Introduction
Menarche and menopause are two major components of the reproductive life of women. The interval between these two events determines the natural reproductive period during which women can procreate. Mounting evidence indicates that early or late occurrences of these events are linked to heightened risks of chronic diseases such as endometriosis, metabolic disorders and breast cancer. Menarche, the first menstrual bleeding, is a sentinel marker for onset of the reproductive lifecycle of a woman and is preceded by a complex cascade of hormonal changes that culminates in reproductive capabilities and results in a complex series of physiological and molecular events, patterns of physical growth and body composition (DiVall & Radovick, Reference DiVall and Radovick2008; Boynton-Jarrett et al., Reference Boynton-Jarrett, Wright, Putnam, Lividoti Hibert, Michels and Forman2013; Yermachenko & Dvornyk, Reference Yermachenko and Dvornyk2014). Menopause refers to the permanent cessation of menstruation resulting from loss of follicular activities of the ovaries and it occurs when the decrease in follicles reaches a critical number (Ginsberg, Reference Ginsberg1991; Gold, Reference Gold2011). Natural menopause, as defined by the World Health Organization (WHO), is ‘the permanent cessation of menstruation, recognized as having occurred after 12 months of amenorrhoea, not attributed to hormone use or surgery for the removal of the uterus or ovaries’ (WHO, 1996).
Sievert (Reference Sievert2014) pointed out that the discipline of anthropology remains well suited to the study of menopause as there is a great deal of variation in age at menopause across populations, along with the meaning of menopause across cultures. Longevity, rather than capacity for menopause, sets humans apart from other primates. A developmental perspective suggests that early childhood may be a critical time for the environment to irreversibly influence the number of oocytes or rate of follicular atresia and, ultimately, age at menopause (Sievert, Reference Sievert2014).
Mounting evidence has established the significance of menarche as both a footprint for chronic disease risks and compass for health and developmental trajectories (Forman et al., Reference Forman, Hong and Ferry2013; Boynton-Jarrett et al., Reference Boynton-Jarrett, Wright, Putnam, Lividoti Hibert, Michels and Forman2013; Li et al., Reference Li, Eriksson, Czene, Hall and Rodriguez-Wallberg2016). An earlier age at menarche is associated with ovarian failure (Chang et al., Reference Chang, Kim, Lee, Kim, Yim and Lim2007), risk of gall stone disease (Ryu et al., Reference Ryu, Chang, Choi, Kwon, Yun and Jung2016), hypertension (Bubach et al., Reference Bubach, Mola, Hardy, Dreyfus, Santos and Horta2018), risk of cancer at adulthood (Werneck et al., Reference Werneck, Coelho-E-Silva, Padilha, Ronque, Cyrino and Szwarcwald2018), cardiovascular disease (Peters & Woodward, Reference Peters and Woodward2018) and metabolic disorders (Kim & Je, Reference Kim and Je2019). Recent studies have indicated an increased risk for endometriosis (Matalliotakis et al., Reference Matalliotakis, Goulielmos, Matalliotaki, Trivli, Matalliotakis and Arici2017; Ottolina et al., Reference Ottolina, Bartiromo, Viganò, Makieva, Schimberni and Candiani2018) and fatty liver disease (Yi et al., Reference Yi, Hwang, Lim, Lee, Kim and Lim2017) with early age at menarche. Studies have also reported that several diseases and conditions are associated with later age at menarche. These include depression (Graber et al., Reference Graber, Seeley, Brooks-Gunn and Lewinsohn2004) and lower bone mineral density (Eastell, Reference Eastell2005). The risks become compounded as there has been a steady decrease or secular trend in the age at menarche in both developed and developing countries (e.g. Park et al., Reference Park, Lim and Park2018; Shen et al. Reference Shen, Chen, Pan and Yu2019). Over the past few decades, a secular trend towards an earlier age at menarche has been established in several countries such as Brazil (Silva & Padez, Reference Silva and Padez2006), Mexico (Marván et al., Reference Marván, Catillo-López, Alcalá-Herrera and Callejo2016) and Switzerland (Lehmann & Scheffler, Reference Lehmann and Scheffler2016). In India too there has been a reduction of nearly one month per decade in the age at menarche, suggesting a secular trend (Bagga & Kulkarni, Reference Bagga and Kulkarni2000; Pathak et al., Reference Pathak, Tripathi and Subramanian2014).
