Introduction
Pubertal maturation generally occurs sequentially, characterized by breast/genital development first, followed by pubic hair growth, with menarche for girls being one of the last markers of pubertal maturity.Reference Euling, Herman-Giddens and Lee 1 – Reference Kaplowitz 3 Extensive evidence indicates that the age at pubertal timing has declined for girls with rising living standards and similar, but less well-documented, declines in boys.Reference Euling, Herman-Giddens and Lee 1 , Reference Biro, Greenspan and Galvez 4 – Reference Tinggaard, Mieritz and Sorensen 6 Early maturation in the human population may be an indicator of chronic environmental exposures and a bioassay of energy availability during childhood.Reference Biro, Greenspan and Galvez 4 , Reference Ellis 7 Understanding contributors to early puberty is critical given its relationship to psychosocial adjustment and increased risk for adult chronic conditions including hormonal cancersReference Golub, Collman and Foster 8 – Reference Charalampopoulos, McLoughlin, Elks and Ong 10 and possibly cardiovascular disease.Reference Golub, Collman and Foster 8 , Reference Charalampopoulos, McLoughlin, Elks and Ong 10 , Reference Prentice and Viner 11
While the age of menarche has fallen substantially since the 19th century,Reference Sorensen, Mouritsen and Aksglaede 12 , Reference Parent, Teilmann and Juul 13 recent secular trends for earlier age at breast development was observed starting in the 1990s in the United States and 15 years later in European countries; though the trend in Europe is less dramatic than the United States.Reference Sorensen, Mouritsen and Aksglaede 12 The US National Health and Nutrition Examination SurveyReference Sun, Schubert and Chumlea 14 and the U.S. Pediatric Research in Office Settings (PROS) studyReference Herman-Giddens, Slora and Wasserman 15 indicate that the median reported age of beginning breast development is ~0.8–1.2 years earlier than previous U.S. population-based studies.Reference Lee 16 U.S. trends of earlier breast development are most marked for racial and ethnic minorities, with African American girls developing the earliest, followed by Hispanic girls.Reference Sun, Schubert and Chumlea 14 , Reference Herman-Giddens, Slora and Wasserman 15 , Reference Wu, Mendola and Buck 17 – Reference Biro, Greenspan and Galvez 20 The Copenhagen puberty study reports breast development is occurring a whole year earlier over a 15-year period and other European countries are currently observing similar trends.Reference Aksglaede, Sorensen, Petersen, Skakkebaek and Juul 21 – Reference Semiz, Kurt, Kurt, Zencir and Sevinc 23 Whether similar trends have occurred elsewhere is less well documented.
Information on boys’ puberty trends is less comprehensive than for girls. Measurement of boys’ pubertal development can be subjective without the assessment of testicular volume through orchidometry, and U.S. population-based studies without volume assessments are therefore difficult to interpret.Reference Euling, Herman-Giddens and Lee 1 , Reference Ahmed, Ong and Dunger 24 The PROS study suggest that genital development is occurring 1.5 years earlier than a landmark UK study,Reference Marshall and Tanner 25 with stronger trends among African American boys; however, the trend is difficult to interpret due to differences in study methodologies and population characteristics.Reference Herman-Giddens, Steffes and Harris 26 European population-based studies suggest from the mid-1960s through 1990s there is no secular trend toward earlier age at genital development;Reference Juul, Teilmann and Scheike 27 , Reference Mul, Fredriks and van Buuren 28 however, over a period of 15–30 years the age at attaining a testicular volume of >3 ml is ~3–5 months earlier.Reference Mul, Fredriks and van Buuren 28 , Reference Sorensen, Aksglaede, Petersen and Juul 29 Studies suggest earlier age at boys’ puberty with economic development over the long term, although the earlier age at boys’ puberty may be less marked in the short-term and less extreme than that observed in girls.Reference Euling, Herman-Giddens and Lee 1 , Reference Tinggaard, Mieritz and Sorensen 6 , Reference Goldstein 30
Unlike breast development, there is less data to determine the trend for pubic hair development. According to an expert panel review of U.S. puberty from 1940 to 1994, the majority of the panel concludes that there is no secular trend over this time period, while a minority conclude that age at pubic hair development has declined for girls and boys by ~6 months.Reference Euling, Herman-Giddens and Lee 1 European findings are varied; however, several studies indicate an earlier age at pubic hair development for girls and boys.Reference Mul, Fredriks and van Buuren 28 , Reference Sorensen, Aksglaede, Petersen and Juul 29 , Reference Kryst, Kowal, Woronkowicz, Sobiecki and Cichocka 31 , Reference Monteilh, Kieszak and Flanders 32
The average age at menarche declined largely between the 1800s and 1900s in the United States and Western Europe with more recent declines in settings where economic development is more recent, such as Asia.Reference Sorensen, Mouritsen and Aksglaede 12 , Reference Parent, Teilmann and Juul 13 Although United States and Western European studies indicate that the age at menarche may have stabilized over the past 50 years, evidence still suggests that in the past 25 years, the median age at menarche has decreased by 2.5–4 months.Reference Euling, Herman-Giddens and Lee 1 , Reference Sorensen, Mouritsen and Aksglaede 12 Stronger trends are observed in racial and ethnic minorities in the United States.Reference Euling, Herman-Giddens and Lee 1 , Reference Sorensen, Mouritsen and Aksglaede 12 , Reference Herman-Giddens, Kaplowitz and Wasserman 33 – Reference Krieger, Kiang and Kosheleva 35
The past several decades have seen a substantial increase in the prevalence of childhood obesity (Fig. 1).Reference Kaplowitz 3 , Reference Ahmed, Ong and Dunger 24 , 41 , Reference Lakshman, Elks and Ong 42 Epidemiological studies have shown that girls with higher body mass index in their childhood years are more likely to undergo earlier pubertal development (i.e. breast development and menarche).Reference Kaplowitz 43 – Reference Wang 46 Body size changes may be a key driver in earlier puberty,Reference Frisch and Revelle 47 but the decline in the average age of menarche occurred before the childhood obesity epidemic, even in settings with lower childhood obesity (e.g. Hong Kong), suggesting that other factors are at play (Fig. 1).Reference Aksglaede, Sorensen, Petersen, Skakkebaek and Juul 21 , Reference Hwang, Shin, Frongillo, Shin and Jo 48 – Reference Rohan, Jain, Howe and Miller 54 Although several genes have been linked to the timing of menarche and height growth,Reference Towne, Czerwinski and Demerath 55 – Reference Spencer, Malinowski and Carty 61 genes alone cannot explain the secular trends. To date, investigation of pubertal timing has focused on changes in (1) environmental exposures [e.g. endocrine disrupting chemicals (EDC)],Reference Walvoord 9 , Reference Diamanti-Kandarakis and Gore 62 – Reference Ulijaszek 66 (2) differences in prenatal exposure and early infant growthReference Yermachenko and Dvornyk 65 , Reference Ibanez, Ferrer, Marcos, Hierro and de Zegher 67 – Reference Ong, Potau and Petry 70 and (3) the social environment (e.g. childhood adversity).Reference Ellis 7 , Reference Walvoord 9 , Reference Yermachenko and Dvornyk 65 , Reference Ulijaszek 66 , Reference Hochberg and Belsky 71 Undoubtedly, all of these play a role, but other factors have also changed that may also explain recent trends.
