Introduction
Several studies have shown that preterm birth predicts an increased risk of mental disorders in childhood, adolescence, and young adulthood (Abel et al. Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010; Loe et al. Reference Loe, Lee, Luna and Feldman2011; El Marroun et al. Reference El Marroun, Zeegers, Steegers, van der Ende, Schenk, Hofman, Jaddoe, Verhulst and Tiemeier2012; Fazel et al. Reference Fazel, Bakiyeva, Cnattingius, Grann, Hultman, Lichtenstein and Geddes2012; Nosarti et al. Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013). This risk extends to different mental disorders across diagnostic boundaries (Abel et al. Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010; Fazel et al. Reference Fazel, Bakiyeva, Cnattingius, Grann, Hultman, Lichtenstein and Geddes2012; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013). However, most of this evidence comes from studies comparing preterm individuals born at the lowest end of gestational age or birth weight distributions with individuals born at term and/or with normal weight. Nevertheless, late preterm births, defined as birth from 34 + 0 to 36 + 6 weeks+days of gestation, constitute over 70% of all preterm births (Davidoff et al. Reference Davidoff, Dias, Damus, Russell, Bettegowda, Dolan, Schwarz, Green and Petrini2006; Moster et al. Reference Moster, Lie and Markestad2008).
Only a few studies to date have assessed the long-term consequences of late prematurity on mental disorders. Previous evidence suggests that late preterm birth predicts an increased risk of any mental disorder and particularly of internalizing disorders in childhood (Talge et al. Reference Talge, Holzman, Wang, Lucia, Gardiner and Breslau2010; Rogers et al. Reference Rogers, Lenze and Luby2013). Significant associations to attention deficit hyperactive disorder have also been reported (Linnet et al. Reference Linnet, Wisborg, Agerbo, Secher, Thomsen and Henriksen2006; Talge et al. Reference Talge, Holzman, Wang, Lucia, Gardiner and Breslau2010), but the findings are inconsistent (Rogers et al. Reference Rogers, Lenze and Luby2013). In a study with a follow-up to young adulthood, individuals born late preterm had increased risks of schizophrenia and of disorders of psychological development, behaviour, and emotion (Moster et al. Reference Moster, Lie and Markestad2008). Another register study showed that a combined group of individuals born moderately (at 32 + 0 to 33 + 6 weeks+days of gestation) or late preterm had increased risks of any severe mental disorder, mood, non-affective psychotic, organic, and stress-related disorders, substance dependence, and of suicide and suicide attempts in adolescence and young adulthood (Lindström et al. Reference Lindström, Lindblad and Hjern2009; Nosarti et al. Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012). However, to our knowledge, no previous study has offered a life-course perspective examining whether the possible effects of late preterm birth on mental disorders extend across adult ages from young to old adulthood.
Some studies suggest that the increased risk for mental disorders associated with preterm birth may characterize especially those preterm individuals who were born small for gestational age [SGA; defined as birth size at ⩽2 standard deviations (s.d.s) or below the 5th or 10th percentile of that predicted by their gestational age; Laursen et al. Reference Laursen, Munk-Olsen, Nordentoft and Bo Mortensen2007; Räikkönen et al. Reference Räikkönen, Pesonen, Heinonen, Kajantie, Hovi, Järvenpää, Eriksson and Andersson2008; Strang-Karlsson et al. Reference Strang-Karlsson, Räikkönen, Pesonen, Kajantie, Paavonen, Lahti, Hovi, Heinonen, Järvenpää, Eriksson and Andersson2008; Monfils Gustafsson et al. Reference Monfils Gustafsson, Josefsson, Ekholm Selling and Sydsjö2009]. In fact, previous studies have shown that at least until young adulthood, individuals born SGA are at increased risk of severe mental disorders independently of their gestational age (Abel et al. Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010; Niederkrotenthaler et al. Reference Niederkrotenthaler, Rasmussen and Mittendorfer-Rutz2012; Nosarti et al. Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012). However, we know of no studies that would have examined whether the effects of SGA birth on mental disorders persist from early to late adulthood.
Finally, scarce evidence suggests that post-term (⩾42 weeks of gestation; Lindström et al. Reference Lindström, Fernell and Westgren2005; El Marroun et al. Reference El Marroun, Zeegers, Steegers, van der Ende, Schenk, Hofman, Jaddoe, Verhulst and Tiemeier2012) and large for gestational age (LGA; ⩾+2 s.d.s or above 90th or 95th percentile; van Lieshout & Boyle, Reference van Lieshout and Boyle2011) births may also predict increased risk of psychopathology in childhood and in adolescence. However, to our knowledge, no studies have systematically assessed the effects of post-term birth on mental health in adulthood. Moreover, for LGA birth, such studies are scarce, and the existing studies have yielded inconsistent findings (Moilanen et al. Reference Moilanen, Jokelainen, Jones, Hartikainen, Järvelin and Isohanni2010; Keskinen et al. Reference Keskinen, Miettunen, Koivumaa-Honkanen, Mäki, Isohanni and Jääskeläinen2013).
Hence, in the current study, we examine whether each of these prenatal risk factors contribute to the risks of mental disorders severe enough to lead to hospitalization or contribute to death from early to late adulthood. More specifically, our first study objective was to examine from a life-course perspective if individuals born late preterm differ from their term-born counterparts in their risks of severe mental disorders across adult ages. As a second study question, we studied if the effects of SGA birth across the gestational age distribution, from late preterm to post-term birth, on mental disorders persist across adult ages. We also add to the literature by examining whether post-term or LGA births increase the risk of severe mental disorders in adulthood. Based on previous findings in younger cohorts, we hypothesized that late preterm and post-term births and SGA and LGA births each set forth predisposing effects on the risks of severe mental disorders.
Method
The study sample
Our study cohort was the Helsinki Birth Cohort Study (HBCS). The HBCS comprises 13 345 singleton live births [6975 men (52.3%) and 6370 women (47.7%)] at the two public maternity hospitals in Helsinki, Finland, between 1934 and 1944. The HBCS, described in detail elsewhere (Osmond et al. Reference Osmond, Kajantie, Forsén, Eriksson and Barker2007), has been approved by the Ethics Committee of the National Public Health Institute. For the current study, we excluded all individuals with biologically implausible values for gestational age, both as absolute values and compared to their birth weight. Namely, we excluded individuals with gestational age below 26 weeks or above 44 weeks, and preterm individuals with disproportionally large birth weight for gestational age (> + 2 s.d.; Kajantie et al. Reference Kajantie, Osmond, Barker and Eriksson2010). These criteria led to the exclusion of 535 (4.0%) individuals. Furthermore, since we focused on late preterm births, and our study sample did not provide sufficient statistical power to study the risks of mental disorders among individuals born very or moderately preterm, we excluded 128 (1.0%) participants born before 34 weeks of gestation. From the current study, we also excluded 54 (0.4%) cohort members with missing or imprecise data in the Finnish Hospital Discharge Register (HDR) or the Causes of Death Register (CDR) or who had died with missing data on year of death or moved abroad with missing data on year of moving abroad, and 31 (0.2%) individuals who had been hospitalized for or who had died from injuries of undetermined intent.