There is also evidence of associations of certain non-communicable diseases and poor health with early age at menopause. It has been shown that early age at menopause is associated with osteoporosis (Kritz & Barrett, Reference Kritz and Barrett1993), lower bone density (Osei-Hyiaman et al., Reference Osei-Hyiaman, Satoshi, Ueji, Hideto and Kano1998) and cardiovascular and coronary diseases (Atsma et al., Reference Atsma, Bartelink, Grobbee and vander2006). A later age at menopause has been linked to breast and endometrial cancers (Ginsberg, Reference Ginsberg1991; Gold, Reference Gold, Bromberger, Crawford, Samuels, Greendale and Harlow2001; Gold et al., Reference Gold2011). A search of the existing literature search showed that a number of determinant factors significantly influence the ages at menarche and natural menopause. These include biological (e.g. genetic, nutritional and reproductive history), socio-cultural (e.g. educational, occupational and rural–urban residence), reproductive (e.g. parity and type of contraceptives used) and lifestyle factors (e.g. smoking and use of tobacco and dietary habits) (Pallikadavath et al., Reference Pallikadavath, Ogollah, Singh, Dean, Dewey and Stones2016; Koukouliata et al., Reference Koukouliata, Nena, Koutlaki, Liberis and Constantinidis2017; Bae et al., Reference Bae, Park and Kwon2018; Park et al., Reference Park, Lim and Park2018; Lawn et al., Reference Lawn, Lawlor and Fraser2018; Shen et al., Reference Shen, Chen, Pan and Yu2019).
Ages at menarche and natural menopause have been implicated in numerous health consequences for women in later life. Hence, an understanding of the association between these two ages and their health risks could help with effective prevention and management of health problems that women may subsequently develop. For example, the most common diseases that pose significant health issues and economic burdens in both developed and developing countries are cardiovascular diseases, osteoporosis, breast cancer and Type 2 diabetes (Sasser et al., Reference Sasser, Rousculp, Birnbaum, Oster, Lufkin and Mallet2005; Qui et al., Reference Qui, Chen, Wen, Zhu, Lin, Huang and Wu2013). An understanding of the relationship between ages at menarche and menopause may also lead to an exploration of the underlying mechanisms of follicular atresia, fertility and disease across the life span, as suggested by Forman et al. (Reference Forman, Hong and Ferry2013). Moreover, the duration of the reproductive period is closely linked to ages at menarche and menopause. For a better understanding of the independent role of reproductive period in disease risk, reliable knowledge about the relation of age at menarche with age at menopause and with duration of the reproductive period is important (Bjelland et al., Reference Bjelland, Hofvind, Byberg and Eskild2018). No consistent results have been reported in previous studies on the association between ages at menarche and natural menopause. On the one hand, studies have reported that women with early menarche experience early menopause (Nagata et al., Reference Nagata, Takatsuka, Kawakami and Shimizu2000; Henderson et al., Reference Henderson, Bernstein, Henderson, Kolonel and Pike2008; Brand et al., Reference Brand, Onland, Eijkemans, Tjonneland, Roswall and Overvad2015; Ruth et al., Reference Ruth, Perry, Henley, Melzer, Weedon and Murray2016; Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson and Kuh2017). On the other hand, other researchers have observed the opposite: that women with early menarche exhibited a late menopause (vanKeep et al., Reference vanKeep, Brand and Lehert1979; Boulet et al., Reference Boulet, Oddens, Lehert, Vemer and Visser1994). Several studies have observed no such associations (Nagel et al., Reference Nagel, Altenburg, Nieters, Boffetta and Linseisen2005; Rizvanovic et al., Reference Rizvanovic, Balic, Begic, Babovic, Bogadanovic and Kameric2013; Bjelland et al., Reference Bjelland, Wilkosz, Tanbo and Gand Eskild2014; Zsakai et al., Reference Zsakai, Mascie-Taylor and Bodzsar2015). If age at menarche is not related to age at menopause, women with early menarche may have a longer time interval between menarche and menopause than women with late or delayed menarche. This interval is often referred to as the woman’s reproductive period. A long reproductive period implies high cumulative exposure to endogenous female sex hormones such as oestrogens and progestogens, and this period has consistently been associated with increased risk of hormone-related cancers, such as breast cancer and endometrial cancer (Bjelland et al., Reference Bjelland, Hofvind, Byberg and Eskild2018). Even though there is evidence of a secular trend in menarche, it is less certain whether a similar trend for menopause exists. However, some trends are available for European countries (Flint, Reference Flint1978; Dratva et al. Reference Dratva, Gómez Real, Schindler, Ackermann-Liebrich, Gerbase and Probst-Hensch2009) and Taiwan (Shen et al., Reference Shen, Chen, Pan and Yu2019).