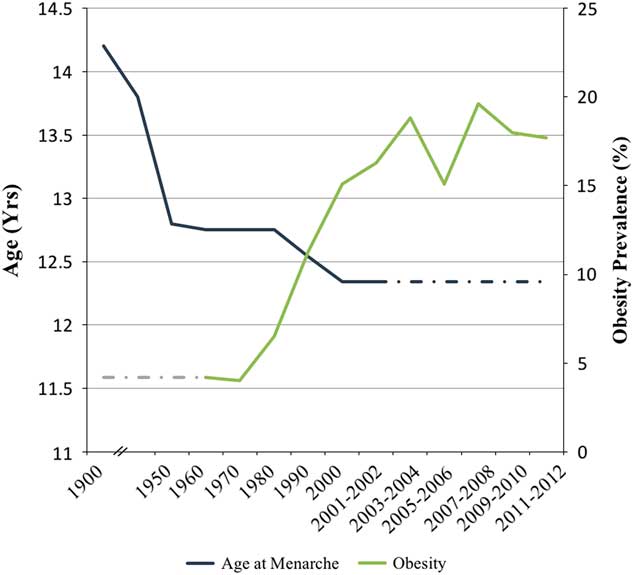
Fig. 1 Secular trends in age at menarche and childhood obesity (ages 6–11 years for boys and girls) in the United States from 1900 to 2012. The dark blue line represents trends in age at menarche and the light green line represents trends in childhood obesity from national data sources. The dashed lines are drawn to approximate trends. Sources: Tanner and EvelethReference Tanner and Eveleth 36 , Gould and Gould 37 , Damon et al. 38 , Anderson et al. Reference Anderson, Dallal and Must 39 , Anderson and Must 40 , National Health and Nutrition Examination Survey II151, Fryar et al. 41 .
Less attention has been given to examining infant and childhood exposures and pubertal timing in the context of other marked secular trends with economic development which maps closely to changes in pubertal timing. Major socio-economic changes and public health initiatives have resulted in vastly reduced exposure (e.g. improved sanitation, decrease in family size) and increased resistance (e.g. widespread antibiotic use, vaccinations) to infectious agents which may influence pubertal timing.Reference Crimmins and Finch 72 , Reference Melosi 73 From a life history perspective, the energetics theory of pubertal timing postulates that in times of critical energy demands an individual will allocate resources for maintenance and survival.Reference Ellis 7 , Reference Hochberg and Belsky 71 The energetics theory has been indirectly assessed by examining the association of puberty with socio-economic status and nutrition.Reference Ellis 7 The most common strategy to cope with energy demands is to reduce energy expenditure of non-essential physiological needs. Therefore, with more infections, there is greater energy investment in the immune system and less energy available for biological systems responsible for reproduction.Reference Rolff 74 , Reference Jasienska 75
The biological basis for the association between infection and pubertal timing lies within the complex interaction between the immune and endocrine systems, where the immune system products modulate hormonal secretions and in turn regulates immune functioning.Reference Bilbo and Klein 76 This is evident in sex differences in immunological responses as females mount a stronger humoral immune response to infection compared with males who are generally more susceptible to infection.Reference Klein 77 , Reference Klein 78 Sex differences are partially attributed to differences in sex-steroids, which influence susceptibility and resistance to infection, most notably by altering host immunity.Reference Klein 77 , Reference Klein 78 Androgens are associated with immunosuppression.Reference Klein 78 Males with androgen deficiencies and gonadectomized mice have greater production of inflammatory cytokines.Reference Klein 78 Androgens also influence disease susceptibility genes (e.g. genes related to competent immune responses and pathogen clearance) and behavior.Reference Klein 78 Estradiol has dual effects, and at low concentrations is associated with proinflammatory activity and at high or sustained concentrations is associated with anti-inflammatory responses.Reference Klein 78 The dual effect makes the precise role of estrogen on immunity unclear.Reference Klein 78 The immune microenvironment in the stroma surrounding mammary gland epithelium is rich in immune cells and, via hormone-mediated communication, drives pubertal and adult mammary gland development.Reference Need, Atashgaran, Ingman and Dasari 79 Murine studies demonstrate that absence or deficiency in key immune cells leads to disruption in terminal end bud ductal branching.Reference Need, Atashgaran, Ingman and Dasari 79
To clarify the potential role of exposure to infections in pubertal timing, we review the epidemiological literature and animal data examining the relationship between infection and pubertal timing – inclusive of breast, genitalia and pubic hair development, and age at menarche – with the hypothesis that infection is associated with later maturation.Reference Kwok, Leung, Lam and Schooling 80 As hypothesized previously, we would expect the greatest effect during periods when the hypothalamic–pituitary–gonadal (HPG) axis is active.Reference Kwok, Leung, Lam and Schooling 80 The HPG axis is active during fetal development and remains active during early infancy (first 12 months) then the HPG axis goes dormant.Reference Grumbach 81 – Reference Chellakooty, Schmidt and Haavisto 84 HPG axis reactivation occurs at the onset of puberty.Reference Melmed, Polonsky, Larsen and Kronenberg 85
Method
Information sources and search criteria
We followed the PRISMA systemic review guidelines. Studies were identified by a systematic search of Medline, Web of Science and EMBASE up until 9 October 2014, with the earliest article identified in 1934.
