INTRODUCTION
Colonial organisms tend to have zooids with simple morphs and few characters are available to discriminate among species. Therefore, species identification is often difficult in the colonial organisms such as cnidarians and tunicates. Ascidian species of the family Didemnidae are always colonial, and some didemnid species inhabiting tropical waters harbour symbiotic cyanophytes, Prochloron and Synechocystis (see Lewin & Cheng, Reference Lewin and Cheng1989). Faunal surveys were recently conducted of the photosymbiotic didemnids in the Ryukyu Archipelago and Bonin Islands, Japan (Hirose et al., Reference Hirose, Kamijoh and Oka2007 and some references therein) and encountered some species with taxonomic debates or problems. Several potentially cryptic species in Didemnum molle were also found; partial mitochondrial gene sequences discriminated four morphotypes, white, brown, grey, and large (Hirose et al., Reference Hirose, Yokobori and Hirose2009b). Moreover, six photosymbiotic didemnids from the Ryukyus were recently described as new species (Oka et al., Reference Oka, Suetsugu and Hirose2005; Hirose & Oka, Reference Hirose and Oka2008; Hirose et al., Reference Hirose, Oka and Hirose2009a; Hirose & Hirose, Reference Hirose and Hirose2009), suggesting that there may be other undescribed species in this area.
Calcareous spicules of various shapes are contained in the tunic of many colonial ascidians of the families Didemnidae and Polycitoridae and some solitary ascidians of Pyuridae. Individual species have spicules of a specific shape and size-range that can be useful features for specific identification (e.g. Turon, Reference Turon1986; Monniot et al., Reference Monniot, Monniot and Laboute1991; Kott, Reference Kott2001), especially among congeners with very similar appearances. For instance, the zooids of Lissoclinum bistratum are very similar in morphology to those of L. timorense (>L. voeltzkowi), but colonies of the former contain only globular spicules and colonies of the latter contain both globular and stellate spicules (see Kott, Reference Kott2001). However, discrimination of species that closely resemble each other based on spicule shape is contested by some taxonomists. For example, for the Lissoclinum species mentioned above, Monniot & Monniot (Reference Monniot and Monniot2001) proposed that L. timorense is a junior synonym of L. bistratum, with the variability of spicules representing intraspecific variation. Similarly, Trididemnum cyclops can be discriminated from T. paracyclops by spicule size, colony size, and some other features of zooids and larvae (see Kott, Reference Kott2001), although Monniot & Monniot (Reference Monniot and Monniot1987) assigned both species to T. cyclops and regarded the spicule differences as intraspecific variation. Kott (Reference Kott2004) described a new species of Didemnum from the north-east coast of the USA based in large part on some differences in spicules from other known species, but it was later proven to be a worldwide invader that happens to produce variable spicules (Lambert, Reference Lambert2009).
Here, we conducted a quantitative study of spicule shape and size in some photosymbiotic didemnid ascidian species. In combination with DNA barcodes, we evaluated the taxonomic significance of tunic spicules in some closely related species.
MATERIALS AND METHODS
Animals
Colonies of photosymbiotic ascidians were collected by snorkelling at shallow coral reefs in the Ryukyu Archipelago, Japan. Species collected were Didemnum molle, Lissoclinum bistratum, L. timorense, Trididemnum cyclops, T. paracyclops, and T. nubilum (Table 1). The identification of each species was primarily based on colony appearance in the field and was confirmed under a stereomicroscope, mainly following Kott (Reference Kott2001). The four morphotypes of D. molle were easily distinguished by colony colour and colony size (Hirose et al., Reference Hirose, Yokobori and Hirose2009b). The specimens were fixed in 10% formalin–seawater for microscopy and in 99% ethanol for DNA analysis. We used different specimens for spicule analyses and DNA sequences. At least two specimens from two or more sites for each species or morphotype were examined.
Table 1. List of the specimens for SEM observation and molecular phylogeny.

