Introduction
Intrauterine and early postnatal development provide a window for metabolic programming that produces long-term effects on offspring’s health. In recent years, it has been demonstrated that maternal environment affects offspring metabolism, modulating the susceptibility of disease developmentReference Hanson and Gluckman1,Reference Harris, Baer and Stanford2 as is encompassed by Developmental Origins of Health and Disease (DOHaD).Reference Gluckman, Hanson and Buklijas3 The phenotype shaping of the developing metabolism is established through plastic processes that enable an adaptation to environmentReference Bateson, Gluckman and Hanson4 by metabolic programming,Reference Bale5 which rely on epigenetic alterations that modulate gene expression and oxidative environment.Reference Barnes and Ozanne6
We and others have demonstrated that maternal exercise during pregnancy improves brain antioxidant defense systemReference Marcelino, Longoni and Kudo7 and induces mitochondrial biogenesisReference Marcelino, Longoni and Kudo7,Reference Klein, Hoppe and Saccomori8 in 7-day-old rats, improves glucose homeostasis and insulin sensitivity in adult mice (>6 weeks old),Reference Carter, Qi, De Cabo and Pearson9 and neurogenesis in newborn ratsReference Akhavan, Emami-Abarghoie and Safari10 and young mice,Reference Bick-Sander, Steiner, Wolf, Babu and Kempermann11 as well as increases brain-derived neurotrophic factor (BDNF) levels in the brain of youngReference Akhavan, Emami-Abarghoie and Safari10 and adultReference Gomes da Silva, de Almeida and Fernandes12 rats. These findings highlight maternal exercise as a modulator of intrauterine environment that favors enhanced brain function. We recently showed that maternal exercise is associated with a high-quality pattern of maternal care during the dark cycle and with increased exploratory behavior of male and female offspring.Reference Klein, Dos Santos Rodrigues and Hozer13 On basis of these wide range of metabolic and behavioral programming effects, different groups have showed that maternal exercise during pregnancy is able to protect adult offspring against chronic metabolic dysfunction.Reference Herring, Donath and Yarmolenko14–Reference Robinson and Bucci19
It has been proposed that the physical exercise improves brain function, including in those with neurodegenerative disorders, such as Alzheimer’s disease (AD).Reference Kim, Han and Min20–Reference Vidoni, Johnson and Morris23 Although human and animal studies have shown that physical exercise during pregnancy improves several brain functions and cognitive performance of the offspring,Reference Akhavan, Emami-Abarghoie and Safari10,Reference Labonte-Lemoyne, Curnier and Ellemberg24–Reference Kim, Lee, Kim, Yoo and Kim28 few studies have addressed the beneficial effects of maternal exercise during pregnancy regarding neuroprotective role against neurodegeneration in the offspring at adult age.Reference Klein, Hoppe and Saccomori8,Reference Herring, Donath and Yarmolenko14 The hallmarks of AD are the amyloid-β (Aβ) peptide accumulation and intracellular neurofibrillary tangles that lead to neuronal damage.Reference Reitz and Mayeux29 In addition, events including oxidative stress, mitochondrial dysfunction, energetic metabolism failure, and neuroinflammation are suggested to be part of the underlying molecular mechanisms of the disease.Reference Dumont, Lin and Beal30,Reference Querfurth and LaFerla31 Increased reactive species (RS) levels and downregulation of the antioxidant system contribute to Alzheimer disease (AD) pathology.Reference Abolhassani, Leon and Sheng32,Reference Wojsiat, Zoltowska, Laskowska-Kaszub and Wojda33 Moreover, oxidative damage occurs in the brain in the presence of amyloid β oligomers (AβO), targeting proteins in a special way, resulting in loss of function.Reference Sultana, Perluigi and Butterfield34 To date, there is no curative treatment to counteract the establishment of the disease since its pathophysiological mechanisms are not fully understood. In this context, the role of the cerebellum in Alzheimer’s disease presents a new frontier of study. Recently, non-motor functions have been recognized in the cerebellum, such as emotional and cognitive associative learning control.Reference Timmann, Drepper and Frings35 Several changes related to exercise adaptive responses in central nervous system such as enhance synaptic plasticity, neurogenesis, and cognition have also been observed in the cerebellum.Reference Mattson36
In this study, we hypothesized that metabolic modulation in the cerebellum of 7-day-old rats by maternal swimming exercise persists up to young adulthood and that in the face of a challenge might be able to protect the rat offspring’s cerebellum from metabolic alterations. Therefore, we set out to investigate whether maternal swimming before and during pregnancy promotes long-lasting metabolic effects on rat offspring’s cerebellum to examine the cellular and molecular underlying mechanisms. Moreover, we investigated the potential of maternal swimming before and during pregnancy in conferring neuroprotection to young adult male rat offspring against Aβ-induced neurotoxicity.
Material and methods
Reagents
Reagents for biochemical assays were obtained from Sigma-Aldrich. Probes for flow cytometric analyses were obtained from Molecular Probes. Antibodies for Western blot were obtained from Millipore, Cell Signaling, Abcam, Santa Cruz Technologies, and GE Healthcare. All the reagents used are listed below in the techniques section.
Animals and ethical approval
Seventy-day-old female (220–260 g) and male (300–350 g) Wistar rats were obtained from in-house breeding colonies at the Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil. Rats facility was under controlled light (12:12 hour light/dark cycle), temperature (22 ± 1°C), and humidity conditions (50–60%). Adult animals were housed in four per cage (approximately 41 × 34 × 16 cm) according to experimental group, while the litter was maintained under maternal care until weaning. All animals had free access to a 20% (w/w) protein commercial chow (CR1 lab chow, Nuvilab Ltda., Curitiba, Brazil) and water.
All experimental procedures were approved by the local Ethics Commission on the Use of Animals (Comissão de Ética no Uso de Animais – CEUA/UFRGS) and were performed in accordance with the National Animal Rights Regulations (Law 11.794/2008), the American National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996), and the Directive 2010/63/EU. ARRIVE guidelines were followed in the preparation of the manuscript. All efforts were made to minimize the number of animals used and their suffering. The number of animals used in each experiment is indicated in the figures captions.