Key words were identified by consulting the literature and using synonyms. Terms used included infection (without restrictions) and ‘puberty,’ ‘pubertal,’ ‘menarche,’ ‘breast development’ and ‘thelarche’ that were restricted to being listed in the title and/or abstract. We used the Boolean operator AND to combine infection and pubertal terms. We only included articles published in English.
Study selection
We included as eligible, primary articles that examined the association between infections including microbial exposures and physical pubertal characteristics (breast, genitalia, and pubic hair development) or age at menarche. We excluded studies that were either published in a language other than English; focused on precocious puberty, which is a diagnostic evaluation;Reference Sorensen, Mouritsen and Aksglaede 12 , Reference Kaplowitz 86 , Reference Gluckman and Hanson 87 were case studies, and/or included youth with autoimmune diseases (including an anemia cohort) because of the difficulty discerning if the pubertal outcome is due to the autoimmune condition or the infection. Among 1372 non-duplicate articles, we excluded 1334 articles during the screening process and an additional 18 articles upon deeper review (Fig. 2).
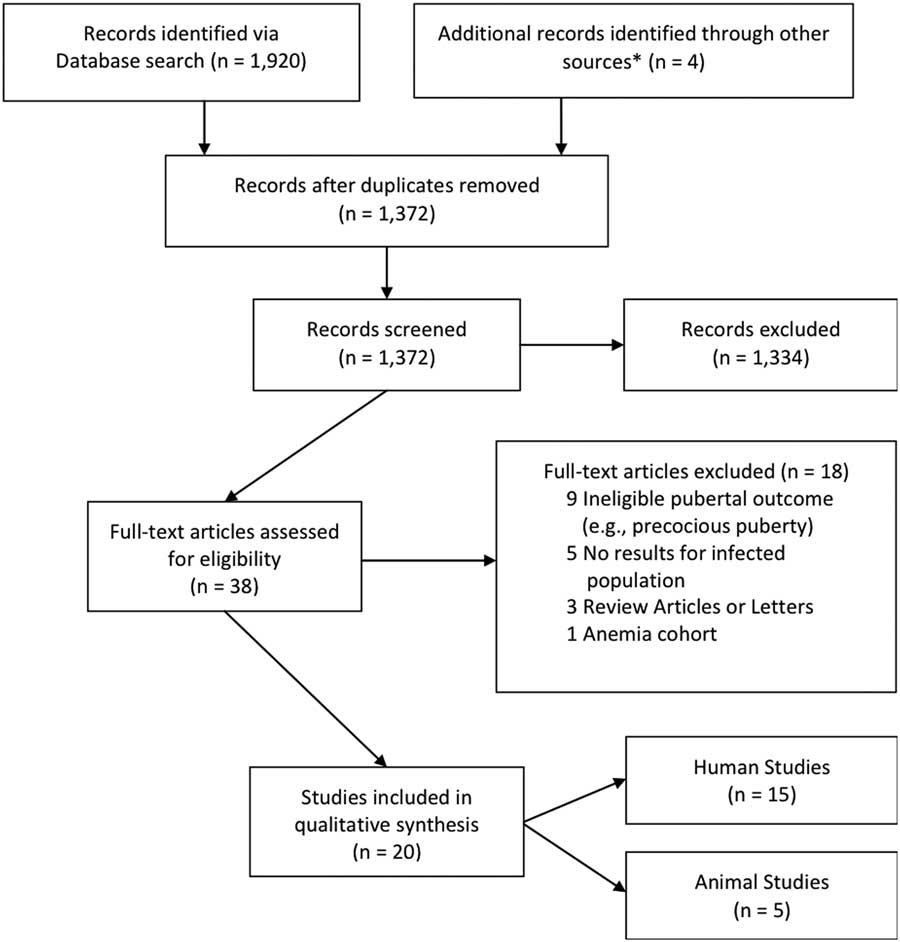
Fig. 2 Schematic of search protocol and results of systematic review. *Identified through review of references of selected papers.
J.A.M. performed all literature searches and the initial screening of article titles and abstracts for eligibility criteria. S.M.E. reviewed 10% of screened articles to validate inclusion and exclusion agreement with J.A.M. J.A.M. identified the full text articles eligible for extensive review and J.A.M. and S.M.E. independently reviewed articles with consensus reached by discussion and by an additional reviewer (M.B.T.).
Data extraction and synthesis
The type of data extracted for qualitative synthesis was decided by J.A.M., M.B.T. and S.M.E. J.A.M., S.M.E. and O.D. independently extracted data for qualitative synthesis and discrepancies between reviewers were resolved through mutual discussion and an additional reviewer (M.B.T.). The summary measures reported were differences in means and proportions, and relative risk ratios which represent hazard ratios or odds ratios depending on study analysis. J.A.M. assessed the quality of the epidemiologic studies using the Newcastle–Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp), which uses a star system to judge study quality across three broad categories: selection of the study groups, the comparability of the groups and the ascertainment of the outcome (cohort study design) or the exposure (case–control study design).