*, References: a, Hirose et al. (2009b); b, this study. Collection site: (1) Seragaki, Okinawajima Island (26°30′30″N 127°51′50″E); (2) Seokojima Island (26°30′00″N 127°51′52″E); (3) Ikei, Ikijima Island (26°22′55″N 127°59′55″E); (4) Bise, Okinawaji Island (26°42′40″N 127°52′30″E); (5) Ara beach, Kumejima Island (26°19′0″N 126°46′30″E); (6) Zanpa, Okinawajima Island (26°26′10″N 127°42′40″E); (7) Odo, Okinawajima Island (26°5′20″N 127°42′30″E); (8) Shiraho, Ishigakijima Island (24°20′55″N 124°15′22″E); (9) Hirakubo, Ishigakijima Island (24°36′50″N 124°19′0″E); (10) Ugachi, Okinawajima Island (26°26′00″N 127°44′00″E); (11) Manza, Okinawajima Island (26°26′30″N 127°50′00″E); (12) Ginoza, Okinwajima Island (26°29′N 128°0′E); (13) Maeda, Okinawajima Island (26°26′30″N 127°46′0″E); (14) Sumiyoshi, Iriomore Island (24°26′20″N 123°46′40″E); (15) Nyu, Fukui (35°42′41″N 135°58′10″E); (16) Shinrihama, Kumejima Island (26°21′0″N 126°42′50″E).
Microscopy
Tunic pieces containing spicules were cut off from the fixed colonies; the lateral side of the D. molle colonies and the colonial margins in the other species. The specimens were immersed in 2–3% sodium hypochlorite for two days. Following a rinse with distilled water, the spicules were air-dried on aluminium stubs 15 mm in diameter. The dried specimens were sputter coated with gold-palladium and observed under a JEOL JSM-6060LV scanning electron microscope (SEM) in low vacuum mode (~30 Pa). In D. molle, T. cyclops, and T. paracyclops, the diameter of spicules was measured from the digital images using ImageJ 1.41 (rsbweb.nih.gov). We examined 100 spicules for each specimen. For the stellate spicules of T. cyclops and T. paracyclops, we used the length between the bases of opposite cones as the spicule diameter, because the tips of the cones were sometimes broken. The variance in spicule diameter among specimens was tested using a Kruskal–Wallis test and Dunn's multiple comparison test, since some data were not normal. Statistical analyses were performed using Instat 3 for Macintosh (GraphPad Software).
DNA extraction, amplification, and sequencing
The preserved colonies were dissected under a stereomicroscope and about 30–40 zooids were suspended in 400 µl CTAB buffer (2% hexadecyltrimethyl ammonium bromide (CTAB), 1.0 M NaCl, 75 mM EDTA pH 8.0, and 35 mM Tris-HCl; pH 8.0) containing 0.1% sodium dodecyl sulphate (SDS) and 0.2% beta-mercaptoethanol, following the methods of Hirose et al. (Reference Hirose, Yokobori and Hirose2009b). The samples were incubated at 65°C for 1 h. Next, proteinase K was added to the samples to a final concentration of 0.1 mg ml−1, and the samples were incubated overnight at 37°C. DNA was then extracted with phenol-chloroform as described by Sambrook et al. (Reference Sambrook, Fritsch and Maniatis1989). The PCR amplification of the cytochrome c oxidase subunit I (COI) was performed using EX Taq DNA polymerase (Takara) and a combination of the degenerate primers UroCox1-244F (5′-CATTTWTTTTGATTWTTTRGWCATCCNGA-3′) and UroCox1-387R (5′-GCWCYTATWSWWAAWACATAATGAAARTG-3′) (Hirose et al., Reference Hirose, Yokobori and Hirose2009b). The PCR amplification was performed under the following conditions: 94°C for 5 minutes; followed by 35 cycles of 94°C for 10 seconds, 40°C for 5 seconds, and 72°C for 2 minutes; with a final extension at 72°C for 7 minutes. For cloning, the TOPO TA cloning kit for sequencing (Invitrogen) was used. Cycle sequencing reactions were performed using DTCS Quick Start Master Mix (Beckman Coulter), and products were analysed using a CEQ8800 (Beckman Coulter) automated DNA sequencing system. At least five clones were randomly selected for each sample and sequenced. The sequences determined in this study were deposited in GenBank, the European Molecular Biology Laboratory (EMBL), and the DNA Data Bank of Japan (DDBJ); Accession numbers are provided in Table 1.