India is a land of enormous genetic, cultural and linguistic diversity (Majumder, Reference Majumder2001). It has been pointed out that the Indian population comprises more than a billion people and consists of 4693 communities with several thousand endogamous groups (e.g. Singh, Reference Singh2002). It is home to a large number of caste and tribal/indigenous populations. A considerable number of scientific papers are available on ages at menarche and menopause from individuals belonging to these ethnic communities/populations (e.g. Singh & Thapar, Reference Singh and Thapar1983; Tyagi et al., Reference Tyagi, Pal and Tewari1983; Chakravarty, Reference Chakravarty1994; Bagga & Kulkarni, Reference Bagga and Kulkarni2000; Purnungla & Sengupta, Reference Purnungla and Sengupta2002; Sidhu, Reference Sidhu2002; Dasgupta & Ray, Reference Dasgupta and Ray2009; Deb, Reference Deb2009; Ahuja, Reference Ahuja2016; Goyal et al., Reference Goyal, Singh and Sethi2016; Sharma & Bansal, Reference Sharma and Bansal2018; Shukla et al., Reference Shukla, Ganjiwale and Patel2018). The northern part of the state of West Bengal is popularly known as North Bengal and comprises the districts of Malda, Uttar Dinajpur, Dakshin Dinajpur, Darjeeling, Cooch Behar, Kalimpong, Alipurduar and Jalpaiguri. A number of indigenous (e.g. Lepcha, Rabha, Meche, Toto and Rajbanshi) and caste (e.g. Bengalee) populations reside in this region. A thorough literature search has shown that studies in menarche and menopause are scarce among these ethnic populations.
The Rajbanshi is the largest indigenous population of North Bengal. A thorough literature search yielded very few investigations on ages at menarche and natural menopause for this population. The age at menarche among Rajbanshi girls was observed to be 14.7 years by Chakravarty (Reference Chakravarty1994). Only two studies were available in the domain of menopause among Rajbanshi women. The different socioeconomic factors affecting menopause among Rajbanshi women were reported by Sinha and Sen (Reference Sinha and Sen2017). The effects of different confounding factors on overweight and obesity among Rajbanshi post-menopausal women were studied by Sinha et al. (Reference Sinha, Mondal and Sen2018).
It remains uncertain whether age at menarche is associated with age at natural menopause. Several research investigations have also confirmed the association of premature and/or early age at menarche with the risk of adverse health outcome in later life with early natural menopause among women (e.g. Mishra et al., Reference Mishra, Pandeya, Dobson, Chung, Anderson and Kuh2017; Andarini & Sujarwoto, Reference Andarini and Sujarwoto2018; Bjelland et al., Reference Bjelland, Hofvind, Byberg and Eskild2018). Apparently, women who experience late menarche can exhibit more significant effects on age at natural menopause than women who have an early menarche. Therefore, the age at menarche appears to be an important contributing factor to age at natural menopause. With these issues in mind, the present investigation aimed to determine the association between an early or later age at menarche with age at natural menopause, along with the effects of different demographic, socioeconomic and lifestyle variables among married post-menopausal women belonging to the Rajbanshi population of North Bengal, India.
Methods
Area of study
This cross-sectional investigation was carried out from January 2015 till May 2015 among 510 post-menopausal Rajbanshi women aged between 45 and 55 years residing in different Rajbanshi-dominated villages located in Kharibari block in the district of Darjeeling, West Bengal, India. The selection of these villages was based on population strength, dominance of the Rajbanshi population and easy road connectivity and accessibility. The Rajbanshi population is chiefly distributed in the north-eastern part of India and mainly concentrated in the state of Assam and few districts in the state of West Bengal (Sanyal, Reference Sanyal1965). In West Bengal they constitute the second largest percentage and number of the Scheduled Caste population. It is generally agreed that ethnically the Rajbanshi show resemblances with the Koch population of neighbouring state of Assam and it has been conjectured that they belong to a mixed race of Australasians/Dravidians and Mongolians (Risley, Reference Risley1891). According to Dalton (Reference Dalton1872), the Rajbanshi originally belonged to a Dravidian stock and later came into contact with the Mongoloid racial strain of Assam, Northeast India. A study on genetic markers has identified the Rajbanshi to be a semi-Hinduized caste group located in-between the clusters of Caucasoid caste populations and Mongoloid tribal populations (Kumar et al., Reference Kumar, Basu and Reddy2004). The Rajbanshi language is a mixture of Tibeto-Burman and Indo-Aryan language speaking families. These people reside in the plain regions and are primarily dependent on agricultural activities. Rice is the staple food for the majority of the population. However, their poor economic condition has created numerous social problems and Rajbanshi women have adopted lower skilled jobs in certain unorganized sectors.