Experimental design
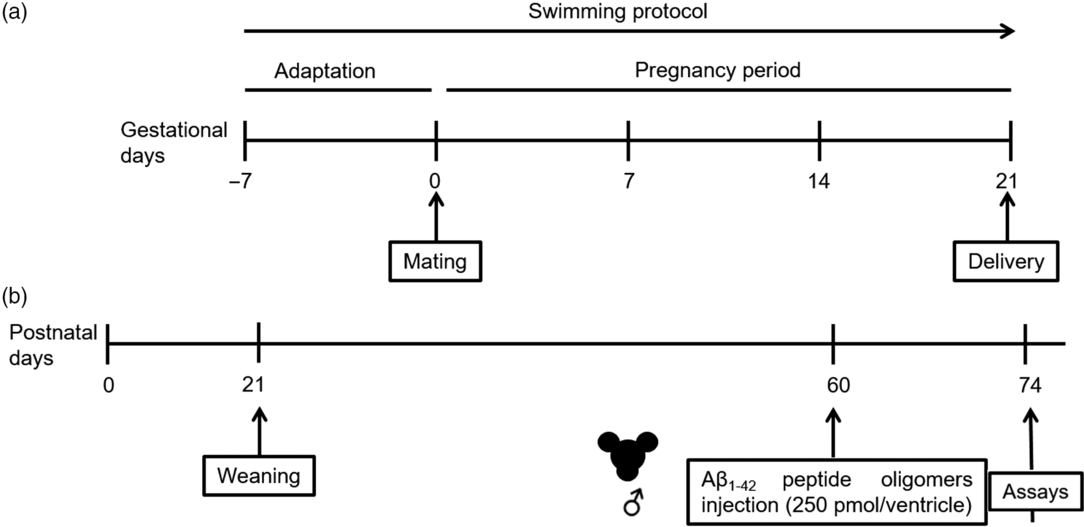
The study design is depicted in Fig. 1. The animals were allocated into different experimental groups by simple randomization. Adult female rats were initially divided into two groups: (1) sedentary control dams, group in which the rats were exposed to aquatic environment stress without exercising and (2) maternal exercise dams, group in which the rats were subjected to a swimming protocol in a pool (30 width × 30 length × 90 depth cm) filled with water at 32 ± 1°C.Reference Marcelino, Longoni and Kudo7 The animals underwent involuntary swimming, 30 min daily (performed between 9 am and 12 pm) for 4 weeks, 5 days/week, 1 week for adaptation to aquatic environment prior to mating, and during entire pregnancy period.Reference Marcelino, Longoni and Kudo7 At the end of first week, two females were mated with one male, and the pregnancy was confirmed by the presence of a vaginal plug or sperm in the vaginal fluid. The pregnant rats underwent the exercise protocol during the entire pregnancy. The animals were left free to swim and were gently stimulated to swim when necessary. After all rats swam, control rats were immersed in water lasting 10 s, carefully dried, and returned to the housing boxes. Beginning on the 20th gestational day, the dams were housed individually and observed twice a day (8 am and 6 pm) to verify litter’s birth. The offspring’s birth was considered postnatal day (PD) 0. Within 24 h after delivery, the litter was adjusted to a number of eight pups per dam. From delivery (PD 0) until weaning (PD 21), each dam was housed together with its litter. On PD 21, female littermates were euthanized, and male littermates were housed in four per cage until the PD 60. On 60 days of age, male rat offspring were subjected to a surgical procedure for bilateral microinfusion of AβO or vehicle into the brain ventricles. Thus, the offspring was subdivided into the following groups: (1) sedentary + vehicle (control group), (2) maternal exercise + vehicle, (3) sedentary + AβO, and (4) maternal exercise + AβO. On the 14th day post-surgery, the rats were euthanized by decapitation for evaluation. The experimenters were blinded at the time of sample preparation, data analysis, and calculation. No animal died or were excluded.

Fig. 1. Experimental design. (a) Maternal swimming protocol and (b) offspring’s timeline.
Aβ1-42 peptide oligomers preparation
Soluble AβO were prepared according to Klein.Reference Klein37 The Aβ (sequence 1-42) peptide (American Peptide Co., Sunnyvale, CA, USA) was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (Sigma-Aldrich, St. Louis, MO, USA) in order to form monomers of Aβ peptide. After incubation lasting 1 h at room temperature and 10 min on ice, the solution was aliquoted, and the tubes containing the Aβ peptide were maintained overnight in the hood to remove HFIP followed by centrifugation in a SpeedVac system to obtain a clear film of monomeric Aβ peptide at the bottom of the tubes, which were stored at −80°C. Twenty-four hours before in vivo infusion, aliquots were solubilized in dimethyl sulfoxide (DMSO), diluted in phosphate buffer saline (PBS) pH 7.4, and incubated at 5°C for 24 h. After incubation, the tubes were centrifuged at 14,000g for 10 min at 4°C, and the AβO-containing supernatants were transferred to a new tube. The vehicle contained DMSO + PBS without any protein. Protein concentration was measured using the Pierce BCA Protein Assay Kit (Thermo Fischer), and structural characterization was performed using the specific Aβ 1-16 antibody 6E10 (Covance) through western blotting.