The current study sample thus comprised 12 597 individuals, 6563 (52.1%) men and 6034 (47.9%) women. Compared to the included participants, the excluded cohort members came more often from families where the father was a manual worker (63.4 v. 56.7%, p = 0.001) or where the mother was unmarried (7.4% v. 4.7%, p = 0.002). In comparison to the included participants, the excluded cohort members with adequate data on gestational age were more often born SGA (7.0% v. 2.3%, p < 0.001), and the excluded cohort members with adequate diagnostic data had a higher risk of severe mood disorders [hazard ratio (HR) = 1.45, p = 0.01].
Gestational age and fetal growth
We extracted data on the date of mothers’ last menstrual period and on infants’ date of birth and birth weight from hospital birth records. Gestational age was calculated by subtracting the date of birth from mothers’ self-reported date of last menstrual period. The participants were divided into three groups by their gestational age: late preterm (34–36 weeks), term (37–41 weeks), and post-term (42–43 weeks) birth. We defined SGA birth in sex-stratified models as birth weight at or below −2 s.d. of that predicted by gestational age, appropriate for gestational age (AGA) birth as birth weight between −2 and +2 s.d. of that predicted by gestational age, and LGA birth as birth weight at or above +2 s.d. that predicted by gestational age (Lee et al. Reference Lee, Chernausek, Hokken-Koelega and Czernichow2003). Since there are no official growth charts available in Finland for 1934–1944, we defined these indices of fetal growth based on the distributions of birth weight and gestational age in our own study cohort.
Diagnoses of mental disorders
We identified the diagnoses of mental disorders from the HDR and CDR. Our diagnostic follow-up extended across 42 years of adult life, from 1969 to 2010. At the end of the follow-up, the participants were aged between 66 and 76 years. The HDR carries the primary and up to three subsidiary discharge diagnoses of all hospitalizations and the CDR carries the primary, underlying, and contributory causes of death for all deaths in Finland. Both registers have been in use since 1969 when a personal identification number was given to each Finnish citizen. In Finland, International Classification of Diseases, eighth revision (ICD-8) was in use in clinical practice between 1969 and 1986; ICD-9, the Diagnostic and Statistical Manual of Mental Disorders, Third Revision was used between 1987 and 1995; and ICD-10 has been in use since 1996. The HDR (Sund, Reference Sund2012) and the CDR (Lahti & Penttilä, Reference Lahti and Penttilä2001) are valid and reliable research tools. Of mental disorders, the HDR diagnoses of schizophrenia (Pihlajamaa et al. Reference Pihlajamaa, Suvisaari, Henriksson, Heilä, Karjalainen, Koskela, Cannon and Lönnqvist2008), bipolar disorder (Kieseppä et al. Reference Kieseppä, Partonen, Kaprio and Lönnqvist2000), and any psychotic disorder (Perälä et al. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä, Pirkola, Partonen, Tuulio-Henriksson, Hintikka, Kieseppä, Härkänen, Koskinen and Lönnqvist2007) each demonstrate high specificity levels. Also the validity of the HDR ICD-8 diagnoses of alcohol dependence and psychosis has gained research support (Poikolainen, Reference Poikolainen1983).
In addition to assessing the associations of late preterm, post-term, SGA, and LGA birth with severe mental disorders as one broad diagnostic category, we assessed associations to seven specific diagnostic categories; substance use, (non-affective) psychotic, mood, anxiety, and personality disorders and suicides and suicide attempts. The diagnostic codes corresponding to the different diagnostic categories are shown in Table 1, together with the number and the percentage of participants with each diagnosis and the median age at and the age range of first diagnosis. Between 1969 and 2010, there were 1660 participants (13.2%; 1058 men and 602 women) who had been hospitalized with a diagnosis of mental disorder as a hospital discharge diagnosis or had died with a diagnosis of severe mental disorder included in the death certificate. The median age at first diagnosis of any severe mental disorder was 43.4 years (range 19.0–76.6 years).
Table 1. International classification of disease diagnostic codes on mental disorders severe enough to warrant or contribute to hospital treatment (HDR) or to be the underlying, intermediate or contributing cause of death (CDR). The prevalence and percentage of subjects with each diagnosis, and median age at first diagnosis for each diagnostic category

HDR, Finnish Hospital Discharge Register; CDR, Cause of Death Register.
a The diagnoses (corresponding to codes F50–F59 or F62–F69 in ICD-10) were included in the ‘Any mental disorder’ category, but were not assessed as a separate category.
b For the diagnoses of substance intoxifications (ICD-9; 305ICD-10: F1x.0, only the primary diagnoses from the HDR and the CDR were included in the diagnostic categories. All other diagnostic entities include primary and up to three subsidiary hospital discharge diagnoses, and primary, underlying and contributory causes of death.
Suicide is often under-diagnosed (Kapusta et al. Reference Kapusta, Tran, Rockett, De Leo, Naylor, Niederkrotenthaler, Voracek, Etzersdorfer and Sonneck2011), and it is uncertain if the diagnoses of injuries of undetermined intent represent suicide in individual cases. Hence, to maximize the reliability of our suicide assessment and to minimize the amount of ‘false negative’ diagnosis, individuals with a diagnosis of injuries of undetermined intent or of poisonings of usually self-inflicted intent with no history of psychopathology were excluded from the study. We identified these exclusion diagnoses with the diagnostic codes E98 from ICD-8, E97 from ICD-9, and Y10–Y34, T39 and T42–T43 from ICD-10.
Furthermore, for the analyses on the specific diagnostic categories, we excluded from the control outcome group all participants with other mental disorders. Thus, the comparison outcome group for all the analyses included 10 937 individuals (5505 men and 5432 women) with no diagnosis of severe mental disorder or of injuries of undetermined intent at any time point. On the other hand, all individuals with a particular diagnosis were included as ‘diagnostic cases’ for that diagnosis, independently of whether or not they had other types of comorbid psychopathology.