Study sample
The participants (i.e. Rajbanshi women) were selected using a stratified random sampling method. At the initial level, Rajbanshi households in the villages were identified. Then, post-menopausal Rajbanshi women in the age group of 45–55 years were singled out. Subsequently, 530 women were approached to take part in the investigation. Details of the research investigation protocol were explained to them. Twenty of them had to be excluded from the investigation as either their age at menarche could not be recalled or they had not experienced natural menopause; rather, they experienced menopause as a result of hormonal therapy or surgery or removal of the uterus. The age at natural menopause was only recorded from those women who reported spontaneous cessation of menstruation for more than one year based on the WHO criterion (WHO, 1996).
Variables
A structured schedule was used to obtain data on age at menarche and age at natural menopause, along with the following demographic, socioeconomic and lifestyle variables: marital status, parity, education, age of first and last pregnancies (in years), duration of breastfeeding of last child (in months), use of oral contraceptives, health status, monthly occupation income and lifestyle factors such as smoking. Age at menarche was ascertained by asking the participants to recall when they experienced menstrual bleeding for the first time. Age at menopause was calculated by subtracting the year of the birth of women from the year of their final menstrual period. Ages at menarche and ages at menopause, along with years elapsed between recalled age at menopause and present age, were calculated in completed years. Face-to-face interviews were conducted to complete the structured schedule. The interviewer (IS) was familiar with the Rajbanshi language and expressions, and had prior field experience in collecting data on reproductive history. Prior to the canvassing of the schedules, the interviewer discussed issues such as sport, literature and movies with the participants to put them at ease and build rapport.
Anthropometric measurements
The anthropometric measurements of height and weight were recorded following standard procedures (Hall et al., Reference Hall, Allanson, Gripp and Slavotinek2007). Height was measured to the nearest 0.10 cm using an anthropometer with the individual standing in erect position with the head oriented in the Frankfort Horizontal place. Weight was recorded to the nearest 0.10 kg with the individual standing motionless on a portable weighing scale. The technical error measurement (TEM) was calculated to determine the accuracy of the anthropometric measurements using the standard method of Ulijaszek and Kerr (Reference Ulijaszek and Kerr1999). To calculate the TEM, height and weight was measured from 50 women other than those covered in the present investigation by two of the authors (IS and PT). The TEM was calculated using the formula:
where D is the difference between the measurements and N is the number of individuals measured.
The co-efficient of reliability (R) was subsequently calculated using TEM using the following equation:
where SD is the standard deviation of the measurements.
The intra-observer and inter-observer TEMs were observed to be within the cut-off value (R=0.95) of Ulijaszek and Kerr (Reference Ulijaszek and Kerr1999). Hence, the anthropometric measurements were recorded by both IS and PT were reliable and reproducible. All the anthropometric measurements in the course of the present investigation were subsequently recorded by one of the authors (IS).
The women’s Body Mass Index (BMI) was calculated using the WHO (1995) formula:
Statistical analysis
The data were statistically analysed using SPSS version 17.0. For a better understanding of the association between age at menarche and age at menopause, the data set was divided into two groups, the first being those women whose menarcheal age was <12 years (i.e. early menarche), and the second comprising those whose menarcheal age was ≥12 years (i.e. later age at menarche), as per the classification proposed by Lakshman et al. (Reference Lakshman, Forouhi, Sharp, Luben, Bingham and Khaw2009), Giles et al. (Reference Giles, Glonek, Moore, Davies and Luszcz2010) and Otero et al. (Reference Otero, Chor, Carvalho, Faerstein, Lopes and Werneck2010). As the information on age at natural menopause was as only available in complete years, the median age at menopause was calculated to be 50 years. A discrete binary logistic regression (BLR) analysis was performed for all of the determinant variables that could be associated with early menarcheal age (<12 years), and the second comprising those whose menarcheal age was ≥12 years. The BLR analysis was fitted to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) so as to examine the effect on individual attained age at menopause (e.g. ≤50 years vs ≥51 years) and allowed the control of the determinant variables, separately. This BLR model allows for controlling the determinant variables by comparing with a reference category. The dependent variables were created by those women whose age at menopause was observed to be ≤50 years and ≥51 years and were coded into ‘0’ and ‘1’ in the BLR model, separately. The predictor variables of age, marital status, parity, education, smoking, health status, age at pregnancy, age at last pregnancy, time of breastfeeding, occupation, monthly income and BMI were entered into the regression models as a set of categorical variables, and results were obtained by comparing them with the reference categories. The p-values of <0.05 and <0.01 were considered to be statistically significant.