Phylogenetic analysis
Phylogenetic trees were built separately for each genus. Initial sequence alignments were performed using CLUSTAL W 1.83 (Thompson et al., Reference Thompson, Higgins and Gibson1994); alignments were then inspected by eye and manually edited. The following analyses were performed on the aligned DNA sequences of the partial COI: maximum likelihood (ML) using TREEFINDER version October 2008 (Jobb, Reference Jobb2008), and maximum parsimony (MP) and neighbour-joining (NJ) using PAUP* 4.0 beta10 (Swofford, Reference Swofford2003). To select an appropriate nucleotide substitution model, Modeltest v. 3.7 (Posada & Crandall, Reference Posada and Crandall1998) was used with PAUP. For data with all three codon positions from Didemnum spp., the Hasegawa, Kishino, Yano 85 (HKY85) model was selected as the best model based on Akaike's information criterion (AIC). For data with all three codon positions from Lissoclinum spp. and Trididemnum spp., transitional model (TIM) was selected under AIC. The MP trees were searched using a heuristic approach with 100 random initial trees. Statistical support for the ML, MP, and NJ trees was evaluated using a non-parametric bootstrap test with 1000 re-sampling events.
RESULTS AND DISCUSSION
Four morphotypes of Didemnum molle
Brown-, grey-, and white-type colonies of Didemnum molle were usually symmetrical domes of 2 cm or less in diameter; the brown- and grey-type colonies exhibited those respective pigmentation colours. Large-type colonies were irregular in shape, 5 cm or more in the long axis, and had grey patches. In all morphotypes, colonies always contained globular spicules and there were no differences in spicule shape among morphotypes (Figure 1). Spicule diameter varied considerably within each colony (Figure 2), and variation among specimens was significant (Kruskal–Wallis test, P < 0.001). A significant difference was found between some specimens in the same morphotype but not between combinations of different morphotypes (Dunn's multiple comparison test, P > 0.05).

Fig. 1. Tunic spicules of the four morphotypes of Didemnum molle. (A) Brown type (Seragaki, Okinawajima Island); (B) grey type (Ara Beach, Kumejima Island); (C) white type (Odo, Okinawajima Island); (D) large type (Seragaki). Scale bars: 10 µm.

Fig. 2. Size variation of the tunic spicules of the four morphotypes of Didemnum molle. Each bar indicates the average of 100 spicules from one specimen with standard deviation. The numbers under the bars indicate the collection site (see Table 1).
A total of eleven haplotypes were found among the 31 specimens of D. molle. The aligned D. molle sequences had no gaps (insertion/deletion) and 68 variable sites over 401 bp. There were 64 parsimony-informative characters. The ML tree of Didemnum spp. based on partial cytochrome c oxidase subunit I (COI) sequences using the HKY+I substitution model (log-likelihood = −1026.993) is presented in Figure 3. Because the topologies of the ML, MP, and NJ trees were nearly identical, the strict-consensus MP tree (tree length = 110, consistency index (CI) = 0.8818, retention index (RI) = 0.9772, rescaled index (RC) = 0.8617, and homoplasy index (HI) = 0.1182) and the NJ tree under the HKY85+I substitution model (proportion of invariable sites = 0.6723, as estimated using the model) are not shown. Monophyly of the grey- and brown-types was supported by high bootstrap values and monophyly of each morphotype (grey, brown, and large) was also supported by high bootstrap values. However, monophyly of the white-type was supported by relatively high bootstrap values. Accordingly, the present results confirmed a previous study indicating potential speciation among morphotypes in D. molle (Hirose et al., Reference Hirose, Yokobori and Hirose2009b), but spicule morphology alone cannot be used to distinguish among these morphotypes.

Fig. 3. Maximum likelihood tree of Didemnum spp. based on partial COI sequences. Didemnum psammatode (AB433972) was used as the outgroup. The HKY + I model was used for the analysis. Bootstrap probabilities larger than 50% are noted for the ML, MP, and NJ trees.