Surgical procedure for AβO infusion
On the PD 60, young adult male rat offspring were anesthetized (100 mg/kg ketamine and 15 mg/kg xylazine, i.p.) and then placed in a stereotaxic frame to the following surgical procedure previously described by Hoppe et al.Reference Hoppe, Coradini and Frozza38 Two trained experimenters performed the surgical manipulation. Using sterile surgical instruments, a mid-sagittal incision was made in the scalp, and bilateral holes were drilled in the skull using a dental drill over the lateral ventricles, according to Paxinos and Watson atlas coordinates: 0.8 mm posterior to bregma and 1.5 mm lateral to the sagittal suture, and the depth of the microinfusion consisted in 3.5 mm beneath the surface of brain.Reference Paxinos and Watson39 Rats received a single intraventricular infusion of 5 µL bilaterally of Aβ peptide oligomers (500 pmol/rat), and control rats received bilateral infusions into each lateral ventricle of equal volume of PBS and 2% DMSO. The microinfusions were performed using a 10 µL Hamilton syringe fitted with a 26-gauge needle in an infusion rate of 1 µL/min over a period of 5 min. At the end of infusion, the needle was left in place for an additional 3 to 5 min before being slowly withdrawn to allow diffusion from the tip and prevent reflux of the solution. After the infusion, the scalp was sutured, and the animals were allowed to recover from the anesthesia on a heating pad to maintain body temperature at 37.5 ± 0.5°C.
Sample preparation for biochemical assays
Rats were euthanized 14 days after the AβO infusion by decapitation, without anesthesia in order to avoid any interference of anesthetic on biochemical assays. For redox state parameters assessment, the cerebellum was rapidly dissected, weighed, and immediately frozen (−80°C) until homogenization (1:10 w/v in 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM KCl). After being homogenized, the samples were centrifuged at 750g for 10 min at 4°C to obtain the supernatant containing cytosol, mitochondria, and other organelles. The assays were performed in duplicate using SpectraMax microplate reader (Molecular Devices, Multi-Mode Analysis Software).
Antioxidant enzymes activities
To examine the endogenous enzymatic antioxidant system, we measured the activity of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). The specific activities of antioxidant enzymes were expressed as U/mg protein.
SOD (EC 1.15.1.1) activity was assayed according to Misra and Fridovich,Reference Misra and Fridovich40 with the assay adapted for 96 wells microplates. We measured the total SOD activity by quantifying the inhibition of superoxide-dependent epinephrine autoxidation at 480 nm at 32°C for 10 min. SOD activity is expressed as the amount (equal to 1 unit) of enzyme that inhibits the oxidation of epinephrine by 50%.
CAT (EC 1.11.1.6) activity was assayed according to Aebi,Reference Aebi41 with the assay adapted for 96 wells microplate. We measured the CAT activity in the sample by the consumption of hydrogen peroxide added to the reaction medium (containing 20 mM H2O2, 0.1% Triton X-100, and 10 mM potassium phosphate buffer, pH 7.0). The decrease in the absorbance at 240 nm was measured using a UV bottom microplate. The CAT unit is defined as 1 µmol of H2O2 consumed per minute at 37°C for 3 min.
GPx (EC 1.11.1.9) activity was assayed according to Wendel,Reference Wendel42 with the assay adapted for 96 wells microplate. We measured the GPx activity in the sample in a reaction medium containing 100 mM potassium phosphate buffer, pH 7.7, containing 1 mM EDTA, 2 mM reduced glutathione (GSH), 0.15 U/mL glutathione reductase (EC 1.8.1.7), 0.4 mM azide, 0.1 mM NADPH, and 0.5 mM tert-butyl hydroperoxide as enzyme substrate. NADPH disappearance was monitored at 340 nm at 25°C for 3 min. The GPx unit is defined as 1 µmol of NADPH consumed per minute.
Reduced GSH content
To examine the most important endogenous nonenzymatic antioxidant in the brain, we measured the content of GSH, whose concentration was measured according to Browne and Armstrong,Reference Browne and Armstrong43 with the assay adapted for 96 wells microplate. Initially, the proteins in supernatant were precipitated with meta-phosphoric acid (1:1, v/v), and after centrifugation, the GSH present in the supernatant was allowed to react with 7.5 mM fluorophore o-phtaldialdehyde present in the reaction medium in addition to 100 mM sodium phosphate buffer, pH 8.0, containing 5 mM EDTA. The fluorescence was measured at excitation and emission wavelengths of 350 and 420 nm, respectively. Standard GSH curve ranging from 0.001 to 1 mM was prepared and a blank sample was performed in parallel. Data are expressed as nmol of GSH/mg protein.
Thiol content
Thiol (SH) content was measured according to Aksenov and Markesbery,Reference Aksenov and Markesbery44 with the assay adapted for 96 wells microplate. The thiol content present in the sample reduces the 50-dithiobis-(2-nitrobenzoic acid) (DTNB), which become oxidized (disulfide), generating a yellow derivative (TNB). The absorbance was measured at 412 nm in a reaction medium containing 20 mM sodium phosphate buffer, pH 7.4, and 10 mM DTNB prepared in a 0.2 M potassium phosphate solution, pH 8.0. Data are expressed as nmol TNB/mg protein.
Carbonyl content
Protein carbonyl content was measured according to Reznick and Packer,Reference Reznick and Packer45 with the assay adapted for 96 wells microplate. Protein carbonyls react with dinitrophenylhydrazine forming dinitrophenylhydrazone, a yellow compound that was measured at 370 nm. Data are expressed as nmol of carbonyls/mg protein.
Respiratory system complexes activities
Rats were euthanized 14 days after the AβO injection by decapitation, without anesthesia in order to avoid tissue chemical contamination. The cerebellum was rapidly dissected, weighed, and immediately frozen (-80°C) until homogenization. The cerebellum was homogenized (1:20 w/v) in SETH buffer, pH 7.4, that contains 250 mM sucrose, 2.0 mM EDTA, 10 mM Trizma base, and 50 IU/mL heparin and centrifuged at 800g for 10 min at 4°C. The pellet was discarded, and the supernatant was collected and submitted to three subsequent freeze–thawing.Reference Grings, Moura and Parmeggiani46 Mitochondrial respiratory chain enzyme activities were measured in homogenates with a protein concentration varying from 1.5 to 5.0 mg protein/mL. The assays were performed in duplicate using SpectraMax microplate reader (Molecular Devices, Multi-Mode Analysis Software).
