With over 386,000 extant species described and over 1.2 million estimated species still awaiting to be discovered, the beetles are the most diverse group of metazoans (Stork Reference Stork2018). Despite their bewildering taxonomic and ecological diversity unparalleled in the animal kingdom, entomologists agree that the title of the most bizarre beetle belongs to one species alone – Micromalthus debilis (Barber Reference Barber1913; Pollock & Normark Reference Pollock and Normark2002; Normark Reference Normark2013).
Micromalthus debilis, the sole living representative of the family Micromalthidae, is a small elongate beetle native to the E of the US and perhaps to parts of Central America (Philips Reference Philips2001). The adults and larvae inhabit decaying hardwoods. Because the beetles are considered to be minor pests of wooden structures exposed to humid conditions such as telephone poles, fences, wooden buildings, and railroad ties, they have become colloquially known as ‘telephone pole beetles' (Ruzzier & Colla Reference Ruzzier and Colla2019). The highly unusual reproductive system of M. debilis is unique among all known animals, although some similarities with marine loriciferans have been noted (Normark Reference Normark2013). Throughout the year, the majority of the population consists of female larvae that give birth to more larvae parthenogenetically without mating. Multiple larvae are often delivered at a time. Males are rare and only produced in the late summer or during periods of drought when female larvae each produce a single unfertilised male egg and then become torpid. The egg hatches into a male larva that enters its mother's body through the genital opening and feasts on it, completely devouring it within a week (Barber Reference Barber1913; Pringle Reference Pringle1938; Normark Reference Normark2013). The male then develops into an adult, but adult males and females are unable to copulate. Adult females are not known to produce fertile eggs and typically live only about six days, while most adult males live only around 13h (Kühne Reference Kühne1972; Perotti et al. Reference Perotti, Young and Braig2016). Moreover, many adults suffer from impaired locomotion, have a weakly sclerotised body, and their internal organs are much reduced when compared to the larvae (Perotti et al. Reference Perotti, Young and Braig2016). The vestigial role of the ‘ghost adults' in Micromalthidae is probably the result of the presence of male-killing endosymbionts such as Wolbachia that imposed a selective pressure for asexual reproduction without males (Normark Reference Normark2013; Perotti et al. Reference Perotti, Young and Braig2016).
Micromalthus debilis is a very old ‘living fossil'. Specimens identical to living representatives of the species have been reported from Miocene Dominican and Mexican ambers (both ca.20–15Ma, cf. Iturralde-Vinent & Macphee Reference Iturralde-Vinent and Macphee2019) (Rozen Reference Rozen1971; Perkovsky Reference Perkovsky2007; Hörnschemeyer et al. Reference Hörnschemeyer, Wedmann and Poinar2010; Normark Reference Normark2013). Two other species belonging to the genus Micromalthus have been reported from Eocene Oise and Rovno ambers (Kirejtshuk et al. Reference Kirejtshuk, Nel and Collomb2010; Perkovsky Reference Perkovsky2016), and a larva described as Cretomalthus acracrowsonorum has been reported from Cretaceous Lebanese amber (Kirejtshuk & Azar Reference Kirejtshuk and Azar2008). Moreover, the Permian Archaeomalthus synoriacos has been recently described by Yan et al. (Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019) based on a compression fossil. Micromalthid compression fossils from the Upper Jurassic of Karatau, Kazakhstan, were mentioned by Kirejtshuk et al. (Reference Kirejtshuk, Nel and Collomb2010) but were never formally described. A checklist of fossil and extant micromalthid beetles is provided in Appendix 1. The long geological record of Micromalthidae should not come as a surprise; the family belongs to the ancient suborder Archostemata that contains taxa with many putatively ancestral characters (Beutel & Haas Reference Beutel and Haas2000; Friedrich et al. Reference Friedrich, Farrell and Beutel2009) that closely resemble the earliest known Palaeozoic beetles (Kukalová Reference Kukalová1965; Ponomarenko Reference Ponomarenko1969; Kirejtshuk et al. Reference Kirejtshuk, Poschmann, Prokop, Garrouste and Nel2014).
