% in our case), nest occurrence remained relatively high (almost 60%; Figure 2c). Although significant, the variances explained by marginal and conditional R2 did not exceed 0.84 (Table 4).
With regards to human presence, the probability of nest occurrence varied exclusively with distance to the edge of the plantation, following a quadratic relationship (Tables 3 and 4, Figure 2f). This probability increased with increasing distance to the edge of the plantation, reached an optimum around 140 m, and then decreased. The maximum variances explained by marginal and conditional R2 did not exceed 0.74 (Table 4). Regarding the spatial autocorrelation, Moran’s I P-values of the three sets of variables were above 0.05 (Table 3), suggesting the absence of spatial autocorrelation in the residuals of the three models.
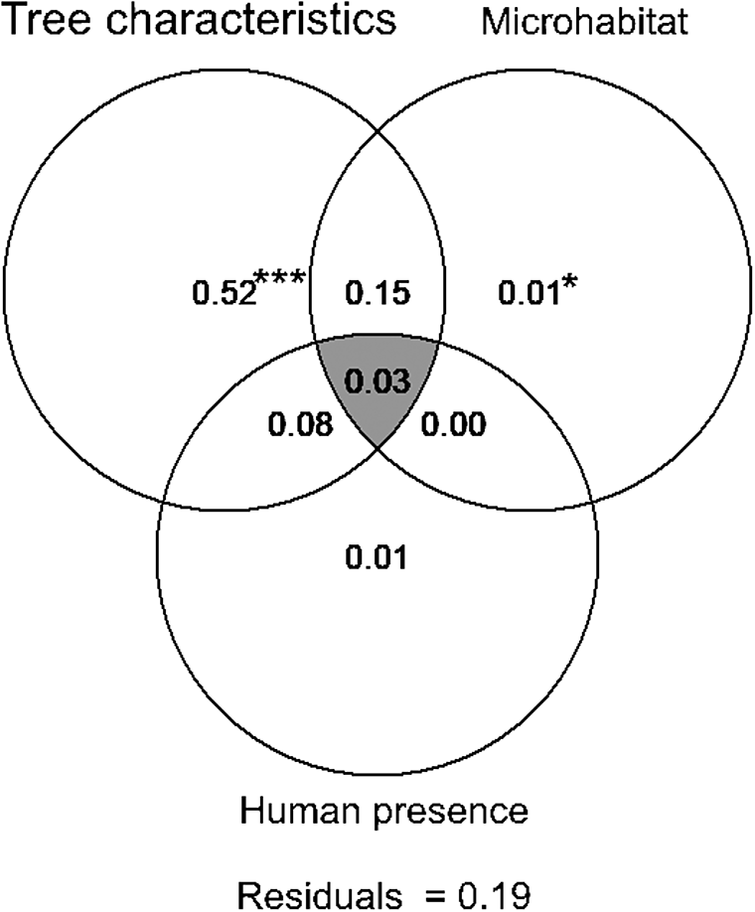
Variation partitioning highlighted the robustness of the unique effect of tree characteristics (52% of the total variance; P = 0.001) in explaining the variation in the probability of Turtle Dove nest occurrence (Figure 3). The joint effect of tree characteristics and microhabitat was also determinant (15%; Figure 3), suggesting that trees surrounded by specific microhabitat features, such as tree and herbaceous layer cover and height of herbaceous vegetation, are suitable for nesting. Although not high enough to be determining (explained variance 8%), the joint effect of tree characteristics and human presence contributes, to some extent, to variation in the Turtle Dove nest occurrence probability within date plam plantations (Figure 3). The pure effects of microhabitat (1%) and human presence (1%) contribute very weakly to this process of selecting nest habitat (Figure 3).