Succinate-2,6-dichloroindophenol (DCIP)-oxidoreductase (complex II) (EC 1.3.99.1) activity was determined according to the method of Fischer et al.Reference Fischer, Ruitenbeek and Berden47 adapted for 96 wells microplate. Complex II enzyme activity in the sample was measured in a reaction medium containing 62.5 mM potassium phosphate buffer, pH 7.4, 250 mM succinate, and 0.5 mM DCIP, at 30°C for 10 min. Complex II activity was determined following the decrease in absorbance due to reduction of DCIP at 600 nm. The activity of complex II is expressed as nmol/min/mg protein.
Cytochrome c oxidase (complex IV) activity was measured according to Rustin et al.Reference Rustin, Chretien and Bourgeron48 adapted for 96 wells microplate. Complex IV enzyme activity in the sample was measured in a reaction medium containing 10 mM potassium phosphate buffer, pH 7.0, 125 mM n-dodecil-β-D-maltoside, and 1% cytochrome c. Complex IV activity was determined following the decrease in absorbance due to reduced cytochrome c oxidation at 550 nm at 30°C for 5 min. The activity of complex IV is expressed as nmol/min/mg protein.
Protein determination
Protein concentration (mg of protein/mL) was measured according to the method described by Lowry et al.,Reference Lowry, Rosebrough, Farr and Randall49 which was adapted for 96 wells microplate, using bovine serum albumin as standard. The absorbance was measured at 750 nm.
Flow cytometry
Flow cytometric analysis was conducted according to Marcelino et al.Reference Marcelino, Longoni and Kudo7 Briefly, the tissue samples (approximately 100 mg) were dissociated in PBS containing 1 mg% of colagenase IV (Sigma-Aldrich, St. Louis, MO, USA, catalog number C5138) and 0.5 mg% of DNase (Sigma-Aldrich, St. Louis, MO, USA, catalog number D5025), filtered using the 40 µm pore size cell strainer (SPL Lifesciences Co., Naechon-Myeon Pocheon, South Korea), and then incubated at 37°C with the molecular probes. Reactive oxygen and nitrogen species levels were measured using 10 µM 2′,7′-dichlorofluorescin diacetate (H2DCF-DA; Sigma-Aldrich, St. Louis, MO, USA, catalog number D6883), nitric oxide (NO•) levels were measured using 10 µM 4-amino-5-methylamino-2ʹ,7ʹ-difluorofluorescein diacetate (DAF-FM; Invitrogen, Molecular Probes, Eugene, OR, USA, catalog number D23841), mitochondrial superoxide were measured using 1 µM MitoSOX® Red (Invitrogen, Molecular Probes, Eugene, OR, USA, catalog number M36008), mitochondrial mass and membrane potential were measured using 100 nM MitoTracker® Green and 100 nM MitoTracker® Red (Invitrogen, Molecular Probes, Eugene, OR, USA, catalog numbers M7514 and M7513), respectively. Cells were gated based on the FSC and SSC pattern of the sample cells, and 30,000 events were acquired per sample in a BD FACSCalibur Flow Cytometry System; a non-labeled sample was used as negative fluorescent control. DCFH, DAF-FM, and MitoSOX data are expressed as mean fluorescence intensity (MFI), and MitoTracker® Green and MitoTracker® Red data are expressed as percentage of double-positive cells. Data were analyzed using the software FlowJo (Tree Star, Ashland, OR, USA).
Western blot assay
Cerebellum is homogenized in ice-cold lysis buffer containing 4% sodium dodecyl sulfate (SDS), 2 mM EDTA, 50 mM Tris, and 1% protease inhibitor cocktail. The homogenates were denatured 100°C for 5 min and then centrifuged at 10,000g for 30 min. After this, the supernatant containing the cytosolic fraction was collected, β-mercaptoethanol was added to a final concentration of 5%, and then the samples were stored at −80°C until use. Equal concentration of protein (50 μg) was loaded and immunodetected as previously described.Reference Hoppe, Coradini and Frozza38 Membranes were incubated for 60 min at 4°C in blocking solution (Tris-buffered saline containing 5% non-fat milk and 0.1% Tween-20, pH 7.4) prior the incubation with the primary antibody. Membranes were incubated overnight at 4°C in blocking solution containing one of the following primary antibodies: rabbit monoclonal anti-synaptophysin (1:2000 dilution, Millipore, catalog number #AB9272), anti-PSD-95 (1:1000 dilution, Cell Signaling, catalog number #2507), anti-sirtuin 3 (SIRT3) (1:1000 dilution, Abcam, catalog number #ab189860), anti-mitofusin 1 (1:1000 dilution, Abcam, catalog number # ab104274), anti-dinamin-related protein 1 (1:1000 dilution, Abcam, catalog number #ab154879), anti-phospho(S9)-glycogen synthase kinase 3β (GSK-3β) (1:1000 dilution, Cell Signaling, catalog number #9336), anti-GSK-3β (1:1000 dilution, Cell Signaling, catalog number #9315), anti-NOS2 (1:250 dilution, Santa Cruz Technologies, catalog number #sc-7271), anti-phospho(S396)-Tau (1:1000 dilution, Cell Signaling, catalog number #9632), anti-Tau (1:1000 dilution, Cell Signaling, catalog number #4019), and rabbit monoclonal anti-β-actin (1:2000 dilution, Cell Signaling, catalog number #4967). After washing, the membranes were incubated with secondary antibody containing peroxidase-conjugated anti-rabbit IgG (1:1000 dilution, GE Healthcare Life Sciences, catalog number #NA934V) and anti-mouse IgG (1:1000 dilution, GE Healthcare Life Sciences, catalog number #NA931V) for 1 h. The chemiluminescence was detected using the digital imaging system Image Quant LAS 4000 (GE Healthcare Life Sciences) and analyzed using the Image J Software. The average optical density for the control group was designated as 100%.
Statistical analyses
The sample size of the study was calculated according to previous works performed in the lab (with α = 0.05, β = 0.20) using the Minitab 16 software (Minitab Inc., State College, PA, USA). Data are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using the GraphPad Prism 6.0 software. All data were tested for normality using Shapiro–Wilk test. Data points outside the 95% confidence interval were treated as outliers and excluded from the data analysis. Two-way analysis of variance (ANOVA) was used to analyze the effect of the two independent variables, maternal exercise, and AβO infusion. Post hoc analysis was carried out using Tukey’s test. Main effects were considered significant if p ≤ 0.05.