Here we describe the first member of the family Micromalthidae from mid-Cretaceous Burmese amber. Protomalthus burmaticus gen. et sp. nov. represents the first adult micromalthid beetle known from the Mesozoic.
1. Material and methods
Amber from northern Myanmar contains probably the most diverse Cretaceous insect fauna (Cai et al. Reference Cai, Huang, Newton, Eldredge and Engel2017; Ross Reference Ross2019). The abundance and quality of the inclusions provides a rare insight into the palaeoecology (Cai et al. Reference Cai, Clarke, Yin, Fu and Huang2019), biogeography (Fu et al. Reference Fu, Cai and Huang2019), phylogeny (Zhou et al. Reference Zhou, Caterino, Ślipiński and Cai2018; Yu et al. Reference Yu, Ślipiński, Lawrence, Yan, Ren and Pang2019b), and even sexual dimorphism (Cai & Huang Reference Cai and Huang2019; Jiang et al. Reference Jiang, Liu and Wang2019) of insects that lived over 100 million years ago.
The material studied herein originated from an amber mine at the summit of the Noije Bum hill [26°20′N, 96°36′E] in the Hukawng Valley, Kachin State, in northern Myanmar. The precise age of the amber from the mine is still a contentious issue. Radiometric dating of the amber-bearing horizon suggested 99 Ma as the minimum age (Shi et al. Reference Shi, Grimaldi, Harlow, Wang, Wang, Yang, Lei, Li and Li2012; Mao et al. Reference Mao, Liang, Su, Li, Rao, Zhang, Xia, Fu, Cai and Huang2018); the presence of pholadid bivalve borings indicates that the amber was redeposited (Smith & Ross Reference Smith and Ross2018). Most recently, the discovery of an ammonite entombed in the amber constrained the maximum age of the amber to the late Albian (Yu et al. Reference Yu, Kelly, Mu, Ross, Kennedy, Broly, Xia, Zhang, Wang and Dilcher2019a). Here we follow a late Albian to early Cenomanian age of the amber. The fossilised resin was probably secreted in a tropical rainforest standing close to the sea, presumably by dawn redwood trees of the genus Metasequoia (Grimaldi & Ross Reference Grimaldi, Ross, Fraser and Sues2017).
The West Burmese block separated from north-western Australia and drifted N to its present position probably sometime between the Late Triassic and Late Jurassic (Metcalfe Reference Metcalfe, Hall and Holloway1998). In line with this, the Burmese amber fauna includes a high number of species that are, today, restricted to Gondwanan areas (Wu et al. Reference Wu, Li and Ding2018; Liu et al. Reference Liu, Tihelka, McElrath, Yamamoto, Slipiński, Wang, Ren and Pang2019), although these findings should be interpreted with caution as future investigations may reveal that the burmite fauna in fact represents a mixture of different biogeographical elements.
The amber piece was prepared using a handheld cutter and polished with different grades of sandpaper and rare earth powder. The specimens were studied under a Zeiss Discovery V20 stereo microscope fitted with an AxioCam MRc 5 camera and a Bresser Advance ICD zoom stereomicroscope. Fluorescence photomicrographs with a green background were taken under a Zeiss Axio Imager 2 microscope with the eGFP mode. Type material is deposited in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (CAS), Nanjing, China. The publication LSID is urn:lsid:zoobank.org:pub:1BCBCD38-46DA-4EED-8D87-BD74FD719D88.
2. Systematic palaeontology
Order Coleoptera Linnaeus, Reference Linnæus1758
Suborder Archostemata Kolbe, Reference Kolbe1908
Family Micromalthidae Barber, Reference Barber1913
Genus Protomalthus gen. nov.
Type species. Protomalthus burmaticus sp. nov.
Diagnosis. Frontoclypeal suture distinct and complete, angulate medially. Labrum and clypeus distinctly separated. Medicranial suture present. Scutellum oval to suboval, not distinctly wide posteriorly. Elytra shortened, truncate apically, leaving four abdominal ventrites exposed.