Trididemnum cyclops and T. paracyclops
Trididemnum cyclops colonies were small oval cushions of about 5 mm in diameter, whereas T. paracyclops colonies were irregularly shaped sheets. Both species contained stellate spicules that were very similar in shape (Figure 4). Spicule diameter varied somewhat (Figure 5); while it was not significantly different within species, it did differ between T. cyclops and T. paracyclops (Dunn's multiple comparison test, P < 0.001).

Fig. 4. (A) Tunic spicules of Trididemnum cyclops from Bise, Okinawajima Island and (B) T. paracyclops from Hirakubo, Ishigakijima Island. Scale bars: 50 µm.

Fig. 5. Size variation of the tunic spicules of Trididemnum cyclops and T. paracyclops. Each bar indicates the average of 100 spicules from one specimen with standard deviation. The numbers under the bars indicate the collection site (see Table 1).
A total of three haplotypes were found in two specimens each of T. cyclops and T. paracyclops. The partial COI sequences (401 bp) from two collection sites of T. paracyclops were identical. The aligned T. cyclops and T. paracyclops sequences had no gaps (insertion/deletion) and 53 variable sites over 401 bp. There were 44 parsimony-informative characters. The ML tree of Trididemnum spp. based on partial COI sequences under the TIM substitution model (log-likelihood = −1213.4027) is presented in Figure 6. Because the ML, MP, and NJ trees had nearly identical topologies, the strict-consensus MP tree (tree length = 171, CI = 0.9181, RI = 0.8955, RC = 0.8222, and HI = 0.0819) and NJ tree under the TIM substitution model are not shown. Monophyly of T. cyclops from Hirakubo (Ishigakijima Island) and T. paracyclops from two collection sites was supported by moderate bootstrap values, whereas monophyly of T. cyclops+T. paracyclops was supported by high bootstrap values.

Fig. 6. Maximum likelihood tree of Trididemnum spp. based on partial COI sequences. Trididemnum savignii (AB499977) was used as the outgroup. The TIM model was used for the analysis. Bootstrap probabilities larger than 50% are noted for the ML, MP, and NJ trees.
Trididemnum paracyclops is distinguished from T. cyclops by characters such as larger colony size, larger zooids, and number of coils in the vas deferens (see Kott, Reference Kott2001). However, Monniot & Monniot (Reference Monniot and Monniot1987, 1991) did not discriminate between the two species in their faunal reports, and probably regarded these differences as intraspecific variation (see Kott, Reference Kott2001). In the present study, the molecular phylogenies based on the COI sequence did not recognize two species, but a more extensive survey is required to conclude the taxonomic debate. Colony size was mainly used as the key character for species identification. Thus, neither colony size nor spicule size should be used to discriminate between these two species, even if they are two valid and distinct species.
Two types of spicules in Trididemnum nubilum
The tunic spicules of T. nubilum were always stellate but could be distinguished into two types. In Type-A spicules, the bases of the spicule cones fused with one another. In Type-B, the bases of the cones did not fuse, and there was a crack between the bases (Figure 7). The composition of the two types of spicules varied among colonies: more than 90% of spicules were Type-A in colonies from Bise, Okinawajima Island (N = 4), while about 65% were Type-B in colonies from Ara Beach, Kumejima Island (N = 2). On the other hand, the partial COI sequences (401 bp) from the two collection sites of T. nubilum were identical. Therefore, differences in the ratio of Type-A and Type-B spicules were probably due to intraspecific variation. It is unclear whether the difference in this ratio is caused by genetic variance, microhabitat, or other factors.

Fig. 7. Two types of stellate tunic spicules in Trididemnum nubilum. (A) The bases of the spicule cones fused with one another (Type-A); (B) the bases of cones not fused with one another (Type-B). Scale bars: 10 µm.