Results
We identified 15 epidemiological studies for qualitative synthesis (Table 1) and five animal studies collectively from the Americas, Africa, Asia, the Caribbean and Europe; we organized these studies by infection type from 1372 screened articles (Fig. 2). Of the 15 epidemiology studies, outcomes were prospectively ascertained in fiveReference Kwok, Leung, Lam and Schooling 80 , Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 , Reference Wu, Tsai and Tung 92 and elicited in a cross-sectional manner at the same time as infection exposure status, in 10.Reference Ferrand, Bandason and Musvaire 90 , Reference Cole, Salem, Hafez, Galal and Massoud 93 – Reference Blell, Pollard and Pearce 101 Physical pubertal characteristics were measured using Tanner and Marshall staging which uses line drawings showing five stages from Tanner stage T1–T5, with stage T2 marking the onset of pubertal development and stages T3–T5 marking increasing maturity.Reference Marshall and Tanner 102 , Reference Marshall and Tanner 103 With one exception where age at menarche was determined through medical records,Reference Wu, Tsai and Tung 92 the start of menarche was recalled by study participants.Reference Ferrand, Bandason and Musvaire 90 , Reference Bernhard, Makunde, Magnussen and Lemnge 96 , Reference Braga, Dourado, Ximenes, Miranda and Alexander 97 , Reference Rosenstock, Jorgensen, Andersen and Bonnevie 99 – Reference Blell, Pollard and Pearce 101 Infectious exposures were assessed diagnostically or through biospecimens, or reported through medical records or by guardian report. We identified four major categories of infection including viral (five studiesReference de Martino, Tovo and Galli 88 – Reference Wu, Tsai and Tung 92 ), bacterial (one studyReference Rosenstock, Jorgensen, Andersen and Bonnevie 99 ), parasitic (10 studiesReference Cole, Salem, Hafez, Galal and Massoud 93 – Reference Fox, Wilson and Addiss 98 , Reference Lacau-Mengido, Mejia and Diaz-Torga 104 – Reference Mukasa-Mugerwa, Kasali and Said 108 ) and non-specific pathogenic infection, termed general infection (three studiesReference Kwok, Leung, Lam and Schooling 80 , Reference Khan, Schroeder, Martorell, Haas and Rivera 100 , Reference Blell, Pollard and Pearce 101 ). For presentation, we have organized the summary of these results by outcome with the human results discussed first followed by the animal evidence.
Table 1 Details of included epidemiological studies of puberty and infection

NHANES, National Health and Nutrition Examination Survey; CI, confidence interval; GSS, Gambian sleeping sickness; HBeAg, Hepatitis B e antigen; RR, relative risk; SMR, sexual maturity rating.
a We used the Newcastle–Ottawa Scale where a study can be awarded a maximum of 4 stars within the selection category, 2 stars within the comparability category and 3 stars within the outcome (cohort study design) or exposure (case–control study design) category based on the answers provided for each item within each category. For those studies with the assessment of multiple pubertal outcomes, the Newcastle–Ottawa Scale was applied to each outcome, independently. The number of stars allocated for each outcome independently was identical; therefore, we present the stars allocated to the outcome/exposure category as a representation of all the pubertal outcomes assessed within each study.
b Data are presented as the age at which 50% of HIV+ girls and boys reached each Tanner stage (stages 2–4) compared with the median (50% percentile) ages of control girls.
c The NHANES III 1988–1994.
d HBeAg seroconversion defined as the spontaneous clearance of serum HBeAg and appearance of anti-HBe for >6 months.
e RR ratios are presented and represent hazard ratios or odds ratios depending on study analysis.
f Criterion for cases was infection with Schistosoma haemotobium only as confirmed by repeated urine and stool analyses. Subjects with other parasitic infections were excluded from study.
g School children were recruited during a campaign against GSS. Cases had school records showing that they had been treated for GSS. Controls were re-tested with the Testryp-CATT to confirm sero-negative status.
h Women recruited from two adjacent villages in northeastern Tanzania and includes women untested for microfilaria.
i Data collected via door-to-door survey in northeastern Brazil.
j Pediatric cohort from the Léoĝane Commune that had participated in longitudinal filariasis studies for ⩾10 years. Study represents cross-sectional diagnostic evaluation that took place in November 2002.
k Ultrasound assessment performed to detect adult worm infections (n=45 girls and 57 boys). Adult worms identified by motility referred as the filarial dance sign.
l Community-based nutritional intervention study carried out between 1969 and 1977. Age at menarche collected between 1991 and 1992.
m Data only includes children where at least 360 days of illness data available.
n Birth cohort was born in 1947 and traced at age 49–51 years where menarche reports were collected.
o Children of 1997 birth cohort recruited from all 49 governmental Maternal and Child Health Centers in Hong Kong. Passive follow-up via record linkage was performed in 2005 and active follow-up with direct contact performed in 2007.