Etymology. From the Greek ‘proto', meaning ‘before the present', and the root of Micromalthus, the type genus of family Micromalthidae.
Protomalthus burmaticus sp. nov.
(urn:lsid:zoobank.org:act:236277BA-0993-4802-A378-1088BE15B2EF)

Figure 1 Habitus photographs of Protomalthus burmaticus gen. et sp. nov. (holotype: NIGP171020): (a) dorsal view under normal reflected light; (b) ventral view under normal reflected light; (c) dorsal view under green fluorescence. Scale bars=500μm.
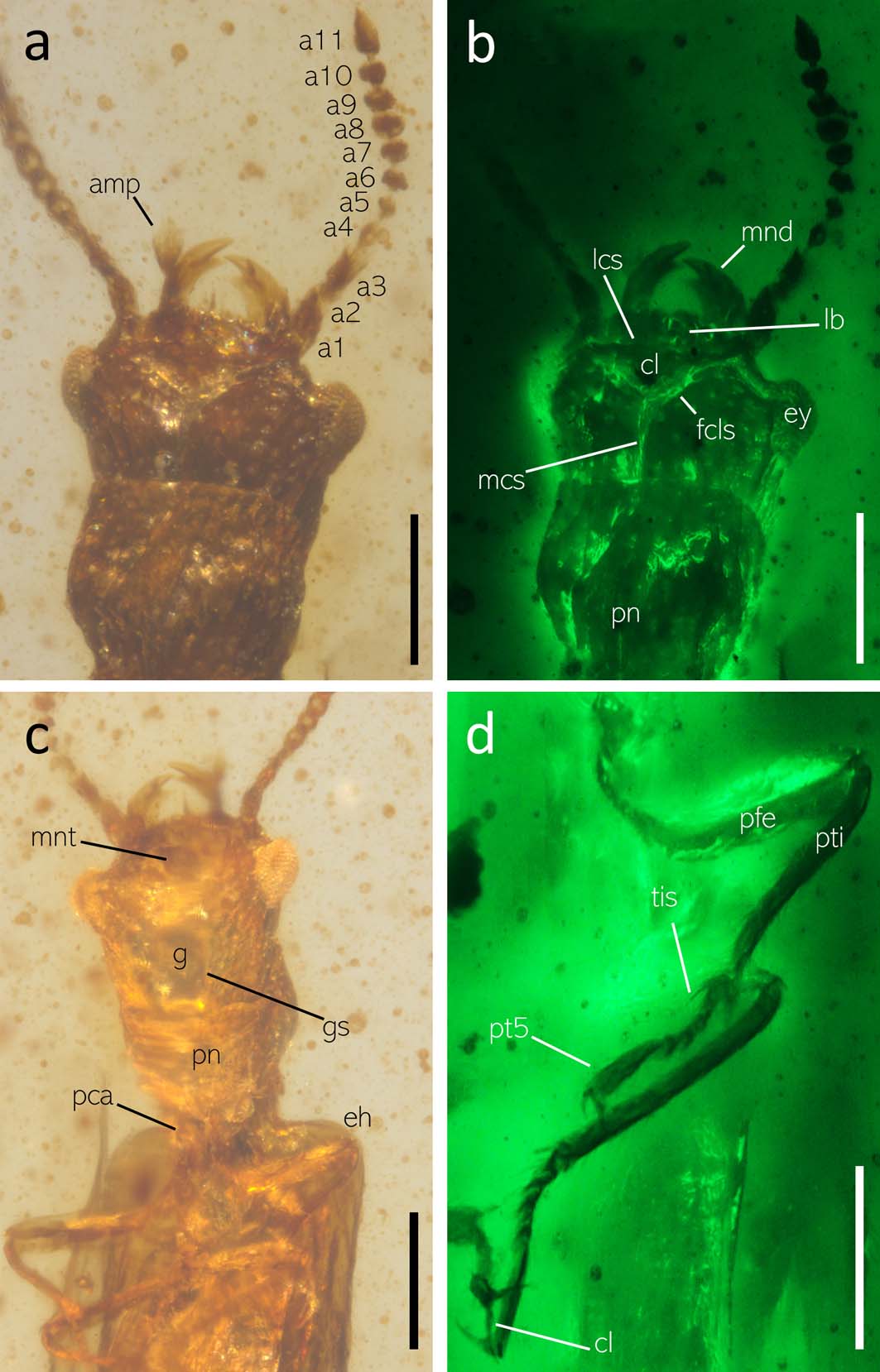
Figure 2 Morphological details of Protomalthus burmaticus gen. et sp. nov. (holotype: NIGP171020): (a) dorsal view of head under normal reflected light; (b) dorsal view of head under green fluorescence; (c) head and prothorax in ventral view under normal reflected light; (d) abdomen in ventral view under green fluorescence. Abbreviations: a1–11=antennomeres 1–11; amp=apical maxillary palpomere; cl=claw; cl=clypeus; eh=elytral humerus; ey=eye; g=gula; fcls=frontoclypeal suture; gs=gular suture; lb=labrum; lcs=labroclypeal suture; mcs=medicranial suture; mnt=mentum; pca=procoxa; pfe=profemur; pn=pronotum; pt5=protarsomere 5; pti=protibia; tis=tibial spur. Scale bars=200μm.
Type material. Holotype NIGP171020. Female, as indicated by the extruded genitalia and the absence of central foveae with tufts of hair on ventrites 2–4.
Diagnosis. As for the genus, with additional characters: antennae reaching to the anterior second third of the pronotum, abdomen with six ventrites, tibial spurs present, body length 2.54mm.
Description. Body small and elongate, flattened, glabrous. Head, pronotum, antennae, and appendages dark brown, elytra light brown and somewhat transparent, apparently weakly sclerotised. Body length 2.54mm from clypeus to abdominal apex, 0.49mm wide across elytral base.
Head 0.41mm long, 1.2 times longer than wide ventrally, widest medially, subhexagonal in dorsal view, approximately as wide as the pronotum, with sparse punctures dorsally. Frontoclypeal suture distinct and complete, angulate at middle, posteriormost section in line with the widest points of the eyes. Mandibles with distal halves strongly curved