Results
Maternal exercise prevents the increase of AβO-induced RS levels
Concerning overall RS measured by DCFH oxidation, two-way ANOVA indicated an effect of maternal exercise (p = 0.013), and Tukey post hoc test indicated that sedentary + AβO group differs from the other groups. Two-way ANOVA also indicated an interaction between maternal exercise and AβO infusion (p = 0.027), indicating that AβO infusion induced an increase of RS levels and that maternal exercise was able to prevent completely such rise (Fig. 2a). Nitric oxide levels were not altered by either maternal exercise (p = 0.473) or AβO infusion (p = 0.653) in the cerebellum of young adult male rat offspring (Fig. 2b). Mitochondrial superoxide levels were not altered by any treatment in the cerebellum of young adult offspring (Fig. 2c). Neither maternal exercise (p = 0.304) nor AβO (p = 0.377) altered cerebellar mitochondrial superoxide levels. Taking together, these results indicated maternal exercise during pregnancy is able to prevent overall RS levels increase induced by AβO infusion, which is mediated mainly via elevation in peroxides, hydroxyl radicals, or peroxynitrite, and not by nitric oxides or mitochondrial superoxide.

Fig. 2. Effects of maternal exercise and AβO infusion on offspring’s cerebellar reactive species levels. (a) Representative histograms of DCFH, DAF-FM, and MitoSox mean fluorescence intensity (MFI). Bars show mean + standard error (n = 6 rats in each group) of (b) DCFH oxidation MFI, (c) DAF-FM MFI, and (d) MitoSOX Red MFI. Two-way ANOVA showed a main effect of maternal exercise and an interaction between maternal exercise and AβO on DCFH oxidation. * P<0.05 sedentary + AβO compared to other groups (indicated by Tukey post hoc test).
Maternal exercise increases GSH levels in young adult male offspring’s cerebellum
Two-way ANOVA indicated a significant effect of maternal exercise on GSH levels (p = 0.0005), which was found increased in the young adult male offspring’s cerebellum (Fig. 3a). No effect of AβO infusion was found on GSH concentration (p = 0.727). Two-way ANOVA indicated that both factors, maternal exercise and AβO infusion, exerted no effect on activities of the enzymes SOD (Fig. 3b; p = 0.470 and p = 0.459, respectively), GPx (Fig. 3c; p = 0.303 and p = 0.107, respectively), and CAT (Fig. 3d; p = 0.170 and p = 0.412, respectively).

Fig. 3. Effects of maternal exercise and AβO infusion on offspring’s cerebellar antioxidant system and oxidative damage. (a) Reduced glutathione (GSH) content; (b) activity of superoxide dismutase (SOD); (c) glutathione peroxidase (GPx); (d) catalase (CAT) enzymes; (e) thiol; and (f) carbonyl contents. Values are expressed as mean + standard error (n = 8 to 11 rats per group for each parameter). Two-way ANOVA showed an effect of maternal exercise on GSH, thiol, and carbonyl contents and a main effect of AβO infusion on carbonyl content. *P<0.05, **P<0.01, and ***P<0.001 effects of maternal exercise (two-way ANOVA), # P<0.05 effect of AβO infusion (two-way ANOVA).
Increased protein carbonylation elicited by AβO is prevented by maternal exercise
Two-way ANOVA indicated an effect of maternal exercise on thiol content (p = 0.029), which was found increased in the offspring’s cerebellum (Fig. 3e); AβO infusion exerted no effect on thiol content (p = 0.532). Concerning carbonyl levels, two-way ANOVA indicated an effect of both maternal exercise (p = 0.002) and AβO infusion (p = 0.028). AβO infusion increased carbonyl levels, while maternal exercise reduced carbonyl levels (Fig. 3f).
Effects of maternal exercise and AβO infusion on mitochondrial parameters
Two-way ANOVA indicated a main effect of maternal exercise on CII (Fig. 4a; p = 0.001) and compled IV (CIV) (Fig. 4b; p = 0.001), evidenced by increased activities and no effect of AβO infusion on both enzymes activities, CII (p = 0.197) and CIV (p = 0.419).

Fig. 4. Effects of maternal exercise and AβO infusion on mitochondrial function in the cerebellum of adult offspring. (a) Complex II, (b) Complex IV enzymes activities, (c) percentage of double-positive Mitotracker Green and Mitotracker Red labeled cells, and (d) representative dot plots of Mitotracker Green and Red-immunolabeled cells. Values are expressed as mean + standard error (n = 6 rats in each group for each parameter). A two-way ANOVA showed an effect of maternal exercise on these parameters. **P<0.01 effect of maternal exercise (two-way ANOVA).
In agreement with the effect of maternal swimming, which activated the mitochondrial complexes, two-way ANOVA indicated a main effect of maternal exercise on MitoTracker Green and Red double-positive cells (p = 0.041), indicating increased mitochondrial mass and membrane potential. In addition, mitochondrial mass and membrane potential were found unchanged by AβO infusion (p = 0.141). It is important to note that AβO infusion did not abolish the effect promoted by maternal exercise on mitochondrial function (Fig. 4c and 4d).
Two-way ANOVA indicated an interaction between maternal exercise and AβO infusion on mitofusin 1 (MFN1) immunocontent in the young adult male offspring’s cerebellum (Fig. 5a) (p = 0.037). Maternal exercise increased offspring’s cerebellar MFN1 levels; however, AβO infusion abolished the effect promoted by maternal exercise. Concerning cerebellar dynamin-related protein 1 (DRP1) immunocontent (Fig. 5b), two-way ANOVA indicated a main effect of AβO infusion (p = 0.042), and a borderline significant effect of maternal exercise on DRP1 levels (p = 0.055). These data indicate that AβO infusion increases DRP1 levels in the offspring’s cerebellum, and rise of DRP1 levels in maternal exercised + AβO group reached values similar to control group.