Breast: all studies that assessed breast development suggested infection was associated with later breast development compared with girls without infection.Reference Kwok, Leung, Lam and Schooling 80 , Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 Three prospective studies suggested perinatal HIV infection was associated with later breast development in girls compared with controls,Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 with the difference ranging from 6 to 25 months (comparing breast Tanner stage 2, B2).Reference de Martino, Tovo and Galli 88 , Reference Williams, Abzug and Jacobson 91 In one of the largest U.S.-based prospective studies of perinatal HIV infection, there was a difference in the mean age at breast development between HIV+ and HIV− exposed but uninfected (HEU) controls; the effect was strongest in girls born after 1997 compared with girls born before 1990.Reference Williams, Abzug and Jacobson 91 HIV disease severity, defined by CD4 counts and viral load, was not associated with breast development.Reference Williams, Abzug and Jacobson 91
The association between infections and girls’ pubertal development may start as early as the first few months of life. The Children of 1997 Hong Kong birth cohort collected information on the number of hospital admissions due to infection using hospital records from 9 days to 8 years of age.Reference Kwok, Leung, Lam and Schooling 80 In 3542 Chinese girls, two or more hospital admissions in the first 6 months of life was associated with later breast development; there was no association with hospital admissions in older ages.Reference Kwok, Leung, Lam and Schooling 80
Genitalia: among the five human studies directly assessing genitalia development,Reference Kwok, Leung, Lam and Schooling 80 , Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 , Reference Cole, Salem, Hafez, Galal and Massoud 93 only the viral studies strongly suggested infection was associated with later development.Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 Perinatal HIV infection was associated with a 6–7 months later genitalia development in boys compared with controls (comparing genitalia Tanner stage 2, G2),Reference de Martino, Tovo and Galli 88 , Reference Williams, Abzug and Jacobson 91 and as observed above with respect to girls and breast development, birth cohort effects were observed for the mean age at genitalia development.Reference Williams, Abzug and Jacobson 91 Boys with greater HIV disease severity had later genitalia development ranging between 2 and 9 months.Reference Williams, Abzug and Jacobson 91 Within a cross-sectional study of parasitic infection in 453 Egyptian boys, Entamoeba hystolytica infection was inconsistently associated with later genitalia development (observed in G2, G4 and G5 stages) compared with non-infected boys.Reference Cole, Salem, Hafez, Galal and Massoud 93 There was no association with overall parasitemia or other individual parasites whose prevalence ranged from 1 to 14% compared with E. hystolytica prevalence of 32–51%. Contrast to the findings with breast development, there was no association between the number of hospital admissions due to infection and genitalia development in 3985 Chinese boys.Reference Kwok, Leung, Lam and Schooling 80 However, one rodent study found that a tapeworm infection at 22 days of age was associated with later sexual development in male rats as defined by testicular and seminal vesicle weight.Reference Ramaley and Phares 105
Pubic hair: while two studies suggested later pubic hair development in HIV-infected youth compared with controls,Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 these studies were limited to a cross-sectional control population and did not consider potential confounders. In contrast, there was no association between HIV-infected youth and pubic hair development compared with HEU controls in girls, with only a small, but not statistically significant, association observed in boys after controlling for confounders.Reference Williams, Abzug and Jacobson 91 However, youth with greater HIV disease severity had later pubic hair development ranging between 3–8 months for girls (CD4 measures only) and 3–9 months later for boys (CD4 and viral load measures) compared with youth with lower disease severity.Reference Williams, Abzug and Jacobson 91
Combined measures of puberty: four studies used a sexual maturity rating (SMR) defined by a combination of Tanner staging for breast, genitalia, testes and/or pubic hairReference Aroke, Asonganyi and Mbonda 95 , Reference Fox, Wilson and Addiss 98 or used Tanner staging without reporting the specific pubertal characteristic assessed.Reference Ferrand, Bandason and Musvaire 90 , Reference Ibrahim, Barakat and Bassiouny 94 Among the one study of viral infectionReference Ferrand, Bandason and Musvaire 90 and the three studies of parasitic infection,Reference Ibrahim, Barakat and Bassiouny 94 , Reference Aroke, Asonganyi and Mbonda 95 , Reference Fox, Wilson and Addiss 98 none provided information on the role of infection on independent physical pubertal characteristics and some did not provide information on initiation of puberty. One study showed a null effect,Reference Aroke, Asonganyi and Mbonda 95 two studies found later timing,Reference Ferrand, Bandason and Musvaire 90 , Reference Ibrahim, Barakat and Bassiouny 94 and one earlier pubertal development.Reference Fox, Wilson and Addiss 98 A cross-sectional Haitian study was the only identified study to observe that infection, defined as having circulating Wucheria bancrofti antigen and the presence of adult worms, was associated with advanced SMR (stages 3–5), compared with those without infection.Reference Fox, Wilson and Addiss 98 However, of 102 youth examined for presence of adult worms, only 11 children had adult worms (n=10 boys) which could be a reflection that detection of adult worms is easier in post-pubertal youth.
Age at menarche: the impact of infection on age at menarche was inconsistent. Three studies suggested infection was associated with later start of menarche.Reference Ferrand, Bandason and Musvaire 90 , Reference Wu, Tsai and Tung 92 , Reference Rosenstock, Jorgensen, Andersen and Bonnevie 99 A Taiwanese cohort [mean (s.d.) age at recruitment 4.6 (3.1) years] that was followed for an average of 24 (3.8 s.d.) years examined Hepatitis B viral infection and menarche.Reference Wu, Tsai and Tung 92 Hepatitis B antigen seroconversion was associated with earlier age at menarche after controlling for viral pathogenic covariates. The two cross-sectional parasitic studies did not show an association with menarche.Reference Bernhard, Makunde, Magnussen and Lemnge 96 , Reference Braga, Dourado, Ximenes, Miranda and Alexander 97 Helicobacter pylori positivity was associated with a 10% increased risk in later age at menarche, compared with H. pylori negative women, after controlling for sociodemographic, metabolic, lifestyle factors and chronic conditions.Reference Rosenstock, Jorgensen, Andersen and Bonnevie 99 Two prospective cohort studies assessed general infection and menarche, including a Guatemalan study where diarrheal and respiratory illness were reported every 2 weeks by maternal or caretaker recall from age 3 months to 3 years and age at menarche was retrospectively collected at approximate ages 15–30 years.Reference Khan, Schroeder, Martorell, Haas and Rivera 100 There was no association with respiratory illness (P>0.10), but marginally (P<0.10), later age at menarche was associated with diarrheal illness after adjusting for confounders.Reference Khan, Schroeder, Martorell, Haas and Rivera 100 The Thousand Family study in the United Kingdom found that infection rate between birth and 8 years of age was not associated with later age of menarche.Reference Blell, Pollard and Pearce 101 Respiratory and intestinal infection were notified by health providers and parents with retrospective recollection of age at menarche by subjects at age 50 years old.Reference Blell, Pollard and Pearce 101 The findings for infection and puberty are also inconsistent among animal studies, while puberty is defined as the first behavioral estrous.Reference Fabre-Nys and Gelez 109 In a Argentinian study of heifers (n=40), those treated with an anti-helminthic reached puberty 3.7 weeks earlier than heifers not treated with an anti-helminthic (statistical significance not reported).Reference Lacau-Mengido, Mejia and Diaz-Torga 104 In two independent ewe lamb studies (n=24 and 112), there was no association between parasitic infections and age at first estrous.Reference Osaer, Goossens, Jeffcoate and Holmes 107 , Reference Mukasa-Mugerwa, Kasali and Said 108