Results
The 510 women were divided into two categories based on their age of menarche: those with early menarche (<12 years) (n=47) and those with later age at menarche (≥12 years) (n=463).
Age at menarche <12 years
In the 47 women with an age at menarche of <12 years, the median age at menopause was 49 years. There was an increase in the median age at menopause with an increase in the age of the women. A BLR model was fitted to find out the odds for different socioeconomic, demographic and lifestyle variables that have the potential to be significantly associated with age at natural menopause (Table 1). The results showed that educational level and health status were not significantly associated with age at natural menopause (p>0.05), but age at first pregnancy was significantly associated with age at natural menopause (p<0.05). Women who were illiterate (odds: 2.50, 95% CI 0.21–29.25; p>0.05) exhibited a higher odds value than those with primary education (odds: 0.80, 95% CI: 0.07–8.75; p>0.05), but neither was statistically significant. The age at first pregnancy in the age group of 17–20 years (odds: 0.04, 95% CI: 0.03–0.78; p<0.05) was significantly associated with age at natural menopause, but health status with major illness (odds: 2.26; 95% CI: 0.292–17.57; p>0.05) and employed women (odds: 3.00; CI: 0.65–13.84; p>0.05) were not significantly associated with age at natural menopause. No significant association was observed among women in the age group of 45–50 years (odds: 1.62, 95% CI 0.29–8.97; p>0.05) with age at natural menopause (p>0.05) (Table 1). No statistically significant associations were observed between age, marital status, parity, age at first pregnancy, duration of breastfeeding, tobacco use or monthly income, BMI and age at menopause (p>0.05).
Table 1. Binary logistic regression analysis of association of socioeconomic, demographic and lifestyle variables with age at menopause among Rajbanshi menopausal women with <12 years age at menarche, N = 47

*p<0.05; **p<0.01.
Age at menarche ≥12 years
In the case of the 463 women who experienced menarche at ≥12 years of age, the median age at menopause was 51 years (Table 2). There was an increase in median age at menopause with an increase in the age of women. Highly significant statistical associations were observed between the age of the women (e.g. 45–50 years) and age at menopause (p<0.001). In addition, there was a significant association between health status and age at menopause (p<0.05). The results also indicated that women in the age group of 45–50 years (odds: 0.019; 95% CI: 0.00–0.06; p<0.001) were significantly associated with age at menarche than women aged 51–55 years. The results further showed that a lower monthly income (<Rs 4000) (odds: 1.95; 95% CI:1.09–3.59; p<0.05), primary education (odds: 7.71; 95% CI: 1.82–23.52; p<0.001), marital status (odds: 9.87: 95% CI: 13.37–7.85; p<0.001), smoking use (odds: 7.40; 95% CI: 3.62–15.13; p<0.001), age at first pregnancy 17–20 years (odds: 10.73; 95% CI: 1.44–17.99; p<0.05), age at pregnancy ≥21 years (odds: 13.48; 95% CI: 1.79–10.02; p<0.05) age at last pregnancy (≥26 years) (odds: 5.77; 95% CI: 6.28–33.21; p<0.001), breastfeeding (odds: 4.74; 95% CI: 5.47–33.40; p<0.001) and health status with major illness (odds: 5.23; 95% CI: 5.41–7.67; p<0.05) had highly significant effects on the age at menopause. Unemployed women (odds: 30.28; 95% CI: 9.42–97.25; p<0.001) showed significantly higher odds than employed with age at menopause. Women with a lower (1–3) number of children or parity (odds: 24.67; 95% CI: 10.53–57.77; p<0.001) were more significantly associated with age at menarche than women with a higher number of children (≥4) (Table 2)
Table 2. Binary logistic regression analysis and association of socioeconomic, demographic and lifestyle variables among Rajbanshi menopausal women with ≥12 years age at menarche with age at menopause, N = 463

*p<0.05; **p<0.01.