inwards, apparently bidentate at apex, but this appearance may be caused by the angle of observation and they may actually be tridentate as in the extant M. debilis. Labrum slightly depressed, very thin and translucent, straight, lacking punctation, separated from clypeus by a distinct suture. Apical maxillary palpomere dilated medially, with an obliquely truncating platform probably with sensory structures, apex narrowly rounded. Mentum subtrapezoidal, as long as the basal two antennomeres combined. Gular sutures subparallel, widely separated along their entire lengths by approximately one third of head width, diverging further anteriorly. Medicranial suture present, fused with the posteriormost point of the frontoclypeal suture and reaching to the posterior end of the head capsule. Antennal sockets visible from above, antennae inserted anterolaterally in front of the eyes. Antennae 11 segmented, reaching to the anterior second third of the pronotum. Antennal segments equally wide throughout; antennomere 1 elongate and twice longer than the following segment; antennomere 2 rounded; antennomere 3 1.4 times as long as the preceding segment; antennomere 4 slightly shorter than the preceding segment and thinner; antennomere 5 as long as wide, broadest medially, 1.2 times as long as the following segment; antennomeres 6–10 subequal, wider than long; antennomere 11 obclavate, pointed apically, 2.3 times as long as the preceding segment. Compound eyes strongly protruding, coarsely faceted, separated by 0.7 time of head width, widest medially. Ocelli apparently absent. Temple region straight to slightly narrowing posteriorly.
Pronotum approximately as wide as the head without eyes, slightly longer dorsally and covering the posterodorsal portion of the head, 0.16mm long ventrally and 0.31mm dorsally. Anterior pronotal margin straight; pronotal sides parallel, narrowing in the posterior third; anterior margin sublinear and slightly raised. Pronotal surface with two slight depressions medially and sparse rounded punctures laterally. Scutellum longer than wide, with a rounded apex, longest medially. Prosternum subtriangular, with a faint median suture.