Discussion
Infection is associated with later breast development, with less consistent evidence for genitalia and pubic hair development and age at menarche (Fig. 3). The consistent association with breast development may be attributed to the fact that secular trends for breast development are more marked than for other pubertal measures. The differences by gender and pubertal marker may be biological or due to measurement issues. The literature is emerging and careful consideration of study design, including exposure and outcome measurements (Table 2), is needed to understand some of the existing inconsistencies and major gaps in the evidence base. Nevertheless, the data are intriguing, particularly given the overall consistency between infections and later breast development as childhood infections have declined over timeReference Cho, Park and Shin 52 , Reference Woolf and Aron 53 and average age at breast development has also declined.Reference Herman-Giddens, Slora and Wasserman 15 , Reference Lee 16 , Reference Biro, Galvez and Greenspan 19 , Reference Aksglaede, Sorensen, Petersen, Skakkebaek and Juul 21 , Reference Ahmed, Ong and Dunger 24 , Reference Anderson, Dallal and Must 39 , Reference Chumlea, Schubert and Roche 110 – Reference Nicolson and Hanley 112 The evidence also supports our hypothesis as the strongest effect between infection and puberty was with infections acquired in early life (perinatal and early infancy), presumably during HPG axis activity.Reference Kwok, Leung, Lam and Schooling 80 , Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 , Reference Khan, Schroeder, Martorell, Haas and Rivera 100

Fig. 3 Results of studies examining infections and pubertal development, 1980–2014. Later refers to studies that the observed infection was associated with a later age of development of the pubertal outcome (indicated in parentheses). Earlier refers to studies that the observed infection was associated with an earlier age of development of the pubertal outcome (indicated in parenthesis). No effect refers to studies that observed no association between infection and the pubertal outcome (indicated in parenthesis). (a) Pubic hair development was later with HIV disease severity in boys. (b) Tanner staging with type unspecified. (c) External sign of puberty in the male rat that is accompanied by testicular and seminal vesicle weight. Animal studies are in italics. SMR; sexual maturity rating.
Table 2 Measurement of pubertal outcome and infection exposure measures and associated challenges

Differences by gender: the inconsistencies observed between infection and pubertal timing may be influenced by sex differences. For example, compared with youth with lower HIV disease severity, there were stronger pubertal trends observed for boys with greater disease severity than with girls.Reference Williams, Abzug and Jacobson 91 As discussed above, sex differences may be due to differences in initial immunological response, where males generally mount a weaker response compared with females, or to differences in levels of circulating sex-steroid hormones.Reference Klein 77 , Reference Williams, Abzug and Jacobson 91 The endocrine system impacts the functioning of the immune system.Reference Bilbo and Klein 76 Immune cells express sex steroid receptors; therefore, sex steroids may modulate activity, expression, and function of immune cells important to cellular and humoral immunityReference Kovats, Carreras and Agrawal 113 and drive mammary gland development.Reference Need, Atashgaran, Ingman and Dasari 79 , Reference Klein and Nelson 114 – Reference Tanriverdi, Silveira, MacColl and Bouloux 116 Parasitic and bacterial infections cause dysregulation of the HPG axis by downregulating sex-steroid hormone production or sex-steroid hormone receptors.Reference Ibrahim, Barakat and Bassiouny 94 , Reference Mavoungou, Lansoud-Soukate and Dupont 117 – Reference Reincke, Arlt and Heppner 123 Thus, greater childhood infectious exposures may result in lower sex-steroid production that results in later age at breast development and menarche. The weaker evidence with genitalia and pubic hair development may be explained because they are more affected by androgens rather than estrogens.Reference Beunen, Rogol and Malina 34 , Reference Biro, Pinney and Huang 124 , Reference Biro, Huang, Daniels and Lucky 125
Challenges in epidemiologic study interpretations: in seven prospective studies,Reference Kwok, Leung, Lam and Schooling 80 , Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 , Reference Wu, Tsai and Tung 92 , Reference Khan, Schroeder, Martorell, Haas and Rivera 100 , Reference Blell, Pollard and Pearce 101 three had inadequate or no control populationReference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Wu, Tsai and Tung 92 and few explicitly report lost to follow-up.Reference Kwok, Leung, Lam and Schooling 80 , Reference Buchacz, Rogol and Lindsey 89 , Reference Blell, Pollard and Pearce 101 In studies that report, the lost to follow-up ranged between 5 and 14%, which could affect small associations through selection bias.Reference Kwok, Leung, Lam and Schooling 80 , Reference Buchacz, Rogol and Lindsey 89 , Reference Blell, Pollard and Pearce 101 The six cross-sectional studies can only infer associations,Reference Ferrand, Bandason and Musvaire 90 , Reference Cole, Salem, Hafez, Galal and Massoud 93 , Reference Bernhard, Makunde, Magnussen and Lemnge 96 – Reference Rosenstock, Jorgensen, Andersen and Bonnevie 99 not causation, and selection bias may be an issue in the two case–control studies based on the control selection.Reference Ibrahim, Barakat and Bassiouny 94 , Reference Aroke, Asonganyi and Mbonda 95 Of the five studies that solely concluded that infection had no association with pubertal development, the NOS quality assessment of the outcome was low (0–2 stars), which stems from the nature of cross-sectional studies lacking follow-up assessments and self-reported menarche introducing recall bias. In contrast, some of the highest quality studies were the viral studies, which consistently found that viral infections resulted in later pubertal development. The viral studies had a high-quality assessment for selection (3–4 stars) and outcome (3 stars, with one study having 0 stars). In contrast, the most heterogeneous findings related to the role of parasitic infections on pubertal development. The heterogeneity within parasitic infections may be attributed to the low quality assessment for both the comparability between groups (0–2 stars) and the outcome (0–2 stars). In the general infection category, the one study that found an association with later pubertal development had the highest NOS assessment ratings.