Fig. 5. Effects of maternal exercise and AβO infusion on mitochondria-related proteins levels in the cerebellum of adult offspring. Immunocontent of (a) mitofusin 1 (MFN1; n = 8 to 9 rats per group), (b) dynamin-related protein 1 (DRP1; n = 8 to 9 rats per group), and (c) SIRT3 (n = 6 rats in each group) expressed as average percentage of control. Representative quantification of proteins immunocontent normalized to b-actin protein (loading control) is shown below the graphs. Values are expressed as mean + standard error. A two-way ANOVA showed interaction between maternal exercise and AβO infusion for mitofusin (p<0.05). *P<0.05 effect of maternal exercise (two-way ANOVA), #P<0.05 effect of AβO infusion (two-way ANOVA).
Two-way ANOVA indicated a main effect of maternal exercise on SIRT3 levels (Fig. 5c; p = 0.039), increasing SIRT3 immunocontent in the cerebellum of young adult male offspring. No effect of AβO infusion was found (p = 0.739). Interestingly, it was observed that AβO infusion did not abolish the SIRT3-mediated adaptive ability of mitochondria to maternal exercise.
Maternal exercise prevented the increment of iNOS immunocontent elicited by AβO infusion in the cerebellum of young adult male offspring
The results concerning the immunocontent of inducible nitric oxide sintase (iNOS) in the offspring’s cerebellum, two-way ANOVA indicated an effect of both maternal exercise and AβO infusion (Fig. 6c). While maternal exercise reduced iNOS levels (p = 0.015), AβO infusion increased iNOS levels (p = 0.008). Consequently, the levels of iNOS in maternal exercised + AβO group reached values similar to control group, suggesting a possible neuroprotective effect.

Fig. 6. Effects of maternal exercise and AβO infusion on synaptic proteins and inducible nitric oxide sintase (iNOS) in the cerebellum of adult offspring. Immunocontent of synaptic proteins (a) synaptophysin (n = 7 to 9 rats per group) and (b) post-synaptic density-95 protein (PSD95) (n = 7 to 8 rats per group). Immunocontent of (c) iNOS (n = 7 to 9 rats per group), (d) phospho-GSK-3β/GSK-3β ratio (n = 6 rats in each group), and (e) phospho-Tau/Tau ratio (n = 8 to 9 rats per group). Data are expressed as the average percentage of control. Representative quantification of proteins immunocontent normalized to b-actin protein (loading control) is shown below the graphs. A two-way ANOVA showed interaction between maternal exercise and AβO infusion for phospho-Tau/Tau ratio (p<0.05). *P<0.05 effect of maternal exercise (two-way ANOVA), ##P<0.01 effect of AβO infusion (two-way ANOVA).
Synaptophysin and PSD-95 immunocontent are not altered in the cerebellum of young adult male offspring in response to AβO infusion
Two-way ANOVA indicated that neither maternal exercise nor AβO infusion exerted any effect on synaptophysin (Fig. 6a; p = 0.146 and p = 0.634, respectively) and PSD-95 levels (Fig. 6b; p = 0.281 and p = 0.325, respectively) in the cerebellum of young adult male offspring.
Maternal swimming during pregnancy prevents the phosphorylation of tau protein in the cerebellum of young adult male offspring AβO-infused
Lastly, we assessed the phosphorylated status of two proteins that are altered in AD.Reference Reddy50 The ratio between phosphorylated and total content of GSK-3β was found unaltered in the cerebellum of all groups (Fig. 6d), in which neither maternal exercise nor AβO infusion exert effect on p-GSK-3β/GSK-3β ratio (p = 0.295 and p = 0.958). Concerning tau phosphorylation status, two-way ANOVA indicated an interaction between both maternal exercise and AβO infusion on p(Ser-396)-tau/tau ratio (p = 0.041). These data indicated that AβO infusion increases tau phosphorylation, in a GSK-3β-independent way, while maternal exercise prevented such increase (Fig. 6e).
Discussion
In a previous work, we demonstrated that maternal exercise prevented learning and memory deficits elicited by AβOs infusion, as well as metabolic alterations in the prefrontal cortex and hippocampus of young adult male rat offspring.Reference Klein, Hoppe and Saccomori8 In the present study, we extended the investigation to cerebellum and observed that maternal exercise during pregnancy promotes adaptive response in developing fetuses through programming cerebellar neurochemistry that remains evident into young adulthood. Importantly, maternal exercise-induced programming was able to prevent some pathologic consequences of AβO peptide infusion, including rise of RS, protein carbonylation, and phosphorylation of tau protein.