Confounders and covariates
The potential confounders of the associations between the prenatal risk factors and mental disorders that were used in the analyses were sex, year of birth, socioeconomic position in childhood, and mothers’ marital status at childbirth. The latter two were used since they have frequently been associated with preterm birth and with fetal growth (Zeitlin et al. Reference Zeitlin, Saurel-Cubizolles and Ancel2002; Blumenshine et al. Reference Blumenshine, Egerter, Barclay, Cubbin and Braveman2010; El-Sayed et al. Reference El-Sayed, Tracy and Galea2012) and with an increased risk of mental disorders (Mäkikyrö et al. Reference Mäkikyrö, Sauvola, Moring, Veijola, Nieminen, Järvelin and Isohanni1998; Fergusson et al. Reference Fergusson, Boden and Horwood2007; Räikkönen et al. Reference Räikkönen, Lahti, Heinonen, Pesonen, Wahlbeck, Kajantie, Osmond, Barker and Eriksson2011; Bjørngaard et al. Reference Bjørngaard, Bjerkeset, Vatten, Janszky, Gunnell and Romundstad2013).
Data on socioeconomic position in childhood, defined as father's highest attained occupational status [manual worker, (lower or upper) clerical worker, or unknown] was extracted from birth, child welfare, and school records. Data on year of birth, on sex and on mothers’ marital status at childbirth (married, unmarried, or divorced, widowed, or unknown) were extracted from birth records. Most (87.3%) of the participants with fathers’ occupation unknown were born to unmarried mothers, suggesting that the missing paternal occupation data was mostly due to the lack of fathers’ presence in early family life. Therefore we decided to treat them as an own socioeconomic category and include them in the analyses.
Statistical analyses
The associations of the sociodemographic covariates (sex, year of birth, socioeconomic position in childhood, and marital status at childbirth) with late preterm and post-term birth, with SGA and LGA birth, and with severe mental disorders were examined with χ 2 and Cox Proportional Hazards models analyses. Next, we examined the associations of late preterm, post-term, SGA and LGA births with severe mental disorders with Cox Proportional Hazards models. The subjects were followed up either until their death, migration, first hospitalization for mental disorders, or until 31 December 2010. Since suicide attempts were encoded to the HDR only during the use of ICD-8, the analyses on suicide attempts comprised a follow-up extending until 31 December 1986.
First we examined the effects of late preterm and post-term birth and of SGA and LGA birth with severe mental disorders in separate models, which were stratified for year of birth and sex, and adjusted for socioeconomic position in childhood and marital status at childbirth. Thereafter, we ran multivariate models where all the independent variables were entered simultaneously, in order to examine whether their effects occurred independently of each other. Since previous studies have shown that there are sex differences in the prevalence of certain mental disorders (Kessler et al. Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005; de Graaf et al. Reference de Graaf, tenHave, van Gool and van Dorsselaer2012: Steel et al. Reference Steel, Marnane, Iranpour, Chey, Jackson, Patel and Silove2014), and in the early life risk factors for mental disorders (Monfils Gustafsson et al. Reference Monfils Gustafsson, Josefsson, Ekholm Selling and Sydsjö2009), sex was used as a potential confounder, and all the analyses were also repeated separately for men and women.
Results
Table 2 shows the birth and the sociodemographic characteristics of the sample. Compared to individuals born in the later years (between 1939 and 1944), individuals born in the earlier years (between 1934 and 1938) had significantly more often been born late preterm (6.3% v. 5.0%, p = 0.01) and less often post-term (8.4% v. 10.1, p = 0.01). The offspring of unmarried mothers were more often born late preterm (8.8% v. 5.1%, p < 0.001) and SGA (4.1% v. 2.2%, p = 0.003) and less often LGA (0.5% v. 2.5%, p = 0.002) than the offspring of married mothers. Our indicators of fetal growth and gestational age were also significantly associated with each other [χ 2(4) = 58.7, p < 0.001]. Compared to individuals born at term, individuals born late preterm were more often born SGA (3.6% v. 1.9%, p = 0.002) and less often born LGA (0.8% v. 2.5%, p = 0.004). Also individuals born post-term were more often born SGA than individuals born at term (4.9% v. 1.9%, p < 0.001).
Table 2. Birth and sociodemographic characteristics of the study sample

SGA, Small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
On the other hand, men had higher risks of any severe mental disorder, substance use disorders, and suicides than women (Table 1, p values <0.001). Individuals born between 1939 and 1944 had higher risks of any mental (HR = 1.18, p = 0.01), substance use (HR = 1.24, p = 0.01), personality (HR = 1.95, p = 0.002), anxiety (HR = 1.44, p = 0.01), and mood (HR = 1.28, p = 0.02) disorders than the individuals born in the earlier years. The offspring of manual worker fathers had higher risks of any severe mental disorder (HR = 1.15, p = 0.005) and of substance use disorders (HR = 1.23, p = 0.003) than the offspring of clerical workers. Marital status at childbirth was not significantly associated with the risks of severe mental disorders (p values ⩾0.13).