Challenges in measurement of outcomes: a major challenge to interpreting the existing literature is heterogeneity in measurement of outcomes. With respect to breast development measurement, palpation assessment is important to avoid misclassification given the rise of childhood obesityReference Marshall and Tanner 102 , Reference Marshall and Tanner 103 but no study reports this method.Reference Kwok, Leung, Lam and Schooling 80 , Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 The gold standard of measuring boys’ puberty includes a visual assessment supplemented by an estimation of testicular volume.Reference Tinggaard, Mieritz and Sorensen 6 The latter provides greater accuracy and less variability across observers; although, the testicular volume threshold indicating pubertal onset is not consistent.Reference Tinggaard, Mieritz and Sorensen 6 One study implied the use of an orchidometerReference Aroke, Asonganyi and Mbonda 95 and three out of nine studies reported orchidometer use,Reference Kwok, Leung, Lam and Schooling 80 , Reference Williams, Abzug and Jacobson 91 , Reference Ibrahim, Barakat and Bassiouny 94 with one of these reporting use on a subset of the cohort.Reference Williams, Abzug and Jacobson 91 Misclassification in Tanner staging can be minimized by training, but studies that provide details on training are scant.Reference Buchacz, Rogol and Lindsey 89 In addition, Tanner stage 2 marks the onset of development; therefore, studies that combine Tanner stages 1/2 are failing to capture initiation.Reference Ferrand, Bandason and Musvaire 90 , Reference Fox, Wilson and Addiss 98 Many of the studies examining age at menarche rely on self-reported age at menarche that may result in misclassification, or at the very least, loss of power if the recall of menarche is in years rather than capturing months.
Challenges in measurement of exposures: potential measurement errors for assessing infectious exposures include the type of measurement and the timing of collection. The majority of studies diagnostically assessed infection measures with only four studies assessing infection before the onset of physical pubertal characteristics.Reference de Martino, Tovo and Galli 88 , Reference Buchacz, Rogol and Lindsey 89 , Reference Williams, Abzug and Jacobson 91 , Reference Wu, Tsai and Tung 92 Infection measures were also assessed through health provider and guardian reports.Reference Khan, Schroeder, Martorell, Haas and Rivera 100 , Reference Blell, Pollard and Pearce 101 Retrospective collection of exposure data, though more feasible, is limited by maternal recall bias. Though bias is expected to be non-differential, this may be of greater consequence in studies of childhood illness where mothers of babies with defects may recall information differentially than mothers of babies without infections.Reference Rothman 126 Medical reports are a valuable alternative. The strength of the Hong Kong cohort was that hospital discharge records accounted for 81.4% of all hospital admissions.Reference Kwok, Leung, Lam and Schooling 80 However, a limitation of medical reports is that they capture serious infections that require hospitalization and physician intervention, missing milder infections. A reduced risk of young adult Hodgkin’s lymphoma has been associated with daycare attendance and a greater number of siblings,Reference Hjalgrim, Smedby and Rostgaard 127 , Reference Chang, Zheng and Weir 128 suggesting the importance of non-medical and early-life infectious exposures for the maturation of cellular responses.
Challenges in temporality: the temporal sequence of acquired infections and puberty is critical to understand causality. In a scenario where infection occurs before pubertal development, there are limitations in data interpretation as there are other factors that affect pubertal timing, such as body size and physical activity. Therefore, prospective studies of infection and pubertal timing should include repeat measures of these relevant constructs. Cross-sectional studies, where the timing of infectious exposure relative to the pubertal outcome is unclear, also presents data interpretation challenges. For example, during pubertal development, the body will focus energy on the endocrine system; thereby, the body may exert less energy on the immune system resulting in greater vulnerability to infection. In this scenario, the infection is subsequent to the pubertal outcome.
Challenges in defining infection and a microbial life cycle: infection is a dynamic process involving a varying degree of immunomodulation during the course of a microbial life cycle – which may range to include an exertion of pathogenesis to a commensal state; whereby, the commensal state can be short lived or prolonged.Reference Casadevall and Pirofski 129 Parasites and bacterial communities are prime examples of microbes living in a commensal state within the human host.Reference Casadevall and Pirofski 129 The literature is limited in examining the relationship between the microbial life cycle and the hosts’ life course relative to puberty. For example, the life cycle of parasitic helminthes, such as schistosomes, are dynamic and within the mammalian definitive host can drive both a proinflammatory and an anti-inflammatory immune response; therefore, eliciting different host responses and host health outcomes.Reference Harn, McDonald, Atochina and Da’dara 130 , Reference McDonald 131 Our literature review observed inconsistent findings between parasitic infections (e.g. filarial, trypanosome, schistosome, protozoan) and pubertal outcomes. In addition to the studies collectively having a low-quality assessment via the NOS scale (discussed above), inconsistency could be due to a simplified definition of infection that does not delineate phases of the microbial life cycle. Methodologically, the effect between high parasitic infection burden and pubertal development may be real, albeit small, and thus require a robust sample size. Moreover, observed effects may require a robust sample of individuals who are experiencing a phase of the microbial life cycle that garners host immune reactivity that would incite changes in hormonal activity.
While we did not include precocious puberty within our eligibility criteria, of the over 80 studies identified in our search that pertained to infections among populations experiencing either constitutionally delayed puberty or precocious puberty, three studies met our criteria for inclusion in the current review.Reference Bhakhri, Prasad, Choudhary and Biswas 132 – Reference Mills, Stolley, Davies and Moshang 134 The single studyReference Mills, Stolley, Davies and Moshang 134 examining the natural history of premature breast development among U.S. girls found no association between this condition and frequency of prenatal general infections. The remaining two studies investigated the prevalence of infections among boys and girls in TurkeyReference Büyükgebiz, Dündar, Böber and Büyükgebiz 133 and IndiaReference Bhakhri, Prasad, Choudhary and Biswas 132 with delayed puberty. Büyükgebiz et al. found an increased prevalence of H. pylori infection among children with constitutional delay in growth and puberty (n=16 out of 24, 66%) compared with healthy, age-matched control children with normal pubertal development (n=12 out of 32, 37.5%)Reference Büyükgebiz, Dündar, Böber and Büyükgebiz 133 , and Bhakhri et al. observed that the highest etiologic proportion (38%) of pubertal delay in their study population was attributable to functional hypogonadotropic hypogonadism owing to chronic illnesses, including chronic infections.Reference Bhakhri, Prasad, Choudhary and Biswas 132 Both of these latter study results are consistent with our hypothesis that the burden of chronic infection delays puberty.