Elytra 6.5 times longer than wide, thin and transparent, truncate apically, leaving four abdominal ventrites exposed. Elytral surface glabrous, impunctate, and lacking veins, at most with shallow disperse grooves. Humeri rounded. Elytra parallel, broadly rounded in the apical tenth, longest medially. Wings present.
Procoxae only very narrowly separated, subconical, oriented posteriorly. Prosternal process not visible, apparently absent. Mesoventrite representing approximately one third of metaventrite length. Mesocoxae elongate and widely separated by a rounded metaventrite process. Metaventrite long, narrowed anteriorly, with a faint median suture. Legs slender and long. Femora equally wide throughout, approximately five times as long as wide, with a shallow groove for the reception of the tibia. Tibia elongate, at least ten times as long as wide, each with apparently a single straight apical spur as long as tarsomere 1. Tarsi 5 segmented, representing approximately 0.7 times of tibia length. Tarsomeres 1–4 subequal. Apical tarsomere 4.2 times as long as the preceding segment, narrowed at base and slightly expanding apically. Claws simple, curved, representing 0.4 of apical tarsomere length, empodia apparently absent.
Abdomen 1.96mm long, with six visible ventrites. Ventrites 1–4 equally wide, apical two ventrites narrowed anteriorly. Ventrites 1–2 and 2–3 subequal, ventrite 3 with an anteromedian depression, possibly with a small rounded glandular opening. Ovipositor extruded. Gonocoxites rather long, gonostyli apparently glabrous.
Type locality and horizon. Amber mine in the Hukawng Valley, Myitkyina District, Kachin State, Myanmar; late Albian to early Cenomanian (mid-Cretaceous).
Etymology. Specific epithet after the type locality in Burma (Myanmar).
3. Discussion
3.1. Systematic position of Protomalthus
The new species can be confidently placed into the family Micromalthidae on the basis of its small size, apparently weakly sclerotised and flattened body, head relatively large and depressed anteriorly, head without protuberances or a constricted neck region, temporal region long and subparallel, antennae inserted anterolaterally in front of the eyes, mesoventrite without a transverse ridge, mesocoxae separated by an anterior mesoventral process, concealed metatrochantins, scutellum oval to suboval, evenly sclerotised elytra truncate anteriorly leaving several abdominal ventrites exposed, and the absence of window punctures, cuticular tubercles, and scales (Beutel et al. Reference Beutel, Ge and Hörnschemeyer2008; Hörnschemeyer Reference Hörnschemeyer2009, Reference Hörnschemeyer, Beutel and Leschen2016; Yan et al. Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019).
Protomalthus burmaticus gen. et sp. nov. can be differentiated from all other micromalthids by the presence of distinct frontoclypeal, labroclypeal, and medicranial sutures. In all hitherto known micromalthids, the labrum, clypeus, and frons are fused without any visible sutures. As such, it is necessary to revise the family diagnosis for Micromalthidae (e.g., Hörnschemeyer Reference Hörnschemeyer, Beutel and Leschen2016) to accommodate the new species. A key to adult micromalthid beetles is provided in Appendix 2.
Despite these differences, P. burmaticus gen. et sp. nov. still closely resembles Palaeozoic, Cenozoic, and recent micromalthids with its weakly sclerotised body lacking surface sculpture, head without protuberances, and short thin elytra (Hörnschemeyer Reference Hörnschemeyer, Beutel and Leschen2016). Yan et al. (Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019) considered these reductional features to be related with low investment into adults due to their apparently vestigial role in the micromalthid reproductive system.