Discussion
The biological implications (i.e. mechanisms) and clinical implications (i.e. consequences) of the three possibilities for associations between the age at menarche and age at natural menopause (i.e. lack of association or direct association or inverse association) are different among different ethnic populations. The mean age at menarche in the present investigation among indigenous Indian Rajbanshi women was 12.52 years, which is in the lower range when compared with those reported for other populations of India. The comparison of the mean age at menarche obtained in the present investigation with those reported for other Indian populations is shown in Table 3. The mean age at menarche among women from India reported in the existing literature ranged from 11.9 to 13.7 years (Tyagi et al. Reference Tyagi, Pal and Tewari1983; Sengupta et al., Reference Sengupta, Gogoi and Chetry1996; Sidhu, Reference Sidhu2002; Deb, Reference Deb2009; Dambhare et al., Reference Dambhare, Wagh and Dudhe2012; Khopkar et al., Reference Khopkar, Kulanthinal, Virtanen and Saavala2015; Goyal et al., Reference Goyal, Singh and Sethi2016; Omidvar et al. Reference Omidvar, Amiri, Bakhtiari and Begum2018). It was observed to be 12.23 years among Brahmin girls from Assam (Sengupta et al., Reference Sengupta, Gogoi and Chetry1996). Singh and Thapar (Reference Singh and Thapar1983) reported the mean age at menarche to be 16.38 years among Bhootia girls from the Mana Valley of Uttar Pradesh. Tyagi et al. (Reference Tyagi, Pal and Tewari1983) observed it to be 12.80 years and 12.76 years, respectively, among Oraon and Munda women, and Chakravarty (Reference Chakravarty1994) observed it to be 14.7 years among the Rajbanshi of North-East India.
Table 3. Age at menarche among different populations

*Mean; **Median.
The influence of age at menarche on age at menopause has been a focus of research in various investigations. Several researchers have reported a direct association, with early menarche leading to early menopause (Cramer & Xu, Reference Cramer and Xu1996; Nichols et al., Reference Nichols, Trentham-Dietz, Hampton, Titus-Ernstoff, Egan and Willett2006; Sioka et al., Reference Sioka, Fotopoulos, Georgiou, Xourgia, Panadopoulos and Kalef-Ezra2009; Li et al., Reference Li, Eriksson, Czene, Hall and Rodriguez-Wallberg2016). This association causes a transition in the entire reproductive period in women towards an early span, but without necessarily shortening it, but the increases in mean age at menopause, with or without shortening of the fertile period, appear to increase the risk of diseases such as osteoporosis (Sioka et al., Reference Sioka, Fotopoulos, Georgiou, Xourgia, Panadopoulos and Kalef-Ezra2009) and cardiovascular disease (Schmidt, Reference Schmidt2017). The inverse association of an early age at menarche and a later age at menopause leads to a lengthening of the reproductive period, greater exposure to circulating endogenous hormones and a subsequent increase in the risk of diseases such as endometrial disease (e.g. endometriosis), cardiovascular disease, metabolic disorders and breast cancer (Nichols et al., Reference Nichols, Trentham-Dietz, Hampton, Titus-Ernstoff, Egan and Willett2006; Schmidt, Reference Schmidt2017). The present investigation also observed similar associations between ages at menarche and natural menopause among the Rajbanshi women. Similar results have been reported in the UK (Hardy & Kuh, Reference Hardy and Kuh2005), United States (Nichols et al., Reference Nichols, Trentham-Dietz, Hampton, Titus-Ernstoff, Egan and Willett2006), Europe (Stepaniak et al., Reference Stepaniak, Szafraniec, Kubinova, Malyutina, Peasey and Pikhart2013) and India (Dasgupta et al., Reference Dasgupta, Pal and Ray2015).
The present investigation reported the median ages at menopause among women who experienced menarche at <12 years and ≥12 years of age and their associations with different dependent and independent variables. In the case of menarche at age <12 years, the median age at menopause was 49 years, and for ≥12 years it was 51 years. This is on the higher side of the reported values from studies carried out in different countries, including India (e.g. Vehid et al., Reference Vehid, Aran, Köksal, Işiloglu and Senocak2006; Cassou et al., Reference Cassou, Mandereau, Aegerter, Touranchet and Derriennic2007; Dasgupta & Ray, Reference Dasgupta and Ray2013). The reported median menopausal age among Lohar Ghadiy as from Madhya Pradesh was 46.34 years (Yadav et al., Reference Yadav, Jain and Sharma2002) and that of the Aao Nagas from Nagaland was 51.33 years (Purnungla & Sengupta, Reference Purnungla and Sengupta2002). In West Bengal, the average menopausal age of Bengali Hindu women has been observed to be 46.14 years (Dasgupta & Ray, Reference Dasgupta and Ray2009) and 44.67 years (Dasgupta & Ray, Reference Dasgupta and Ray2013). The overall median age at natural menopause (i.e., 50 years) in the present investigation was close to the values observed in developed countries such as Finland (51 years) (Luoto et al., Reference Luoto, Kaprio and Uutela1994), United States (51 years) (Kato et al., Reference Kato, Toniolo, Akhemedkhanov, Koenig, Shore and Zeleniuch-Jacquotte1998), France (52 years) (Cassou et al., Reference Cassou, Mandereau, Aegerter, Touranchet and Derriennic2007) and Italy (51.2 years) (Parazzini & Progetto Menopausa Italia Study Group, Reference Parazzini2007). A population-specific comparison of the median age at menopause from different populations with the present investigation is shown in Table 4.