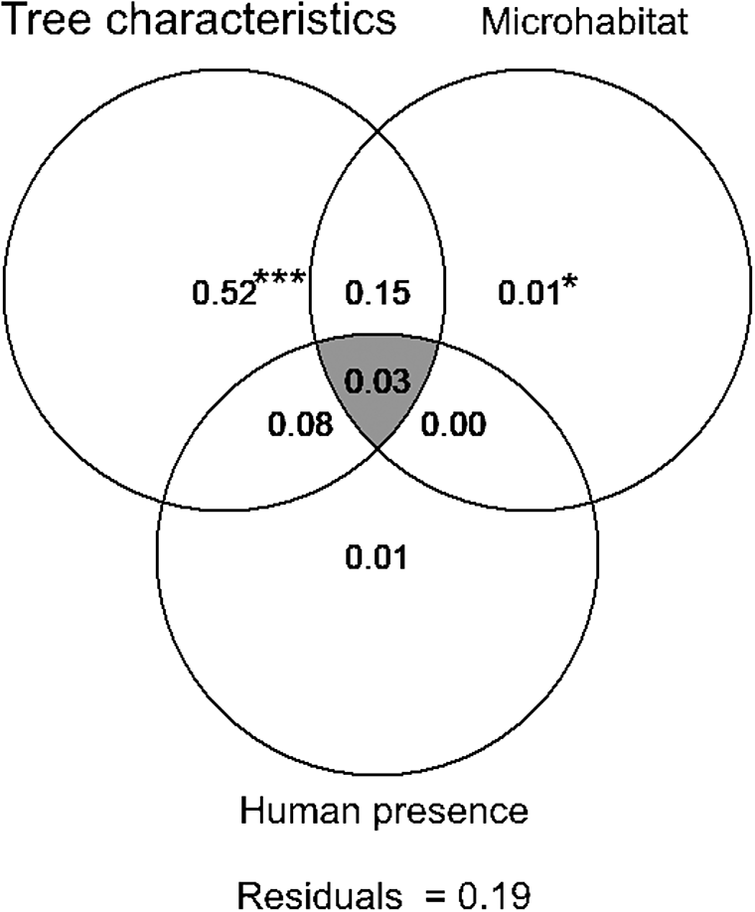
Figure 3. Venn diagram for variation partitioning showing the percentage contribution of characteristics of trees, microhabitat features, and human presence, as well as the shared amount of explained variation (intersection of ellipses), in explaining the Turtle Dove nest occurrence probability. Each number shows the fractions of variation explained by variables included in each component of scales.
Discussion
In the present study, we investigated the effects of tree characteristics, microhabitat, human presence, and space variables on the selection of nest habitat by Turtle Doves. Nest occurrence probability is positively related to the height of trees and herbaceous layer and is higher at DBH ranging between 1.5 and 2.5 m, tree cover between 60 and 75%, and distance to the edge of plantation between 20 and 140 m. To our knowledge, this is the first study that investigates the Turtle Dove nest habitat selection in the date palm plantations.
In the Biskra date palm plantations, Turtle Dove selects trees higher than 10 m in height and having a DBH between 1.5 and 2.5 m for nesting. Such characteristics of trees would protect them from (i) climbing predators, such as common genet Genetta genetta and black rat Rattus rattus (Khoury et al. Reference Khoury, Janaydeh and Al-Hmoud2009, Hanane and Baâmal Reference Hanane and Baâmal2011, Saâd et al. Reference Saâd, Hanane, Farhi and Khemis2020), (ii) human disturbance (e.g. agricultural activities: forage crops and irrigation) (De Buruaga et al. Reference De Buruaga, Onrubia, Fernandez-Garcia, Campos, Canales and Unamuno2012, Dias et al. Reference Dias, Moreira, Beja, Carvalho, Gordinho, Reino, Oliveira and Rego2013, Hanane Reference Hanane2012, 2018), and (iii) inclement weather (Sadoti Reference Sadoti2008). The selection of tall trees allows good visibility of the surroundings. Date palm trees having a DBH of more than 2.5 m are most often not occupied, likely because these trees generally have dense and tangled palm fronds that are not suitable for establishing nests. The same also applies to predators, which are often attracted by such conditions (Huhta et al. Reference Huhta, Aho, Jäntti, Suorsa, Kuitunen, Nikula and Hakkarainen2004, Seibold et al. Reference Seibold, Hempel, Piehl, Bässler, Brandl, Rösner and Müller2013). For instance, the dense and tangled palm fronds with high DBH (>2.5 m) are resting places for the Common genet (N. Saâd pers. obs.). Overall, by choosing date palm trees with a DBH between 1.5 and 2.5 m, Turtle Doves seek a trade-off that would allow reaching a good breeding success.