Similarities between Protomalthus gen. nov., the Permian Archaeomalthus, and the extant Micromalthus point towards a prolonged morphological stasis in Micromalthidae. Members of some beetle taxa have changed little since the Cretaceous, as evidenced by the existence of Burmese amber inclusions that could be placed into extant genera (e.g., Liu et al. Reference Liu, Shi, Cai, Liang and Huang2015; Cai & Huang Reference Cai and Huang2016, Reference Cai and Huang2017). Traditionally, morphological stasis in the fossil record has been explained by the supposed stability of the microhabitat that members of a given taxon inhabit, which would result in low selection pressure for morphological change, similar mode of life, and similar ecological requirements (Clarke & Chatzimanolis Reference Clarke and Chatzimanolis2009). Yan et al. (Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019) argued that the structural simplifications of micromalthid beetles resulting from their unusual reproductive system may be irreversible, thus resulting in morphological stasis.
The range of intraspecific morphological variability in Micromalthidae is still poorly understood. Individuals assigned to the extant species M. debilis collected in different localities show varying degrees of morphological disparity. For example, Paterson (Reference Paterson1938) reported a population of M. debilis living in structural timber in gold mines in South Africa some 6000 feet below the surface (Pringle Reference Pringle1938). He remarked that the South African beetles examined by him differed from North American specimens by their greater size, slightly shorter elytra reaching only to the second abdominal segment, the presence of tibial spurs, and wing venation. The South African beetles had seven visible ventrites, while American beetles seen by Paterson only has six visible ventrites. Marshall & Thornton (Reference Marshall and Thornton1963) described further differences between M. debilis specimens collected in their native range, South Africa, and in Hong Kong. Among other differences, the triungulin larvae from Hong Kong possessed a single stemma on each side of the head, which is reportedly absent in all other populations. Despite these differences, no author has yet described these aberrant specimens as a separate species. Lawrence (Reference Lawrence and Stehr1991) considered that at least the Hong Kong specimens may be deserving of a separate species status, while Grimaldi & Engel (Reference Grimaldi and Engel2005) remarked that M. debilis may in fact represent a complex consisting of several cryptic species. This hypothesis is presently under investigation.
3.2. Palaeobiogeography
The discovery of micromalthids in Cretaceous ambers from Lebanon (Kirejtshuk & Azar Reference Kirejtshuk and Azar2008) and Myanmar suggest a Gondwanan distribution of the family during this period.
That Micromalthidae had a Gondwanan distribution in the Cretaceous but today is restricted to North and Central America (Philips Reference Philips2001) suggests two possible scenarios. Either the family suffered extinctions throughout much of its original range in the Cenozoic, or micromalthids still occur in the Gondwanan region to the present day, but have been overlooked. With regards to the latter possibility, it is interesting to return to Paterson's account of Micromalthus infesting structural timber in South African mines. It is unclear whether the timber was imported from North America or was sourced locally, but the former seems unlikely since M. debilis only inhabits very old wood in late stages of decay, which would probably not be brought into the mines in the first place (Pollock & Normark Reference Pollock and Normark2002). This leaves the possibility that Micromalthus populations may have occurred, or still occur, in South African forests. Whether micromalthids are native to South Africa as Pringle (Reference Pringle1938) proposed, or whether the beetles were introduced into the local forests through intercontinental timber trade, remains unknown, but the finding that micromalthid beetles had a Gondwanan distribution in the Mesozoic suggests that the first option may not be entirely out of the question. It seems possible that Micromalthus may still be occurring in this part of the world but has remained undetected, perhaps due to low sampling effort, its small size, and cryptic life history.
3.3. Palaeoecology
It is impossible to determine if adult micromalthid beetles in the Permian were vestigial based solely on compression fossils, but the presence of clearly vestigial features in P. burmaticus gen. et sp. nov., such as the weakly sclerotised and at times transparent body parts that are otherwise unusual for archostematan beetles (Fig. 1), may indicate that the adults of this species possibly played a minimal role in reproduction and, consequently, investment into adults was low (Yan et al. Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019). As such, P. burmaticus gen. et sp. nov. indicates that the peculiar micromalthid asexual reproductive system, and possibly also male-killing insect endosymbionts likely responsible for its emergence (Perotti et al. Reference Perotti, Young and Braig2016), may have been present since the Cretaceous.