Lissoclinum bistratum and L. timorense
Both Lissoclinum bistratum and L. timorense formed sheet- or cushion-like colonies of irregular shapes. Lissoclinum timorense colonies had linguiform projections of tunic around the colony periphery (Figure 8A&C) and sometimes on the colony surface, whereas such projections never occurred in L. bistratum. The tunic spicules of L. bistratum were always globular (Figure 8B). In contrast, those of L. timorense varied from globular to stellate. Spicules of typical shapes are shown in Figure 8D, E; some intermediate shapes were also observed. Therefore, we could not calculate the composition ratio for each specimen of L. timorense. It should be noted that the colonies of L. timorense always contained stellate spicules, which were never found in L. bistratum.

Fig. 8. Colonies of Lissoclinum bistratum (A) containing only spherical spicules (B), and those of L. timorense (C) containing both spherical (D) and stellate (E) spicules. Scale bar: 20 µm.
A total of five COI haplotypes were found among four specimens of L. bistratum and three specimens of L. timorense. The aligned L. bistratum and L. timorense sequences had no gaps (insertion/deletion) and 67 variable sites over 401 bp. There were 64 parsimony-informative characters. The ML tree under the TIM substitution model (log-likelihood = −1027.8732) is presented in Figure 9. Because the topologies of the ML, MP, and NJ trees were nearly identical, the strict-consensus MP tree (tree length = 171, CI = 0.9181, RI = 0.8955, RC = 0.8222, and HI = 0.0819) and the NJ tree under the TIM substitution model are not shown. The partial COI sequences of two distant collection sites of L. timorense from Ara Beach and Odo were identical. The difference of DNA sequences between L. bistratum from Ara beach and Bise was 2 bp over 401 bp, and the differences between the haplotype from the L. timorese (from Ara beach and Odo) and L. bistratum from Ara beach and Bise were 1 bp, respectively. Monophyly of L. bistratum from Ara Beach and Bise and L. timorense from Ara Beach and Odo was supported by high bootstrap values. The partial COI sequences of L. bistratum from Shinrihama and L. timorense from Bise were identical. The difference of DNA sequences between the haplotype and L. bistratum from Maeda was 2 bp over 401 bp. Monophyly of L. bistratum from Maeda and Shinrihama and L. timorense from Bise was supported by high bootstrap values. The sequence difference within each monophyletic group was very low (0–2 bp, 0–0.5%). On the other hand, the difference between the ‘L. bistratum from Ara Beach and Bise and L. timorense from Ara beach and Odo’ and ‘L. bistratum from Maeda and Shinrihama and L. timorense from Bise’ groups was high (64–65 bp, 16.0–16.2%).

Fig. 9. Maximum likelihood tree of Lissoclinum spp. based on partial COI sequences. Lissoclinum puctatum (AB433976) was used as the outgroup. The TIM model was used for the analysis. Bootstrap probabilities larger than 50% are noted for the ML, MP, and NJ trees.
The COI phylogeny of L. bistratum–L. timorense specimens discriminated two clades, but each clade included both L. bistratum and L. timorense (Figure 9). Therefore, the presence or absence of stellate spicules does not discriminate the two clades of L. bistratum–L. timorense. Monniot & Monniot (Reference Monniot and Monniot2001) proposed that L. timorense and L. voeltzkowi are junior synonyms of L. bistratum, considering the large variability of spicules among colonies inhabiting the same site and the absence of distinct zooid morphology. On the other hand, it is possible that the two clades recognized in Figure 9 represent two distinct species, but we could not find any morphological features with which to distinguish them.