Late preterm and post-term birth
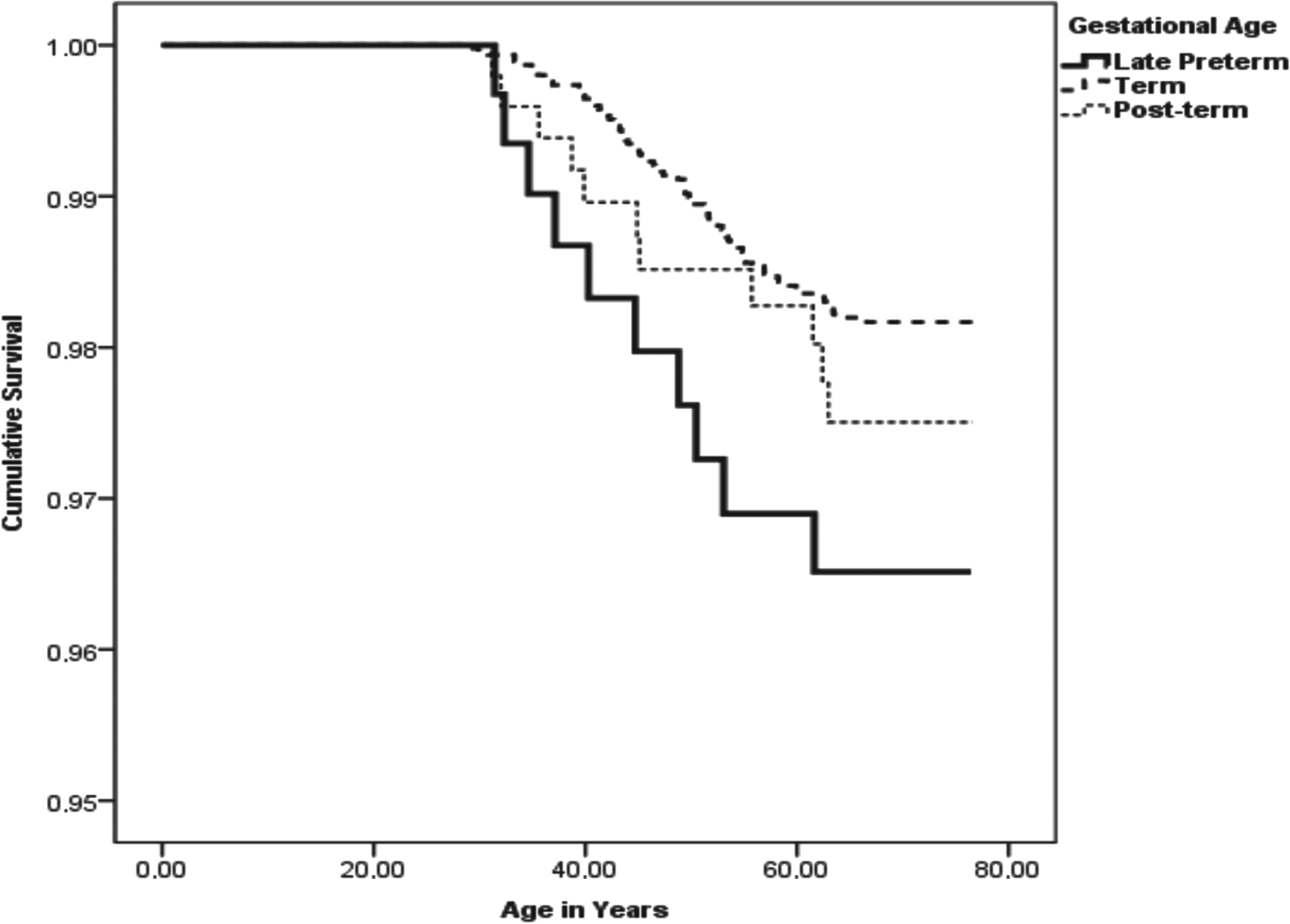
Table 3 shows that stratifying for year of birth and adjusting for socioeconomic position in childhood and marital status at childbirth, late preterm birth was not significantly associated with the risks of any severe mental disorder or of the specific mental disorders in the analyses of all participants (Table 3, p values ⩾0.09) or among women (p values ⩾0.23). However, compared to men born at term, men born late preterm had a significantly increased, twofold risk of suicides (p = 0.03), Fig. 1 shows the survival function to committed suicides for men born late preterm, term, and post-term. As shown in Table 3, this association remained significant after further adjustment for SGA/AGA/LGA status as an indicator of fetal growth (p = 0.04).
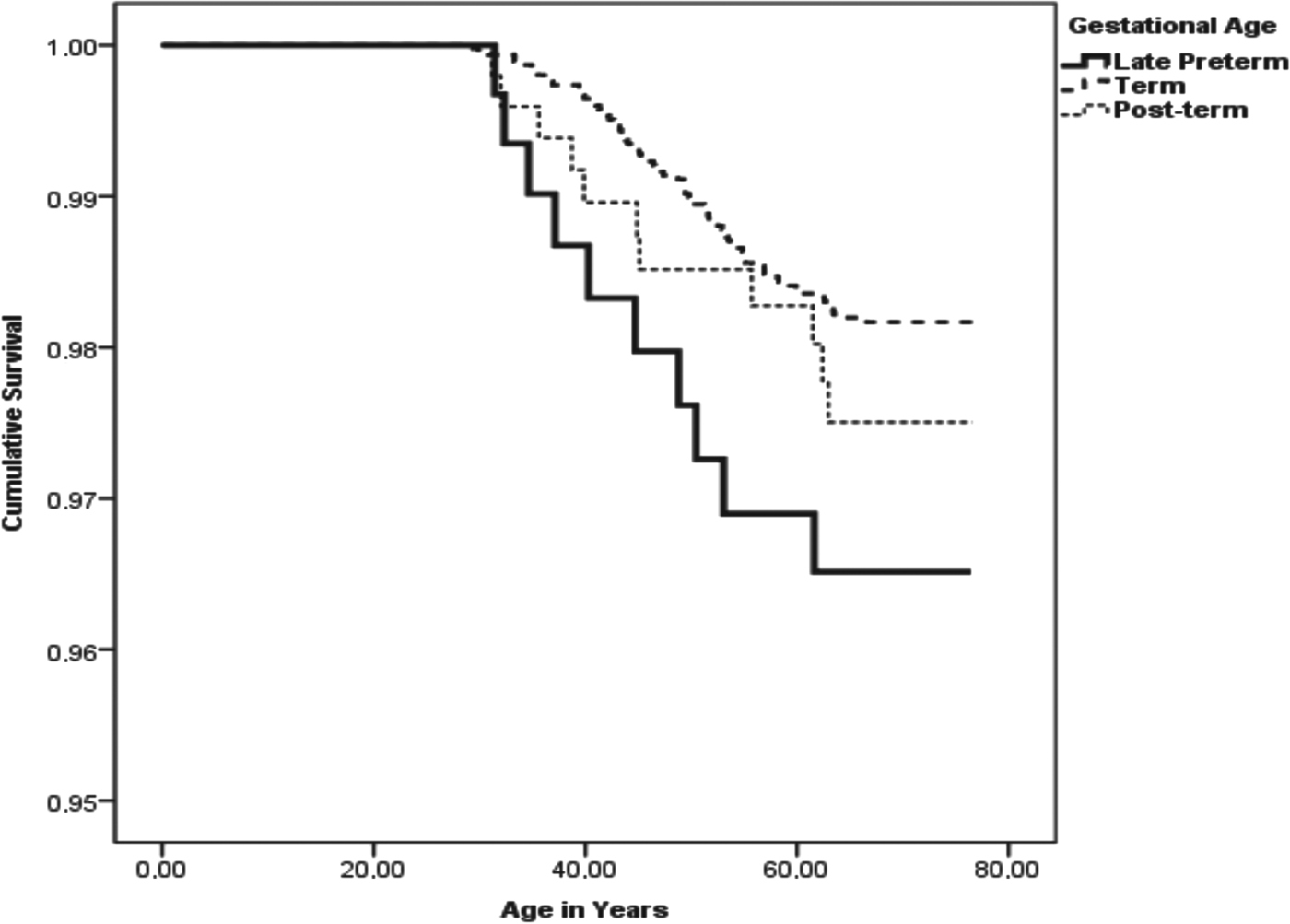
Fig. 1. Gestational age and survival function to committed suicide among men. This figure shows the Kaplan–Meier survival function plots to committed suicide for men born late preterm, term, and post-term.