The 255-million-year-old history of Micromalthidae (Yan et al. Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019) begs the question of whether the beetle's unique reproductive system could play a part in the family's persistence. Comparisons are difficult to make since the micromalthid reproductive system is unique among metazoans (Normark Reference Normark2013), but marine loriciferans that likewise have a complex reproductive system with paedogenic larvae and matriphagy, also have a very long fossil record dating back to the Cambrian (Peel Reference Peel2010). Asexual reproduction would be expected to contribute to persistence due to the low cost of male production but would not lead to the recruitment of genetically new individuals into the population, thus presumably lowering the likelihood of survival in the face of environmental change (Short et al. Reference Short, Polidoro, Livingstone, Carpenter, Bandeira, Bujang and Erftemeijer2011). As such, it would be expected that in stable environments, such as those inhabited by micromalthids, asexuality may indeed support the persistence of taxa over long periods of geologic time.
4. Concluding remarks
Micromalthidae represents one of the oldest extant coleopteran families. They have been present since the Permian, but their Mesozoic fossil record has, until now, been limited to just a single larva in Lebanese amber (Kirejtshuk & Azar Reference Kirejtshuk and Azar2008). Protomalthus burmaticus gen. et sp. nov. represents the first micromalthid beetle from Burmese amber and the first adult individual from the Cretaceous. The weakly sclerotised body, similar to the extant M. debilis, suggests low investment into adults, implying that the micromalthid asexual reproductive system originated at least in the mid-Cretaceous and points towards a long history of insect-associated endosymbiotic microorganisms. The finding confirms a Gondwanan distribution of Micromalthidae in the Cretaceous, raising the possibility that yet undescribed species may occur in the region to the present day. The remarkable morphological similarity between P. burmaticus gen. et sp. nov. and M. debilis reveals a morphological and probably behavioural stasis in micromalthids for over the last 100 million years.
5. Note
While this paper was in press, Yamamoto (Reference Yamamoto2019) published a report of a micromalthid beetle from Burmese amber. The single poorly preserved specimen was tentatively assigned to the extant genus Micromalthus but left without a formal description. In light of our findings, it is possible that this fossil in fact belongs to the genus Protomalthus gen. nov. This is difficult to verify, however, since the specimen illustrated by Yamamoto (Reference Yamamoto2019) appears somewhat deformed and does not preserve crucial diagnostic characters of the head.
6. Acknowledgements
We thank two anonymous reviewers for their insightful comments. Financial support was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000 and XDB18000000), the National Natural Science Foundation of China (41688103 and 41672011), and the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0706).
7. Appendix 1: Catalogue of telephone pole beetles (Micromalthidae)
The following is a list of all presently known fossil and extant micromalthid beetles. The list of species is arranged alphabetically, stating the type depository, geological range, and known distribution for each species. A dagger symbol (Ԡ') is used to indicate extinct taxa, holotypes are abbreviated as HT, paratypes as PT. Type localities are cited as given by the original authors, missing data not reported in the original publications are supplied inside square brackets.
†Archaeomalthus Yan et al., Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019, pp. 1–9
Type species. Archaeomalthus synoriacos Yan et al., Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019 by monotypy.
synoriacos Yan et al., Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019, pp. 1–9
HT. compression fossil, Babiy Kamen, Tom River, Kuznetsk Basin, S Siberia [Russia], PIN 4887/102 – Borissiak Paleontological Institute, Russian Academy of Sciences, Moscow, Russia.
Distribution. Known only from the Late Permian Babiy Kamen locality.
†Cretomalthus Kirejtshuk & Azar, Reference Kirejtshuk and Azar2008: 17–20
Type species. Cretomalthus acracrowsonorum Kirejtshuk & Azar, Reference Kirejtshuk and Azar2008 by original designation.
acracrowsonorum Kirejtshuk & Azar, Reference Kirejtshuk and Azar2008, pp. 17–20
HT. Larva in Cretaceous Lebanese amber [Lebanon], JS27 Acra Coll. – Muséum national d'Histoire naturelle, Paris, France.
Distribution. Known only from Cretaceous Lebanese amber (Barremian to lowermost Aptian).