Table 4. Age at menopause among different populations
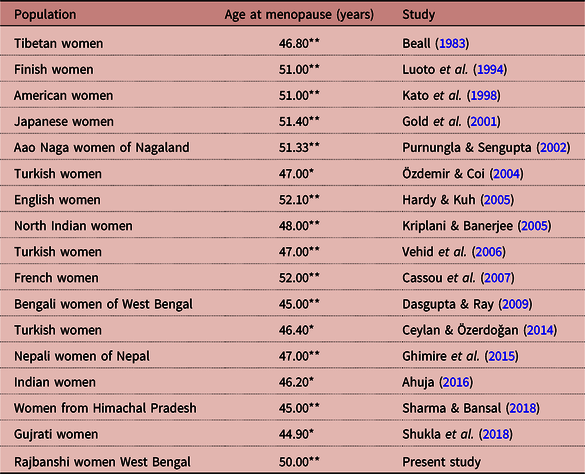
*Mean; **Median.
The median age at menopause was observed to be higher among women with early menarche (<12 years) than in those with later age at menarche (≥12 years). Therefore, there appeared to be some association between ages at menarche and menopause among these women. Women who experienced a later age at menarche (≥12 years) had more significant effects on age at natural menopause than women who experienced an early age at menarche (<12 years). Age of the women appears to be an important factor related to age at menopause. Those aged 45–50 years exhibited greater significant effects on age at menopause than those aged 51–55 years. For parous women, age at natural menopause occurred significantly later compared with nulliparous women, concurring with the results of Bromberger et al. (Reference Bromberger, Matthews and Kuller1997). A trend of increasing age at menopause with increasing number of live births was observed, but the trend was not strongly monotonic, unlike that reported in a previous report (Hardy & Kuh, Reference Hardy and Kuh1999). A study among Chinese women observed early menarche, younger age at first live birth, older age at last live birth and higher parity to be associated with late onset of menopause (Dorjgochoo et al., Reference Dorjgochoo, Kallianpur, Gao, Cai, Yang and Li2008).Women who have given birth to at least one child have a larger reserve of oocytes and longer exposure to oestrogens (Nikolaou & Templeton, Reference Nikolaou and Templeton2004; Santoro et al., Reference Santoro, Brockwell, Johnston, Crawford, Gold and Harlow2007). Thus, increasing parity may lead to slower depletion of ovarian follicles resulting in a later age at menopause (Ginsberg, Reference Ginsberg1991) because onset of menopause is theorized to be related to the rate of loss of oocytes and thus to the occurrence of ovulatory cycles.
The results of the present investigation add to a growing body of literature showing that regular and irregular smoking is significantly associated with an earlier age at menopause after adjustment for confounding factors. Some studies have reported that heavy smokers experience an earlier menopause than light smokers (Torgerson et al., Reference Torgerson, Avenell, Russell and Reid1994; Parazzini & Progetto Menopausa Italia Study Group, Reference Parazzini2007; Li et al., Reference Li, Eriksson, Czene, Hall and Rodriguez-Wallberg2016; Rumianowski et al., Reference Rumianowski, Rotter, Brodowska, Adler, Kowalski and Karakiewicz2016), while another investigation has shown that former smokers had no different, or only a slightly earlier, age at menopause than those who had never smoked (Cooper et al., Reference Cooper, Sandler and Bohlig1999). The effect of smoking may not be permanent – a finding inconsistent with a toxic effect leading to the atrophy of ovarian follicles. Polycyclic aromatic hydrocarbons in cigarette smoke are toxic to ovarian follicles and could result in their loss and thus lead to earlier menopause among smokers. An appreciable number of studies have observed smoking to have a consistent association with menopausal age (e.g. Elias et al., Reference Elias, van Noord, Peeters, den Tonkelaar and Grobbee2003; Sievert & Hautaniemi, Reference Sievert and Hautaniemi2003; Sapre & Thakur, Reference Sapre and Thakur2014; Ertunc et al., Reference Ertunc, Tok, Aytan and Gozukara2015).