In this North African Saharan area, Turtle Doves occupy the date palm trees surrounded (10-m radius) by a cover of trees between 60 and 75%. This selection pattern, based on a high cover of trees, was also recorded in Greece (Bakaloudis et al. Reference Bakaloudis, Vlachos, Chatzinikos, Bontzorlos and Papakosta2009), Spain (De Buruaga et al. Reference De Buruaga, Onrubia, Fernandez-Garcia, Campos, Canales and Unamuno2012), Portugal (Dias et al. Reference Dias, Moreira, Beja, Carvalho, Gordinho, Reino, Oliveira and Rego2013), and Morocco (Hanane Reference Hanane2018). However, in the present study, a cover of trees exceeding 75% is synonymous with a weak probability of nest occurrence. Indeed, beyond this limit, conditions no longer seem to be suitable for the Turtle Doves, possibly due to obscured view around nest-trees and difficulty to escape from nest-trees for both adults and fledged young doves). Furthermore, the selection of nest habitat by this vulnerable dove species is positively affected by the herbaceous layer of up to 70 cm high. This level of height often reflects a weak human frequentation around selected nest-trees. This is true because the majority of species constituting the herbaceous vegetation are perennial, such as Bermuda grass Cynodon dactylon, cocon grass Imperata cylindrica, and purple nudsedge Cyperus rotundus. Several studies conducted in agricultural areas reported the use of less human disturbed areas by Turtle Doves in the breeding period (Peiro Reference Peiro1990, Browne and Aebischer Reference Browne and Aebischer2003, Hanane and Baâmal Reference Hanane and Baâmal2011, Hanane Reference Hanane2015, Reference Hanane2017, Reference Hanane2018). Besides, the presence of herbaceous vegetation in the neighborhood of the nest trees can contribute to satisfying the feeding requirements for Turtle Doves as shown in Portugal by Dias et al. (Reference Dias, Moreira, Beja, Carvalho, Gordinho, Reino, Oliveira and Rego2013), in Spain by Gutiérrez-Galán et al. (Reference Gutiérrez-Galán, Lopez Sanchez and Alonso2019), and in Italy by Chiatante et al. (Reference Chiatante, Porro and Meriggi2020).
In the studied agricultural area, Turtle Doves select the nesting habitats that are not far away from the edge of date palm plantations. This pattern has been previously recorded by Hanane (Reference Hanane2018) who studied the multi-scale nesting habitat selection in a Moroccan agroforestry system, and Reino et al. (Reference Reino, Beja, Osborne, Morgado, Fabiao and Rotenberry2009), who examined the effect of the forest edge on farmland birds. The configuration of the date palm plantations of the Biskra region would explain this outcome. Indeed, the presence of a livestock (goast, sheeps, and cows) stable at each date palm plantation would also contribute to satisfying the food requirements of Turtle Doves (Saâd et al. Reference Saâd, Hanane, Khemis and Farhi2021). Indeed, the daily availability of cereals, mostly barley at the time of feeding goats, sheep, and cows, turns out to be very useful for this vulnerable species. The non-relevance of distance to cereal crops to explain the occurrence of Turtle Dove nests supports our interpretation. Therefore, Turtle Doves move back and forth between stables (outside orchards) and the nesting trees (inside orchards) without spending much energy. Closer proximity to feeding areas is also known to increase food acquisition efficiency in adult doves while allowing them to spend more time caring for their broods (Pearse et al. Reference Pearse, Cavitt and Cully2004, Dunn et al. Reference Dunn, Hamer and Benton2010, Hanane Reference Hanane2015, Reference Hanane2018, Kafi et al. Reference Kafi, Hanane, Bensouilah, Zeraoula, Brahmia and Houhamdi2015). Interestingly, the statistical analysis has not highlighted a relevant role for the distance to water in Turtle Dove nest habitat selection in this Saharan agroecosystem. This last finding may be explained by the ability of the species to travel long distances to reach water points (Hanane Reference Hanane2018), or by the availability of water at the plot-scale through irrigation.