A way forward: in summary, given the intriguing, but limited epidemiological and animal data, we propose suggestions for future studies to explore the role of infection and puberty.
-
(1) Studies that carefully measure anthropometry: teasing apart factors that contribute to earlier pubertal maturation is difficult because childhood obesity exists alongside the changing environmentReference Kaplowitz 3 , Reference Ahmed, Ong and Dunger 135 and emerging evidence is examining the role of infection as a cause of obesity.Reference McAllister, Dhurandhar and Keith 136 – Reference Schooling, Jones and Leung 138 Epidemiologic studies will need to examine these associations in pubertal cohorts that span the continuum of body size.
-
(2) Studies that have measures of additional exposures that are also changing with time: EDCs and the immune system interact with the endocrine system affecting hormone production;Reference Diamanti-Kandarakis and Gore 62 – Reference Özen and Darcan 64 therefore, EDCs and infectious exposures could interact synergistically or antagonistically depending on the EDC. Future studies should consider the interaction between exposures to EDCs and infection on pubertal timing.
-
(3) Studies that measure home and community environment that may impact both infection exposure and pubertal outcomes: psychosocial factors contribute to pubertal timingReference Ellis 7 and mounting evidence links the immune and neuroendocrine system.Reference Bilbo and Klein 76 Animal and human studies suggest adaptation to the social environment leads to a complex interaction between immune and reproductive functioning.Reference Klein and Nelson 114 , Reference Ellis, Shirtcliff, Boyce, Deardorff and Essex 139 Future studies should examine the complex interaction between the home and social environment (e.g. familial relationships, stress), infection and puberty.
-
(4) Studies able to assess multiple pubertal outcomes: large prospective studies with wide age ranges are most desirable for the study of infections and pubertal timing where the timing of pubertal outcomes and infection are known or adequately approximated and confounders can be ascertained.Reference Euling, Herman-Giddens and Lee 1 Including more studies on the association of infection with pubic hair development, pubertal tempo (time between maturational stages) and childhood height would be valuable. Given that shorter adult stature has been associated with later pubertal timingReference Schooling, Jiang and Lam 140 and childhood infections have been shown to impair height,Reference Hwang, Mack and Hamilton 141 – Reference Patel, Mendall, Khulusi, Northfield and Strachan 144 future studies should examine infection and pubertal height.
-
(5) Studies that assess multiple common infections through diverse measures across windows of susceptibility: future studies should include a variety of infectious exposures with pre-pubertal measures including diagnostic and biospecimen assessment, medical records and prospective and retrospective health provider and/or guardian reported data. When collecting infectious exposure data, three factors should be considered. First, infection severity should be captured biologically or by infection frequency. Second, timing and type of infection should be considered within windows of susceptibility from birth throughout childhood, including acute and chronic infections and if possible the microbes life cycle, given the different factors that determine infant, childhood and pubertal growth.Reference Karlberg 145 Third, in populations where infectious exposures are limited, measures should be carefully selected to represent a range of prevalence to adequately test hypotheses.
-
(6) Studies that assess the interactions between the endocrine and immunoregulatory systems: sex steroids not only underlie the physical manifestations of puberty, they also increase years before physical signs that may be attributed to differences in centrally or peripherally produced hormones.Reference Biro, Pinney and Huang 124 , Reference Houghton, Cooper and Bentley 146 Studies need to investigate the intersection between the complexities of the endocrine system (with comprehensive sex-hormone measures) and the plausible influence of the immunoregulatory system. A key consideration here is the use of measures which capture sex hormones comprehensively.
-
(7) Animal studies: the animal literature is scant but generally consistent with the human evidence. Pending results of human epidemiological studies, consideration of animal models to disentangle exposure effects will be needed.
If large studies replicate the intriguing findings that exposure to childhood infections may delay pubertal timing, the impact on public health is clear. There should not be changes to public health policies like vaccinations and sanitation improvements that have reduced the spread of infectious disease, but rather there may be a greater need to discourage excessive environmental sterility and use of antibacterial lotions and products.
Understanding infection across the continuum of maturation can inform whether infant/childhood public health policies, such as vaccinations, or other practices, such as use of antibacterial product use, may affect long-term risk of breast cancer and other hormone-related diseases.Reference Golub, Collman and Foster 8 , Reference Walvoord 9 , Reference Kwok, Leung, Lam and Schooling 80 , Reference Evans, Sany and Pearmain 147 The incidence of late-stage breast cancer risk is rising in young women (<40 years)Reference Johnson, Chien and Bleyer 148 and there is a rise in incidence of testicular cancer, for which pubertal timing is a risk factor.Reference Huyghe, Matsuda and Thonneau 149 With rapid declines observed in age at onset of breast development and age at menarche remaining relatively stable, pubertal tempo is increasing that may be related to breast cancer.Reference Bodicoat, Schoemaker and Jones 150 Globally, as countries develop and infectious agents decrease, the age of puberty declines; thus, future research that explores common childhood microbes and the underlying biological mechanisms (i.e. endocrine system) are important to mitigate population-level health risks.Reference Golub, Collman and Foster 8
Acknowledgments
The authors would like to sincerely thank Dr Lauren Houghton for reviewing earlier versions of the manuscript and Dr Barun Mathema for intellectual conversation regarding the revision of the manuscript.
Financial Support
The authors greatly acknowledge the funding by the National Cancer Institute at the National Institutes of Health (J.A.M., grant number K01 CA186943, M.B.T. grant number R01 CA138822).
Conflicts of Interest
None.