Brain development, including growth and maturation, extends from gestational period beyond early PDs.Reference Rice and Barone51 The majority of literature data focus on cortical and hippocampal modulatory effects promoted by maternal exercise in the offspring’s brain,Reference Akhavan, Emami-Abarghoie and Safari10–Reference Gomes da Silva, de Almeida and Fernandes12,Reference Park, Kim and Eo52 and little is known about prenatal exercise effects on cerebellum. Data of adaptive changes in the cerebellum induced by maternal exercise were recently published by our group.Reference Marcelino, Longoni and Kudo7,Reference Marcelino, de Lemos Rodrigues and Klein26 We have shown that swimming during pregnancy enhances mitochondrial function and antioxidant defenses in the cerebellum, parietal cortex, hippocampus, and striatum of 7-day-old pups.Reference Marcelino, Longoni and Kudo7 Increased GSH and SH content and reduced protein carbonylation in young adult male offspring’s cerebellum presented reinforce our previous findings, evidencing long-term positive influence of maternal exercise on brain development. In addition, similar levels of oxidants in the cerebellum of young adult male offspring, when compared to control, contrasted to increased oxidants levels in the cerebellum of 7-day-old pups.Reference Marcelino, Longoni and Kudo7 Taken together, these findings suggest that increased oxidant levels on PD7 precede the metabolic adaptation improving antioxidant system efficiency that persists in young adulthood. In accordance, Akhavan et al.Reference Akhavan, Emami-Abarghoie and Safari10 demonstrated that involuntary swimming exercise represents a stressful experience by mother at the end of pregnancy resulting in increased corticosterone levels in blood, which contribute to the adaptive effects of improved cell function observed in the offspring. The adaptive changes in metabolism triggered by mild levels of stress, such as forced exercise, fit the hormesis concept,Reference Webb, Hughes, Thomas and Morris53 in which is defined as an adaptive response to low/mild levels of stress resulting in improved aptitude for physiological systems.Reference Calabrese and Baldwin54
Exercise-induced metabolic adaptation in individuals arises to regulate bioenergetics demand.Reference Marques-Aleixo, Oliveira, Moreira, Magalhaes and Ascensao55 Likewise, fetal adaptive responses to maternal exercise are engaged to deal with energetic challenge by modulating mitochondrial function.Reference Marcelino, Longoni and Kudo7,Reference Park, Kim and Eo52 Mitochondria are crucial to maintain the constant supply of energy required by brain and other organs.Reference Knott, Perkins, Schwarzenbacher and Bossy-Wetzel56 The cerebellum suffers marked metabolic adaptation in response to exercise,Reference Chalimoniuk, Chrapusta, Lukacova and Langfort57,Reference Marques-Aleixo, Santos-Alves and Balca58 even during pregnancy.Reference Marcelino, Longoni and Kudo7 We found that maternal exercise increased Complex II and cytochrome c oxidase activities, which are in agreement with increased mitochondrial mass and membrane potential as well as MFN1 and SIRT3 levels, and unchanged DRP1 found in young adult male offspring’s cerebellum. Currently, there are two reports in the literature that investigated the effect of maternal exercise on mitochondrial enzymes activities and biogenesis in offspring’s brain.Reference Klein, Hoppe and Saccomori8,Reference Park, Kim and Eo52 Park et al.Reference Park, Kim and Eo52 reported that pregnant mice undergoing treadmill exercise affect positively hippocampal mitochondrial function and biogenesis of 3-day-old pups. In addition, our group recently demonstrated that maternal swimming enhances mitochondrial function in the 60-day-old offspring’s prefrontal cortex and hippocampus by increasing mitochondrial mass and membrane potential and the activity of cytochrome c oxidase.Reference Klein, Hoppe and Saccomori8 It has been shown that mitochondrial SIRT3 increases in response to different exercise modalities.Reference Marques-Aleixo, Santos-Alves and Balca58 SIRT3 expression modulates adaptive responses of hippocampal neurons to exercise and strengths resistance to oxidative stress and apoptosis.Reference Cheng, Yang and Zhou59 To the best of our knowledge, this is the first study to demonstrate that mitochondrial SIRT3 and dynamic proteins in young adult male offspring’s cerebellum are modulated by maternal exercise.
NO• in the brain acts as a signaling molecule that regulates cerebral blood flow,Reference Garry, Ezra, Rowland, Westbrook and Pattinson60 and as a neuromodulator that regulates synaptic transmission through NO•/cyclic guanosine monophosphate (NO•/cGMP) pathway.Reference Chalimoniuk, Chrapusta, Lukacova and Langfort57,Reference Furini, Rossato, Bitencourt, Medina, Izquierdo and Cammarota61,Reference Llansola, Hernandez-Viadel, Erceg, Montoliu and Felipo62 Brain adaptive response to exercise seems to be mediated by NO• through upregulation of endothelial nitric oxide synthase (eNOS)Reference Chalimoniuk, Chrapusta, Lukacova and Langfort57,Reference Gertz, Priller and Kronenberg63 and downregulation of the calcium-independent inducible NOS (iNOS),Reference Liu, le Yang, Fan, Jiang and Pan64 whose effects can be neurotoxic.Reference Garry, Ezra, Rowland, Westbrook and Pattinson60 In the present study, we reported unaltered NO• levels and reduced iNOS immunocontent in the cerebellum of young adult male offspring delivered to exercised dams. On basis of these results, it seems that increased NO• levels are only necessary to induce metabolic adaptation in early postnatal brain of pups born to exercised dams, as previously demonstrated by our group.Reference Marcelino, Longoni and Kudo7
Different exercise regimens have shown to increase proteins related with neuroplasticity. Treadmill exercise in a schedule of 40 min/day, 3 days/week for 4 weeks, or 30 consecutive days increased synaptophysin immunocontent in the cerebellum of rats.Reference Real, Garcia, Britto and Pires65 Similarly, Liu et al.Reference Liu, Xue, Xia, Liu and Qi66 demonstrated that swimming exercise (60 min/day, 5 days/week for 4 weeks) is able to reverse the reduction of synaptophysin levels in the hippocampus of mice previously submitted to a chronic unpredictable mild stress. Here, we observed that maternal exercise does not modify pre-and post-synaptic protein levels in the cerebellum of 74-day-old young adult male rat offspring, at least in our experimental conditions.
Few studies have examined the effect of AD-associated Aβ neurotoxicity in the cerebellum.Reference Kuwabara, Ishizeki and Watamura67–Reference Kozuki, Kurata and Miyazaki69 We found an increase in overall cerebellar RS levels and tau phosphorylation following AβOs infusion, which were prevented by maternal exercise. Whereas H2DCF is oxidized mainly by hydrogen peroxide (H2O2), hydroxyl radical (•OH), and peroxynitrite (ONOO-),Reference Myhre, Andersen, Aarnes and Fonnum70,Reference Kalyanaraman, Darley-Usmar and Davies71 we sought to investigate the contribution of others RS, such as nitric oxide (•NO) and mitochondrial superoxide (mO2•-). We observed no change in mitochondrial superoxide and NO•, as well as antioxidant enzymes after AβOs infusion, despite increased RS levels. It is possible that the overall RS measured are hydrogen peroxide (H2O2) species, which might be interacting with NO• yielding peroxynitrite explaining the increased iNOS immunocontent and absence of alteration in NO• levels. The positive augmentation of some metabolic marks induced by maternal exercise in the male offspring’s cerebellum, such as GSH and SH content, was not abolished by AβOs infusion. AβOs infusion increased protein carbonylation, DRP1, and iNOS levels. While these parameters were significantly higher in the male rat offspring’s cerebellum born to sedentary dams than male control offspring, protein carbonylation, DRP1, and iNOS levels reached control values in the offspring born to exercised dams. These data indicated that AβO infusion promoted protein oxidation in cerebellum, and maternal exercise prevented the oxidative effect of AβO, suggesting a neuroprotective potential of maternal exercise against AD-changes induced by neurotoxic AβOs in the cerebellum of young adult male offspring. Similarly, Herring et al.Reference Herring, Donath and Yarmolenko14 reported that running during pregnancy alleviated cerebral oxidative stress and inflammation associated with decreased Aβ plaque in 5-month-old offspring carrying APP transgene.