Contrary to our prediction, results of the variation partitioning analyses rather evidenced the robustness of the pure fraction of tree characteristics in explaining the probability of Turtle Dove nest occurrence within date palm plantations. The shared variation between tree characteristics and microhabitat contributes also contributes by 15% to this selection. This suggests that trees, which are surrounded by specific microhabitat features, such as tree and herbaceous layer covers and height of herbaceous vegetation, are somehow appropriate for nesting. The implication of microhabitat in selecting Turtle Dove nest habitat has also been described in Moroccan forest (Hanane Reference Hanane2018). Overall, in the date palm plantations of Biskra, it seems like Turtle Doves first of all seek to ensure a good security level to complete their reproduction successfully, which occurs at a tree height exceeding 10 m and a DBH between 1.5 and 2.5 m. In our study site, human presence was not a significant factor in terms of nest habitat selection. Such a result could have three potential explanations. First, the production of dates is the major production system in the Biskra region and almost all plantations are well-protected and access is strictly prohibited with permanent day and night presence of guards, thus making the human presence (plantation employees only) very limited. Second, a certain degree of human is tolerated in particular because of the beneficial impact of feeding goats, sheep and cows on Turtle Doves and the absence of hunting, and (iii) their adopted strategy in selecting nest trees is helps reduce the impacts of predation, disturbance, and nest predation.
Conclusions, recommendations, and perspectives
This study indicated that nest habitat selection by Turtle Doves is mainly due to tree characteristics, rather than microhabitat or human presence. In the date palm plantations of the Biskra region, Turtle Doves select the tallest trees (more than 10 m) with DBH between 1.5 and 2.5 m.
In this context, it is important to manage in an informed, sustainable way date palm plantations of the Biskra region. To reach this goal, two steps are to be considered: (1) encouraging agricultural managers and orchard owners to place a greater emphasis on ensuring the presence of high-sized class of date palm trees at each date palm plantion of this region. This remains feasible since the majority of tall date palm trees of the Biskra region are still at the begening of their production age (18 years ± 2.35, it remains more than 20 years to reach the end of date production at ideal conditions), but also because the government encourages plantation owners (by reducing water and electricty load and providing the necessary equipments) to use old date palm trees as a source of organic compost (e.g., from palm leaves and petioles), which will be very useful for the growth of the young plantations, (2) start to implant young date palm plants at each exploitation, so that in 15 years, once older date palm trees will no longer be productive, the substitute trees will be ready to accommodate the Turtle Dove nests. It goes without saying that for efficient and rapide growth of young date palm plants, it is important (1) to increase the irrigation frequency (switch to two irrigations per month instead of one), (2) to constantly check if the plants are in good condition (phytosanitary treatments if necessary), and (3) maintain date palm trees (e.g., pruning, compost). In parallel, carrying-out awareness campaigns with the local growers would also be of crucial importance in the aim to guarantee the persistence of this North African population. The implementation of the conservation measures would undoubtedly help enhance prevailing conditions in date palm plantations, which will have a good impact on the population size of this vulnerable species in this Saharan area.
It has to be recalled that our combined models explained 81% of the variance; However, despite this percentage, there are still several other non-evaluated variables that may also influence the selection of nesting habitats, including (i) food resources, (ii) predation, (iii) age and date palm tree variety, (iv) competition with other dove species, such as the laughing dove and Eurasian collared dove, and (v) landscape composition. Therefore, future research works have to understand the bio-ecology of this vulnerable dove species and enhance conservation actions. Estimating the Turtle Dove population size in this Saharan part of North Africa is a challenge that researchers should met to know whether the implementation of conservation measures has been relevant for this population. Overall, in the absence of other plantation types with the same scope, date palm plantations of Biskra remain of paramount importance as they offer good opportunities for consolidating and improving the knowledge on this threatened species at the edge of the Sahara.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S0959270921000290.
Acknowledgements
We thank the owners of the farms for kindly allowing us to work on their properties. We are grateful to Kamal Saâd for its help during the fieldwork and Tarek Moussai for elaborating the map. We also thank two anonymous reviewers and the Editor of Bird Conservation Journal for their comments and advice.