Spicules as a taxonomic character
Didemnid species, other than Diplosoma spp., usually possess calcareous spicules. There are various forms of spicules (e.g. globular, stellate, burr-like; see Kott, Reference Kott2001), and each species has a particular type or types of spicules of a specific size range. Therefore, tunic spicules are undoubtedly an important character for taxonomy in didemnid ascidians. However, our results, from a combination of quantitative and molecular studies, revealed the presence of substantial intraspecies variation in shape and size of tunic spicules. A similar finding was reported for Didemnum vexillum (Lambert, Reference Lambert2009). Therefore, spicules are not always crucial features discriminating species, particularly among closely related species. For instance, the four morphotypes of D. molle, which were supported by the molecular phylogeny, possess spicules of almost the same shape and size. On the other hand, the size of stellate spicules differed significantly between T. cyclops and T. paracyclops, but the molecular phylogeny did not support this species level distinction. Phenotypic plasticity is a delicate problem, and the divergence of the spicule form and colony form may result from the different microhabitats in Lissoclinum bistratum–L. timorense. By contrast, Dias et al. (Reference Dias, Abreu, Silva and Solferini2009) showed the genetic differentiation between morphotypes from intertidal and subtidal zones in Trididemnum orbiculatum. We also encountered, albeit infrequently, some malformed spicules (Figure 10) as did Lambert (Reference Lambert2009). The presence of malformed spicules indicates that ascidian colonies cannot always produce spicules accurately and that there could be variation in their shape and size. In the present study, we could not well quantify the shape of the spicules. The variability of the spicules causes the occurrence of intermediate forms, and they are difficult to be classified into discrete categories. Evaluation of shape parameters, such as ray number, may provide useful characters for taxonomy. In conclusion, tunic spicules are potentially valuable features for didemnid taxonomy, but intraspecific variation should be taken into account if they are to be used as key characters for species identification.
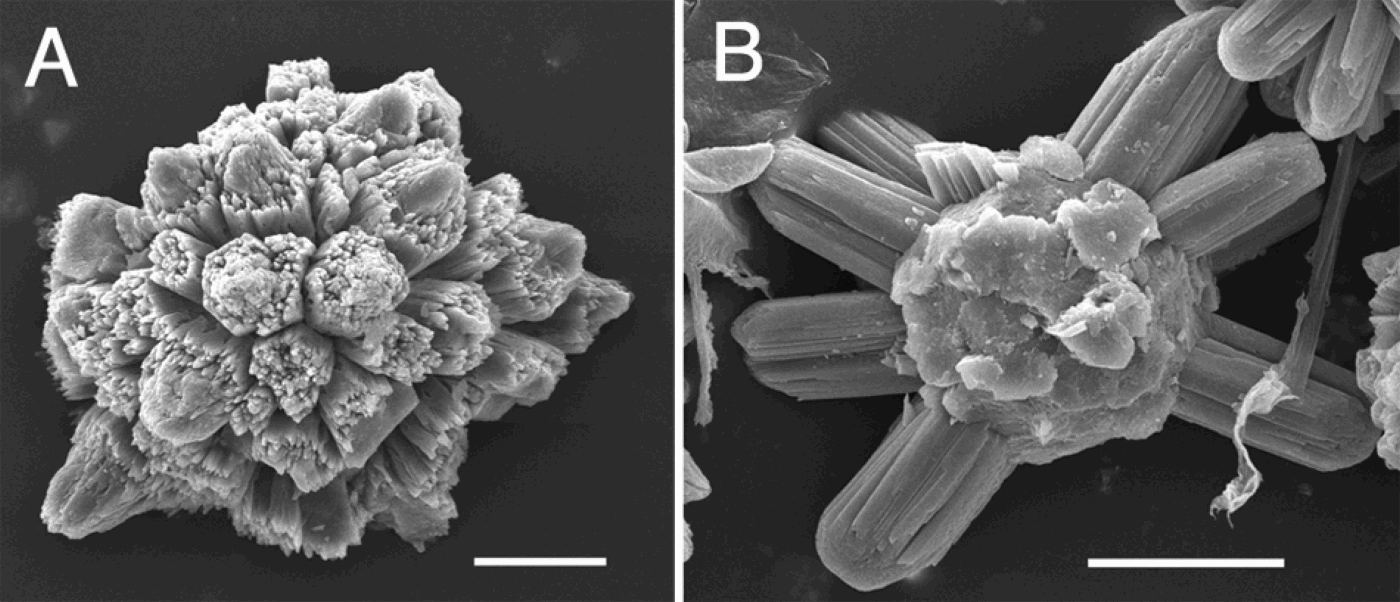
Fig. 10. Malformed tunic spicules in (A) Lissoclinum timorense and (B) Trididemnum cyclops. Scale bar: 10 µm.
ACKNOWLEDGEMENT
This study was supported by a Grant-in-Aid for Scientific Research (No. 20570092) from the Japan Society for the Promotion of Science.