Table 3. Gestational age and severe mental disorders. Hazard ratios (HR) and 95% confidence intervals (CI) for the associations between late preterm and post-term birth with severe mental disorders. Term-born subjects are the reference group.

n.a., Not applicable.
a The analyses are stratified for sex and year of birth and adjusted for socioeconomic position in childhood and mothers’ marital status at childbirth.
b The analyses are stratified for sex and year of birth and adjusted for fetal growth, socioeconomic position in childhood and mothers’ marital status at childbirth.
Bold font indicates significant effects.
Conversely, compared to term-born participants, individuals born post-term had 1.2-, 1.3-, and 1.4-fold significantly increased risks of any severe mental disorder (p = 0.04), substance use disorders (p = 0.01), and anxiety disorders (p = 0.03) disorders, respectively (Table 3). Sex-specific analyses showed that post-term birth predicted the risks of severe mental disorders especially among men, such that men born post-term had a 1.2-fold risk of any severe mental disorder (Table 3, p = 0.02), a 1.3-fold risk of substance use disorders (p = 0.02), and a 1.6-fold risk of anxiety disorders (p = 0.04). Further adjustments for fetal growth did not lead to noticeable changes in the previously significant findings (all p values ⩽0.05). Post-term birth had no significant effects on mental disorders among women (p values ⩾0.32).
SGA and LGA – status at birth
Table 4 shows that compared to individuals born AGA, individuals born SGA had a 1.4-fold increased risk of any severe mental disorder (p = 0.02) and a 1.7-fold increased risk of substance use disorders (p = 0.01). The association between SGA birth and substance use disorders was more evident and statistically significant among women, for whom SGA birth predicted a 2.4-fold increased risk of substance use disorders (p = 0.01). These effects of SGA birth on mental disorders remained significant after adjustments for gestational age (Table 4, all p values ⩽0.01). In contrast, SGA birth had no significant effects on severe mental disorders among men (p values ⩾0.07). LGA birth was not significantly associated with the risks of severe mental disorders in the analyses of all participants (p values ⩾0.19, Table 4) or among men (p values ⩾0.17). However, women born LGA had at a 2.4-fold, significantly increased risk of psychotic disorders (p = 0.02), compared to women born AGA. Also this association remained significant after adjustment for gestational age (p = 0.01).
Table 4. Fetal growth and severe mental disorders. Hazard ratios (HR) and 95% confidence intervals (95% CI) for individuals born with birth weight for gestational age at below – 2 s.d.: [small for gestational age (SGA)] or above >2 s.d. (large for gestational age (LGA)]. Individuals born with birth weight appropriate for gestational age (AGA; between −2 and 2 s.d.) are used as the reference group

n.a., Not applicable.
a The analyses are stratified for sex and year of birth and adjusted for socioeconomic position in childhood and mothers’ marital status at childbirth.
b The analyses are stratified for sex and year of birth and adjusted for gestational age, socioeconomic position in childhood and mothers’ marital status at childbirth.
Bold font indicates significant effects.
Interactions by sex
Since some of the findings were more evident among one or the other sex, we examined in separate Cox models whether there were significant interactions of late preterm or post-term birth and/or of SGA or LGA births with sex in predicting the risks of severe mental disorders. We found a significant interaction between LGA status at birth and sex in predicting the risk of psychotic disorders (p = 0.04), but there were no other significant interactions by sex (all p values ⩾0.21).
Discussion
In this longitudinal cohort study from birth to late adulthood, late preterm birth was not associated with the risk of any severe mental disorder, but it predicted a significantly increased risk of suicides among men. On the other hand, both birth as SGA and as post-term emerged as significant risk factors for any severe mental disorder and for substance use disorders across adult life. Of other mental disorders, post-term birth also predicted the risk of anxiety disorders. LGA birth predicted an increased risk of psychotic disorders among women. These effects of late preterm and post-term birth and of SGA and LGA status at birth on severe mental disorders occurred independently of each other. Our study with its long diagnostic follow-up adds significantly to the previous literature by suggesting that these prenatal risk factors exert long-lasting effects on severe mental disorders throughout adult ages.
Late preterm birth predicted an increased risk of suicides among men. Correspondingly, a previous study showed that moderate or late preterm birth predicted an increased risk of suicides and/or suicide attempts in young adulthood (Lindström et al. Reference Lindström, Lindblad and Hjern2009) but whether this effect was more pronounced on committed or attempted suicide was not specified. However, in contrast to studies in younger cohorts showing predisposing effects of late preterm birth on any mental disorder and on internalizing disorders in childhood (Talge et al. Reference Talge, Holzman, Wang, Lucia, Gardiner and Breslau2010; Rogers et al. Reference Rogers, Lenze and Luby2013), or of moderate or late preterm birth on severe mental disorders across diagnostic boundaries in young adulthood (Moster et al. Reference Moster, Lie and Markestad2008; Lindström et al. Reference Lindström, Lindblad and Hjern2009; Nosarti et al. Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012), here late preterm birth was not associated with other types of severe psychopathology from early to late adulthood.
As a novel contribution, we demonstrated that individuals born post-term had increased risks of any severe mental disorder and particularly of substance use and anxiety disorders in adulthood. Corresponding effects have been reported previously on psychopathology risk in childhood. Namely, El Marroun et al. (Reference El Marroun, Zeegers, Steegers, van der Ende, Schenk, Hofman, Jaddoe, Verhulst and Tiemeier2012) found that children born post-term had more emotional and behavioural disorders at the ages of 1.5 and 3 years, and another study found that children born post-term have an increased risk of neurodevelopmental disorders (Lindström et al. Reference Lindström, Fernell and Westgren2005). Collectively these findings highlight the importance of assessing post-term birth as a possible independent risk factor for mental disorders. Nevertheless, studies on psychopathology risk among individuals born post-term are still scarce and the findings warrant replication.