Mitochondrial dysfunction is an early event that accompanies AD pathologic features underlying the major metabolic changes verified in this disease.Reference Abolhassani, Leon and Sheng32 It has been shown that AβO accumulates in mitochondria,Reference Manczak, Anekonda, Henson, Park, Quinn and Reddy72 thus AβO can target directly essential metabolic enzymes impairing its function.Reference Kandimalla and Reddy73 Immunoprecipitation analyses of mitochondrial dynamics showed that AβOs interact with DRP1 leading to abnormal mitochondrial dynamics and increased mitochondrial fragmentation, thus culminating in neuronal damage.Reference Manczak, Calkins and Reddy74 We observed that MFN1 and DRP1 levels in maternal exercised offspring infused with AβOs remained at control values, indicating a balance between fusion and fission processes despite the extinction of maternal exercise-induced MFN1 increase. In our previous work, we demonstrated that maternal exercise increased MFN1 levels in the 74-day-old male rat offspring’s prefrontal cortex and prevented the increase of DRP1 levels induced by AβOs in the hippocampus.Reference Klein, Hoppe and Saccomori8 Moreover, the main regulator of mitochondrial energy homeostasis, SIRT3,Reference Giralt and Villarroya75 was found increased together with enhanced mitochondrial enzymes activities, mass, and membrane potential. The increased SIRT3 might be leading to an elevation of mitochondrial activity.Reference Palmeira, Teodoro, Amorim, Steegborn, Sinclair and Rolo76 On the other hand, an unbalance of mitochondrial dynamic seems to occur in the maternal sedentary male rat offspring once DRP1 levels were increased by AβOs, while no effect was identified on MFN1 levels. The increased mitochondrial fission protein might be responsible by the removal of damaged mitochondriaReference Palmeira, Teodoro, Amorim, Steegborn, Sinclair and Rolo76 and, as postulated by Demetrius et al.,Reference Demetrius, Magistretti and Pellerin77 it is possible that a compensatory mechanism, in which occurs an upregulation of oxidative phosphorylation in some mitochondria to compensate energy production and to maintain impaired cells viable, is being operated.
As increased phosphorylated tau is also involved in AD pathology,Reference Reitz and Mayeux29 the rise in pTAU/TAU ratio in the cerebellum of maternal sedentary male offspring, but not in the maternal exercised male offspring, is another indicative of neuronal damage following AβOs infusion. Despite GSK is the main enzyme phosphorylating TAU protein, herein we observe no alteration in the more active status of GSK, its phosphorylated form. In AD, beyond altered status of kinases enzymes, it is common the altered activity of phosphatases acting over TAU protein, thus contributing to the hyperphosphorylation of TAU in the pathology.Reference Querfurth and LaFerla31
The work conducted by Thal et al.Reference Thal, Rub, Orantes and Braak78 demonstrates that cerebellar pathological features of Aβ deposition in the cerebellum and related clinical manifestation are evident in later stages of AD. In accordance, Kuwabara et al.Reference Kuwabara, Ishizeki and Watamura67 demonstrated that cerebellar neuropathology and accumulation of Aβ appear with disease progression in APPswe/PS1dE9 double transgenic mice. The authors also revealed that Aβ accumulation in the cerebellum was associated with impaired long-term depression but unaffected synaptic transmission,Reference Kuwabara, Ishizeki and Watamura67 which might help us to explain why AβOs did not induce reduction of synaptic proteins in the offspring’s cerebellum in the present work. We previously demonstrated that AβOs reduced significantly synaptophysin levels in the hippocampus of 74-day-old male rats and that maternal exercise was able to mitigate this Aβ-induced synaptophysin reduction.Reference Klein, Hoppe and Saccomori8 In this way, it is possible that we could not observe other alterations following AβO infusion as function of the time we assessed the parameters, but we cannot exclude the possibility of later appearance of AβO-associated pathological features in the cerebellum of 74-day-old male rat offspring. So far, one suggested molecular mechanism responsible by maternal exercise-promoted protective effects observed against biochemical changes caused by AβOs could be the improved mitochondrial bioenergetics.
Conclusion
In summary, we report for the first time that exercise during pregnancy exerts long-lasting effects on the cerebellar metabolism of male rat offspring by inducing adaptive changes in utero. Remarkably, these metabolic programming effects are still evident during young adulthood, on PD 74. Our findings reveal that the long-term influence of maternal exercise on offspring metabolism may offer some protection against AD changes induced by neurotoxic AβO (Fig. 7). Our study provides another example of the benefits of exercising during pregnancy to the offspring and a promising neuroprotective approach to modify the course of disease later in life.

Fig. 7. Summary effects of maternal exercise and AβO infusion found on the cerebellum of adult offspring.
Acknowledgments
This study was supported by the Pró-Reitoria de Pesquisa/Universidade Federal do Rio Grande do Sul (PROPESQ/UFRGS).
Financial support
CPK is a PhD postgraduate student in Biological Sciences – Biochemistry receiving grants from the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). CM received grants from CNPq [Universal 442406/2014-2 and INCT 465671/2014-4].
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (CONCEA) and has been approved by the institutional committee (Comissão de Ética no Uso de Animais – CEUA/UFRGS, under the protocol number 27349).