The results of the present investigation showed that ages at first (i.e. ≥21 years) and last pregnancy (e.g. ≥26 years) had more significant associations with age at natural menopause among women who experienced later age at menarche (i.e. ≥12 years) than those with an early age at menarche (i.e. <12 years) (p<0.001). These results partially corroborate the finding from Indian national-level data, which have shown that women who begin and end childbearing at an earlier stage of their reproductive life reach menopause earlier (Shyamala & Sivakami, Reference Shyamala and Sivakami2005). Several studies have also shown menarcheal age, duration of breastfeeding and ages at first and last pregnancies to be significantly associated with the onset of menopause (e.g. Gold et al., Reference Gold, Bromberger, Crawford, Samuels, Greendale and Harlow2001; Chang et al., Reference Chang, Kim, Lee, Kim, Yim and Lim2007; Parazzini & Progetto Menopausa Italia Study Group, Reference Parazzini2007; Dorjgochoo et al., Reference Dorjgochoo, Kallianpur, Gao, Cai, Yang and Li2008), suggesting a role for these variables in influencing the ovarian store of the body. It has been suggested that an increased duration of breastfeeding may prevent follicle depletion and preserve ovarian function, thereby delaying the onset of menopause (Chang et al., Reference Chang, Kim, Lee, Kim, Yim and Lim2007).
The results showed that women with a higher level of education and employment were significantly associated with an earlier age at menopause as compared with women with a lower level of education and unemployment. Several research studies found that lower educational attainment and/or socioeconomic status, often determined by occupational status of the woman or her husband, were associated with an earlier age at menopause (Torgerson et al., Reference Torgerson, Avenell, Russell and Reid1994; Vehid et al., Reference Vehid, Aran, Köksal, Işiloglu and Senocak2006; Parazzini & Progetto Menopausa Italia Study Group, Reference Parazzini2007; Koukouliata et al., Reference Koukouliata, Nena, Koutlaki, Liberis and Constantinidis2017). Social and physical stresses are associated with amenorrhoea and reproductive dysfunction, and low socioeconomic status or low educational level may be markers for elevated stress. In the present investigation, BMI was observed to be significantly associated with age at menopause. Some studies have observed that women with lower BMI experienced an earlier natural menopause (e.g. Willett et al., Reference Willett, Stampfer and Bain1983; Al-Safi & Polotsky, Reference Al-Safi and Polotsky2015), as documented in the present investigation. In humans, caloric restriction and nutritional deficiencies are associated with amenorrhoea. The production of oestrogens in adipose tissue, which is greater in more-obese women, may result in higher levels of circulating oestrogens, which may contribute to longer reproductive functioning. However, some studies have also argued that body composition had no relationship with menopausal age (e.g. Akahoshi et al., Reference Akahoshi, Soda, Nakashima, Tominaga, Ichimaru and Seto2002; Ku et al., Reference Ku, Kang, Kim, Ku, Lee and Suh2004).
The present investigation has its limitations. A major limitation is that it was cross-sectional in nature and did not focus on the reproductive health of the Rajbanshi women. Memory bias could have affected the results as the data were retrospective in nature. There was also a lack of information on the duration and regularity of the women’s menstrual cycles. The other limitations include lack of information on diet and body composition measures (like waist–hip ratio) prior to menopause and total duration of breastfeeding (for all children).
In conclusion, this investigation observed that among Rajbanshi women from North Bengal, India, the median age of menopause among women who experienced menarche at <12 years of age was higher (≤49 years) in comparison to that of women who attained menarche at ≥12 years of age (≥50 years). There appears to be an indirect association between different variables and age at menarche and natural age at menopause. Factors such as marital status, parity, educational status, tobacco use, duration of breastfeeding of the last child and age at first pregnancy discriminate the menopausal age of women. Duration of breastfeeding and age at first pregnancy are essentially culturally determined behaviours and are not uniform across ethnic groups/populations. Age at menarche, despite being largely controlled by biological processes, varies from one population to another. As India is a multi-ethnic country, it would be interesting to conduct similar investigations in order to identify how these factors discriminate the age of menopause for other ethnic populations.
Acknowledgments
The authors acknowledge the help and co-operation of the village local authorities, community leaders and participants. The extensive help of the Department of Anthropology, North Bengal University, is also acknowledged.
Funding
The financial assistance of the University Grants Commission, Government of India, in the form of a National Fellowship for Scheduled Caste [(F1-17.1/2017-18/RGNF-2017-18-SC-WES-42802/ (SA-III)/Website)] is also acknowledged.
Conflicts of Interest
The authors have no conflicts of interests to declare.
Ethical Approval
Research permission/ethical clearance was obtained from the Department of Anthropology, University of North Bengal.