In agreement with a representative study with a follow-up extending to young adulthood (Abel et al. Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010), SGA birth predicted increased risks of any severe mental disorder and of substance use disorders throughout adulthood in our study. However, in the study by Abel et al. (Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010), SGA birth also predicted increased risks of schizophrenia and of anxiety disorders while we found no significant effects of SGA birth on non-affective psychotic disorders or on anxiety disorders. The differences in the findings may be due to the different ages covered in the diagnostic follow-ups; our follow-up extended to between 65 and 75 years of age, while Abel et al. (Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010) followed up the subjects only until young adulthood. In contrast, in our study, among women, LGA birth predicted an increased risk of psychotic disorders. Previous studies have shown that LGA birth may predict psychopathology risk in childhood and adolescence (van Lieshout & Boyle, Reference van Lieshout and Boyle2011), but findings on adulthood effects are scarce and inconsistent (Moilanen et al. Reference Moilanen, Jokelainen, Jones, Hartikainen, Järvelin and Isohanni2010; Keskinen et al. Reference Keskinen, Miettunen, Koivumaa-Honkanen, Mäki, Isohanni and Jääskeläinen2013).
We also examined whether the associations varied by sex. The effects of SGA and LGA birth on severe mental disorders were more evident among women, while late preterm and post-term birth had stronger effects on psychopathology risk among men. Previous findings on the sex-specificity of the effects of these prenatal risk factors on mental disorders are inconsistent (Monfils Gustafsson et al. Reference Monfils Gustafsson, Josefsson, Ekholm Selling and Sydsjö2009; Abel et al. Reference Abel, Wicks, Susser, Dalman, Pedersen, Mortensen and Webb2010). Importantly, however, here significant interactions with sex emerged only for LGA birth in predicting psychotic disorders, not for the other examined risk factors. Hence, the potential sex-specificity of our findings must be interpreted with much caution.
Potential contributing factors to our significant findings may include exposure to prenatal malnutrition, to substance use during pregnancy, and to perinatal complications, overexposure to glucocorticoids as a consequence of maternal psychosocial stress during pregnancy, and exposure to pregnancy disorders such as pre-eclampsia and gestational diabetes. Of pregnancy disorders, gestational diabetes predicts increased risks of preterm (Hedderson et al. Reference Hedderson, Ferrara and Sacks2003; Fadl et al. Reference Fadl, Östlund, Magnuson and Hanson2010; Zhang et al. Reference Zhang, Liu, Gao, Wang, Gu, Zhang, Zhou and Li2012) and LGA (Hedderson et al. Reference Hedderson, Ferrara and Sacks2003; Fadl et al. Reference Fadl, Östlund, Magnuson and Hanson2010) births, while hypertensive disorders during pregnancy are associated with an increased likelihood of preterm (Clausson et al. Reference Clausson, Cnattingius and Axelsson1998; Hedderson et al. Reference Hedderson, Ferrara and Sacks2003; Kajantie et al. Reference Kajantie, Eriksson, Osmond, Thornburg and Barker2009; Ferrazzani et al. Reference Ferrazzani, Luciano, Garofalo, D'Andrea, De Carolis, De Carolis, Paolucci, Romagnoli and Caruso2011; Zhang et al. Reference Zhang, Liu, Gao, Wang, Gu, Zhang, Zhou and Li2012) and SGA (Clausson et al. Reference Clausson, Cnattingius and Axelsson1998; Ferrazzani et al. Reference Ferrazzani, Luciano, Garofalo, D'Andrea, De Carolis, De Carolis, Paolucci, Romagnoli and Caruso2011; Leviton et al. Reference Leviton, Fichorova, O'Shea, Kuban, Paneth, Dammann and Allred2013) births. Also maternal smoking (Clausson et al. Reference Clausson, Cnattingius and Axelsson1998; Heaman et al. Reference Heaman, Kingston, Chalmers, Sauve, Lee and Young2013) and psychosocial stress (Zhang et al. Reference Zhang, Liu, Gao, Wang, Gu, Zhang, Zhou and Li2012; Heaman et al. Reference Heaman, Kingston, Chalmers, Sauve, Lee and Young2013) during pregnancy are both associated with increased risks of preterm and SGA deliveries. Particular types of malnutrition during pregnancy have been shown to contribute to the risks of SGA (Dwarkanath et al. Reference Dwarkanath, Barzilay, Thomas, Thomas, Bhat and Kurpad2013), LGA (Knudsen et al. Reference Knudsen, Heitmann, Halldorsson, Sørensen and Olsen2013) and preterm (Zhang et al. Reference Zhang, Liu, Gao, Wang, Gu, Zhang, Zhou and Li2012; Leventakou et al. Reference Leventakou, Roumeliotaki, Martinez, Barros, Brantsaeter, Casas, Charles, Cordier, Eggesbø, van Eijsden, Forastiere, Gehring, Govarts, Halldórsson, Hanke, Haugen, Heppe, Heude, Inskip, Jaddoe, Jansen, Kelleher, Meltzer, Merletti, Moltó-Puigmartí, Mommers, Murcia, Oliveira, Olsen, Pele, Polanska, Porta, Richiardi, Robinson, Stigum, Strøm, Sunyer, Thijs, Viljoen, Vrijkotte, Wijga, Kogevinas, Vrijheid and Chatzi2014) births. On the other hand, compared to term-born individuals, certain perinatal complications (e.g. asphyxia) are more common both among individuals born post-term (Thorngren-Jerneck & Herbst, Reference Thorngren-Jerneck and Herbst2001; Olesen et al. Reference Olesen, Westergaard and Olsen2003) and among individuals born preterm (Gouyon et al. Reference Gouyon, Vintejoux, Sagot, Burguet, Quantin and Ferdynus2010). All these factors have also been associated with increased risks of certain mental disorders (Dalman et al. Reference Dalman, Thomas, David, Gentz, Lewis and Allebeck2001; Gardener et al. Reference Gardener, Spiegelman and Buka2009; Räikkönen et al. Reference Räikkönen, Pesonen, Heinonen, Lahti, Komsi, Eriksson, Seckl, Järvenpää and Strandberg2009; Ekblad et al. Reference Ekblad, Gissler, Lehtonen and Korkeila2010; Rice et al. Reference Rice, Harold, Boivin, van den Bree, Hay and Thapar2010; Tuovinen et al. Reference Tuovinen, Räikkönen, Kajantie, Pesonen, Heinonen, Osmond, Barker and Eriksson2010; Bao et al. Reference Bao, Ibram, Blaner, Quesenberry, Shen, McKeague, Schaefer, Susser and Brown2012; Fazel et al. Reference Fazel, Bakiyeva, Cnattingius, Grann, Hultman, Lichtenstein and Geddes2012).