Micromalthus LeConte, Reference LeConte, Hubbard and Schwarz1878, p. 613
Type species. Micromalthus debilis LeConte, Reference LeConte, Hubbard and Schwarz1878 by monotypy.
debilis LeConte, Reference LeConte, Hubbard and Schwarz1878, p. 613
=Micromalthus anansi Perkovsky, Reference Perkovsky2007, pp. 626–628
HT. ♀, in Miocene Dominican amber [Dominican Republic], Do-632-K – Staatliches Museum für Naturkunde, Stuttgart, Germany.
Placed in synonymy by Hörnschemeyer et al. (Reference Hörnschemeyer, Wedmann and Poinar2010, pp. 300–311).
HT. ♀, [USA, Michigan, Wayne] Detroit, Type 3684 – Harvard Museum of Comparative Zoology, Cambridge, MA.
Distribution. Presumably native to eastern US and Belize; introduced to Alabama, Austria, Brazil, British Columbia (Canada), Cuba, Florida, Gibraltar, Hawaii, Hong Kong, Italy, Japan, New Mexico, Oregon, South Africa; fossil representatives known from Miocene Dominican and Chiapas (Mexican) ambers.
†eocenicus Kirejtshuk et al., Reference Kirejtshuk, Nel and Collomb2010. pp. 223–225
HT. ♀, in Eocene Oise amber [France], PA 7870 – Musée of Menat, Puy-de-Dôme, France.
PT. ♀, in Eocene Oise amber [same locality as HT], PA 1286.
Distribution. Known only from Eocene Oise amber.
†priabonicus Perkovsky, Reference Perkovsky2016, pp. 293–296
HT. ♀, in Eocene Rovno amber [Ukraine, Rivne Oblast], Klesov, SIZK, K7760 – Schmalhausen Institute of Zoology, National Academy of Sciences of Ukraine, Kiev, Ukraine.
PT. ♀, in Eocene Rovno amber [same locality as HT] SIZK, K7758g.
PT. 74 larvae in one piece of Eocene Rovno amber [same locality as HT], K7757–K7763.
PT. Larva in Eocene Rovno amber [same locality as HT], K1069a.
Distribution. Known only from Eocene Rovno (Ukrainian) amber.
†Protomalthus gen. nov. Tihelka, Huang & Cai
Type species. Protomalthus burmaticus gen. et sp. nov. Tihelka, Huang & Cai by original designation.
burmaticus sp. nov. Tihelka, Huang & Cai
HT. ♀, in Cretaceous Burmese amber, Noije Bum hill amber mine, Hukawng Valley, Kachin State, N Myanmar, NIGP171020 – Nanjing Institute of Geology and Palaeontology, CAS, Nanjing, China.
Distribution. Known only from Cretaceous Burmese amber.
8. Appendix 2: Key to adult telephone pole beetles (Micromalthidae)
1(2) Head with distinct frontoclypeal, labroclypeal, and medicranial sutures †Protomalthus burmaticus gen. et sp. nov. Tihelka, Huang & Cai
2(1) Head not as above, with labrum, clypeus, and frons fused without visible sutures. 3
3(2) Procoxae not contiguous and narrowly separated, scutellum triangular with a narrowed and rounded apex.†Archaeomalthus synoriacos Yan et al., Reference Yan, Beutel, Lawrence, Yavorskaya, Hörnschemeyer, Pohl, Vassilenko, Bashkueva and Ponomarenko2019
4(3) Procoxae and scutellum not as above 5
5(4) Antennae as long as head, with antennomere 3 elongate and antennomere 4 not transverse †Micromalthus priabonicus Perkovsky, Reference Perkovsky2016
6(5) Antennae longer than head. 7
7(6) Apical antennomere oval. Apical maxillary palpomere with an acute apex. Scutellar apex bilobed to sublinear Micromalthus debilis LeConte, Reference LeConte, Hubbard and Schwarz1878
8(7) Apical antennomere subacute. Apical maxillary palpomere rounded apically. Scutellum distinctly oval, longest medially †Micromalthus eocenicus Kirejtshuk et al., 2010