Previous studies have found that neurological impairments are more common among individuals born late preterm (Petrini et al. Reference Petrini, Dias, McCormick, Massolo, Green and Escobar2009) post-term (Lindström et al. Reference Lindström, Fernell and Westgren2005; Moster et al. Reference Moster, Wilcox, Vollset, Markestad and Lie2010) and SGA (Leviton et al. Reference Leviton, Fichorova, O'Shea, Kuban, Paneth, Dammann and Allred2013). Altered neurodevelopment as a consequence of prenatal adversity and contributing to the risk of mental disorders may hence have contributed to our findings (Loe et al. Reference Loe, Lee, Luna and Feldman2011).
More specifically, neurobiological explanations underlying our findings may involve possibly epigenetic changes in the functioning of the brain's stress system, as a consequence of prenatal adversity, and contributing to an increased risk of mental disorders (Lupien et al. Reference Lupien, McEwen, Gunnar and Heim2009; Bock et al. Reference Bock, Rether, Gröger, Xie and Braun2014). The brain's stress system includes the hypothalamus-pituitary-adrenal (HPA) axis and the interconnected brain regions amygdala, hippocampus, and the prefrontal cortex (Lupien et al. Reference Lupien, McEwen, Gunnar and Heim2009; Bock et al. Reference Bock, Rether, Gröger, Xie and Braun2014). SGA (De Bie et al. Reference De Bie, Oostrom, Boersma, Veltman, Barkhof, Delemarre-van de Waal and van den Heuvel2011; Parikh et al. Reference Parikh, Lasky, Kennedy, McDavid and Tyson2013) and preterm (Parikh et al. Reference Parikh, Lasky, Kennedy, McDavid and Tyson2013; Thompson et al. Reference Thompson, Adamson, Roberts, Faggian, Wood, Warfield, Doyle, Anderson, Egan and Inder2013) births, individual differences in birth size (De Bie et al. Reference De Bie, Oostrom, Boersma, Veltman, Barkhof, Delemarre-van de Waal and van den Heuvel2011; Reynolds et al. Reference Reynolds, Walker, Syddall, Andrew, Wood and Phillips2005), and particular prenatal adversities (Entringer et al. Reference Entringer, Kumsta, Hellhammer, Wadhwa and Wüst2009; Buss et al. Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; O'Connor et al. Reference O'Connor, Bergman, Sarkar and Glover2013; Rifkin-Graboi et al. Reference Rifkin-Graboi, Bai, Chen, Hameed, Sim, Tint, Leutscher-Broekman, Chong, Gluckman, Fortier, Meaney and Qiu2013; Bock et al. Reference Bock, Rether, Gröger, Xie and Braun2014) have each been associated with long-lasting structural and functional changes in the brain regions implicated in the stress system. Furthermore, maternal cortisol levels during pregnancy have been shown to predict psychiatric symptoms in the offspring (Buss et al. Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012), and this effect was partially mediated by changes in child's amygdala volume. In addition, altered functioning of the brain's stress system has been found among patients with different mental disorders, for example among patients with depression (Vreeburg et al. Reference Vreeburg, Zitman, van Pelt, Derijk, Verhagen, van Dyck, Hoogendijk, Smit and Penninx2010; Stetler & Miller, Reference Stetler and Miller2011; Carvalho Fernando et al. Reference Carvalho Fernando, Beblo, Schlosser, Terfehr, Otte, Löwe, Wolf, Spitzer, Driessen and Wingenfeld2012), anxiety disorders (Ipser et al. Reference Ipser, Singh and Stein2013), post-traumatic stress disorder (Morris et al. Reference Morris, Compas and Garber2012; Patel et al. Reference Patel, Spreng, Shin and Girard2012), and borderline personality disorder (Carrasco et al. Reference Carrasco, Díaz-Marsá, Pastrana, Molina, Brotons, López-Ibor and López-Ibor2007; Carvalho Fernando et al. Reference Carvalho Fernando, Beblo, Schlosser, Terfehr, Otte, Löwe, Wolf, Spitzer, Driessen and Wingenfeld2012; Ruocco et al. Reference Ruocco, Amirthavasagam and Zakzanis2012).
On a cellular level, the effects may be mediated by epigenetic changes affecting gene expression. Epigenetic DNA methylation changes are found among patients with mental disorders (Dempster et al. Reference Dempster, Pidsley, Schalkwyk, Owens, Georgiades, Kane, Kalidindi, Picchioni, Kravariti, Toulopoulou, Murray and Mill2011; Sabunciyan et al. Reference Sabunciyan, Aryee, Irizarry, Rongione, Webster, Kaufman, Murakami, Lessard, Yolken, Feinberg and Potash2012). Such methylation changes are also associated with individual differences in birth size (Shams et al. Reference Shams, Kilby, Somerset, Howie, Gupta, Wood, Afnan and Stewart1998; Dy et al. Reference Dy, Guan, Sampath-Kumar, Richardson and Yang2008; Michels et al. Reference Michels, Harris and Barault2011; Börzsönyi et al. Reference Börzsönyi, Demendi, Pajor, Rigó, Marosi, Agota, Nagy and Joó2012; Liu et al. Reference Liu, Murphy, Murtha, Fuemmeler, Schildkraut, Huang, Overcash, Kurtzberg, Jirtle, Iversen, Forman and Hoyo2012; Marsit et al. Reference Marsit, Maccani, Padbury and Lester2012) and gestation length (Demendi et al. Reference Demendi, Börzsönyi, Pajor, Rigó, Nagy, Szentpéteri and Joó2012; Marsit et al. Reference Marsit, Maccani, Padbury and Lester2012) and with specific prenatal or neonatal adversities (Oberlander et al. Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008; Liu et al. Reference Liu, Murphy, Murtha, Fuemmeler, Schildkraut, Huang, Overcash, Kurtzberg, Jirtle, Iversen, Forman and Hoyo2012; Wehkalampi et al. Reference Wehkalampi, Muurinen, Wirta, Hannula-Jouppi, Hovi, Järvenpää, Eriksson, Andersson, Kere and Kajantie2013), possibly particularly in genes regulating HPA axis function (Shams et al. Reference Shams, Kilby, Somerset, Howie, Gupta, Wood, Afnan and Stewart1998; Dy et al. Reference Dy, Guan, Sampath-Kumar, Richardson and Yang2008; Oberlander et al. Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008; Börzsönyi et al. Reference Börzsönyi, Demendi, Pajor, Rigó, Marosi, Agota, Nagy and Joó2012; Demendi et al. Reference Demendi, Börzsönyi, Pajor, Rigó, Nagy, Szentpéteri and Joó2012; Liu et al. Reference Liu, Murphy, Murtha, Fuemmeler, Schildkraut, Huang, Overcash, Kurtzberg, Jirtle, Iversen, Forman and Hoyo2012; Marsit et al. Reference Marsit, Maccani, Padbury and Lester2012; Wehkalampi et al. Reference Wehkalampi, Muurinen, Wirta, Hannula-Jouppi, Hovi, Järvenpää, Eriksson, Andersson, Kere and Kajantie2013). For example, reduced expression of the placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) gene regulating the 11β-HSD2 enzyme that protects the fetus from circulating maternal glucocorticoids is found among individuals born SGA (Shams et al. Reference Shams, Kilby, Somerset, Howie, Gupta, Wood, Afnan and Stewart1998; Dy et al. Reference Dy, Guan, Sampath-Kumar, Richardson and Yang2008; Börzsönyi et al. Reference Börzsönyi, Demendi, Pajor, Rigó, Marosi, Agota, Nagy and Joó2012) and among individuals born preterm (Demendi et al. Reference Demendi, Börzsönyi, Pajor, Rigó, Nagy, Szentpéteri and Joó2012). Evidence from animal studies and from epigenetic and observational studies in humans suggest that lower placental 11β-HSD2 activity is also associated with poorer neurobehavioural development in the offspring (Welberg et al. Reference Welberg, Seckl and Holmes2000; Räikkönen et al. Reference Räikkönen, Pesonen, Heinonen, Lahti, Komsi, Eriksson, Seckl, Järvenpää and Strandberg2009; Marsit et al. Reference Marsit, Maccani, Padbury and Lester2012). Hence, epigenetic changes in gene expression as a consequence of prenatal adversity, influencing neurodevelopment and thereby affecting the risk of mental disorders may possibly have played an underlying role in our findings. Furthermore, there is evidence of differential prenatal epigenetic programming effects for boys and girls (Liu et al. Reference Liu, Murphy, Murtha, Fuemmeler, Schildkraut, Huang, Overcash, Kurtzberg, Jirtle, Iversen, Forman and Hoyo2012), which may help in explaining the potential sex-specificity of the findings. However, since there is a hereditary component affecting gestation length and prenatal growth (Clausson et al. Reference Clausson, Lichtenstein and Cnattingius2000; Silventoinen et al. Reference Silventoinen, Pietiläinen, Tynelius, Sørensen, Kaprio and Rasmussen2008; Oberg et al. Reference Oberg, Frisell, Svensson and Iliadou2013) and the risk of mental disorders (Kendler et al. Reference Kendler, Aggen, Knudsen, Røysamb, Neale and Reichborn-Kjennerud2011), genetic predispositions may also have contributed to our findings.
The strengths of our study include the hospital birth record-based data on gestational age and on birth size and the long diagnostic follow-up period enabling us to examine effects on psychopathology throughout a wide age interval in adulthood. However, there are also limitations. Since we extracted data on mental disorders diagnoses only from the HDR and CDR, our findings generalize best to severe mental disorders requiring hospitalization or contributing to death. Using only these registers misses the outpatient diagnosis, and the generalizability of our findings to less severe psychopathology is limited. The use of only hospital discharge and causes of death registers for diagnosis identification also accounts for the rather low prevalence of anxiety and mood disorders in our sample. It is also of note that we did not find the sex-difference in the prevalence of mood or anxiety disorders that is usually consistently observed in population-based epidemiological studies (Kessler et al. Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005; Steel et al. Reference Steel, Marnane, Iranpour, Chey, Jackson, Patel and Silove2014).
Furthermore, since we had diagnostic data available only from 1969 onwards, we were unable to identify those individuals who were hospitalized or who had died with mental disorders before this year, before they reached the ages 24–35 years and were not hospitalized again thereafter. Many mental disorders have their onset in childhood, adolescence, or early adulthood, and we lack information from those age periods in our sample. Our findings hence best generalize to mental disorders that lead to hospitalization or contribute to death between the ages 24–35 and 65–76 years. There are hence some false negatives in our sample: participants who had severe mental disorders early but not later in adulthood. A bias introduced to the findings by this lack of information is possible. However, considering that a major aim of the current study was to study if corresponding effects to those found previously in younger cohorts are also found on mental disorders later in life, then for this purpose the age range for the diagnostic follow-up is well-justified. Corresponding effects to younger ages were indeed found, especially for SGA and post-term births. On the other hand, the number of individuals in the prenatal risk conditions was too small to reliably study interactions between these factors in predicting mental disorders, although such interactions have been suggested by some previous research (Laursen et al. Reference Laursen, Munk-Olsen, Nordentoft and Bo Mortensen2007; Monfils Gustafsson et al. Reference Monfils Gustafsson, Josefsson, Ekholm Selling and Sydsjö2009). Furthermore, we had no data on parental mental disorders that may modify the effects of prenatal adversity on mental disorders (Niederkrotenthaler et al. Reference Niederkrotenthaler, Rasmussen and Mittendorfer-Rutz2012; Keskinen et al. Reference Keskinen, Miettunen, Koivumaa-Honkanen, Mäki, Isohanni and Jääskeläinen2013). Because suicide attempts were encoded to the HDR only until 1986, the diagnostic follow-up period for them was not comparable to the other diagnoses. This probably also explains the lower number of suicide attempts than committed suicides in our study sample. Overall, the two changes to the diagnostic classification system during the follow-up may have created heterogeneity to the diagnostic groups, which is an inherent problem to register-based studies with long follow-ups.
In conclusion, although late preterm birth predicted the risk of suicides among men, it did not have more widespread effects on severe mental disorders from early to late adulthood. On the other hand, SGA birth exerted predisposing effects on severe mental disorders across adult ages. Post-term birth emerged as a novel prenatal risk factor for severe adult mental disorders. LGA birth predicted the risk of psychotic disorders among women. Our longitudinal study findings suggest that the effects of prenatal developmental adversity on severe mental disorders may extend throughout adulthood.
Acknowledgements
This study was supported by grants from the Academy of Finland (grant numbers 261661, 140278, 135132, 132614, 129911, and 129457), University of Helsinki, the Finnish Foundation of Cardiovascular Research, the Finnish Diabetes Research Foundation, the Finnish Medical Society, Finska Läkaresällskapet, the National Doctoral Programme of Psychology, the Päivikki and Sakari Sohlberg Foundation, the Juho Vainio Foundation, the Yrjö Jahnsson Foundation, Samfundet Folkhälsan, the Signe and Ane Gyllenberg Foundation, the Jalmari and Rauha Ahokas Foundation, the Emil Aaltonen Foundation, the Finnish Ministry of Education and the Finnish Foundation for Paediatric Research. The funders were not involved in the conduct of the study or in the collection, management, analysis or interpretation of the data.
Declaration of Interest
None.