Congenitally corrected transposition of the great arteries is a rare cardiac anomaly in patients with CHD.Reference Connelly, Liu and Williams 1 This anomaly is often associated with other defects such as ventricular septal defect. Although only 1–2% of patients have no coexisting anomalies, structural abnormalities of the anatomical tricuspid valve are frequently found.Reference Allwork, Bentall and Becker 2 The superiority of the double-switch operation to conventional repair remains uncertain, especially in patients with only tricuspid valve insufficiency associated with Ebstein’s anomaly.Reference Hraska, Duncan and Mayer 3 Replacement of the left-sided systemic tricuspid valve is an option, but the available data are currently insufficient.
We report successful tricuspid valve replacement in a 3-month-old boy with this cardiac disease with Ebstein’s anomaly as the only associated cardiac anomaly.
Case report
A male baby with a suspected fetal diagnosis of congenitally corrected transposition of the great arteries was born by normal spontaneous vaginal delivery at 39 weeks of gestation, weighing 2648 g. Transthoracic echocardiography revealed atrioventricular and ventriculoarterial discordance with mild left-sided systemic tricuspid valve regurgitation. We initially planned to perform pulmonary artery banding as a prelude to performing a double-switch operation. During follow-up, however, the occurrence of a 2:1 atrioventricular block and increased tricuspid regurgitation resulted in worsening heart failure symptoms. At 3 months of age, pulmonary artery banding, tricuspid valve plication, and pacemaker implantation were performed.
During the operation, the left-sided atrioventricular valve was confirmed as a morphological tricuspid valve. Coaptation of the tricuspid valve leaflets was very poor owing to downward displacement of the attachments of the scanty septal leaflet. This was repaired in three ways by plicating the atrialised segment, placing artificial chordae tendineae, and reducing the annular circumference. The main pulmonary artery was banded loosely because the directly measured pulmonary arterial pressure was approximately equal to the systemic blood pressure. The surgery was completed by implanting a pacemaker to improve the atrioventricular block.
The tricuspid regurgitation, however, did not improve, and the chest X-ray showed increasing pulmonary congestion (Fig 1a). Although the left-sided morphological right systemic ventricle had good contractility, the heart failure symptoms gradually worsened. We decided to perform tricuspid valve replacement because we felt that reducing the tricuspid regurgitation was vital to improve his overall clinical condition.
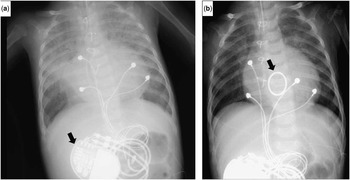
Figure 1 Chest X-ray before and after tricuspid valve replacement (TVR). ( a ) Chest X-ray after pulmonary artery banding demonstrated pulmonary congestion. The black arrow indicates the implanted pacemaker. ( b ) Post-TVR chest X-ray demonstrated normal pulmonary blood flow. The black arrow indicates the mechanical valve.
Intraoperative findings included marked dilation of the left atrium and pulmonary veins. The tricuspid valve did not appear to be amenable to a second plication. Every component of the valve structure was removed for replacement with a 16-mm diameter ATS-AP mitral valve (Medtronic, Minneapolis, Minnesota, United States of America); however, implanting into the native annulus was impossible because the motion of the mechanical leaflets was restricted by the ventricular wall preventing complete opening. The top-hat method of implantation was required to address this problem (Fig 2).Reference Nishida, Tamura and Yamazaki 4 An end of a 20-mm-diameter by 7-mm-long expanded polytetrafluoroethylene graft was reduced to 17-mm diameter. This 17-mm-diameter end of the graft was sutured to the native annulus of the tricuspid valve, and the 16-mm mechanical valve was sutured to the remaining 20-mm end of the graft. This supra-annular valve replacement technique allowed the mechanical leaflets to open smoothly. The cardiopulmonary bypass time and aorta cross-clamp time were 130 and 98 minutes, respectively.

Figure 2 Tricuspid valve replacement with expanded polytetrafluoroethylene (ePTFE) graft. Both sides of an ePTFE graft (20-mm diameter) were cut and sewn into sides of 17-mm diameter. This ePTFE graft (17-mm diameter, 7-mm height) was set into the native annulus as a base. The mechanical valve (ATS-AP mitral valve Φ16 mm) was set onto this base as supra-annular valve replacement (top-hat method).
The postoperative course was uneventful. Echocardiography disclosed good wall motion of the systemic ventricle and good function of the prosthetic valve without stenosis or regurgitation. Chest X-ray revealed improvements in cardiomegaly and pulmonary congestion (Fig 1b). The patient is doing well 3 years after the operation, and is taking an anticoagulation regimen consisting of warfarin sodium and aspirin.
Discussion
The double-switch operation is thought to be an adequate treatment for congenitally corrected transposition of the great arteries with significant tricuspid regurgitation.Reference Imai, Seo and Aoki 5 Before the double-switch operation, pulmonary artery banding is commonly performed; however, patients with tricuspid insufficiency without ventricular septal defects tend to be intolerant to this banding because it causes deterioration of tricuspid regurgitation.Reference Hraska, Duncan and Mayer 3 Our patient had already demonstrated severe heart failure before the banding, which we felt was primarily due to the significant tricuspid regurgitation. The deterioration of this tricuspid regurgitation after the banding further exacerbated the heart failure symptoms. We believe that the lack of a ventricular septal defect also contributed to worsening of the tricuspid regurgitation.
The prognosis in congenitally corrected transposition of the great arteries mainly depends on the severity of the tricuspid regurgitation.Reference Prieto, Hordof, Secic, Rosenbaum and Gersony 6 In an attempt to improve this tricuspid regurgitation, valve plication was attempted during the first surgery. Although performing a single-staged, double-switch operation was one option because of the presence of systemic pulmonary artery pressures, we felt that the patient might not tolerate the longer bypass and cross-clamp times owing to the severe preoperative heart failure. Therefore, we decided to perform a loose pulmonary artery banding, with concomitant tricuspid valve plication, expecting the pulmonary arterial pressure to decrease with the improvement in tricuspid regurgitation. Unfortunately, the tricuspid regurgitation did not improve postoperatively.
Tricuspid valve replacement can be indicated in patients with heart failure caused by tricuspid regurgitation due to structural leaflet malformations. Short-term results show that this procedure improves systemic right ventricular function.Reference Scherptong, Vliegen and Winter 7 This procedure should be performed before the systemic ventricular ejection fraction falls below 40%.Reference Mongeon, Connolly, Dearani, Li and Warnes 8 After the first tricuspid valve plication, unfortunately, the regurgitation remained significant and his condition did not improve despite intensive medical management. As a result, we decided to perform tricuspid valve replacement while the systemic ventricular function was maintained. Surgical technique plays an important role in tricuspid valve replacement. His native annulus was too small for the implantation of a mechanical valve, but the left atrium was large enough to accommodate the supra-annular placement of the mechanical valve while still avoiding pulmonary venous obstruction.
Further careful observation is required because replacement with a larger mechanical valve will be needed in the future. Lubiszewska et alReference Lubiszewska, Rozanski and Szufladowicz 9 reported that a mechanical valve of >23-mm diameter in the atrioventricular position in the systemic ventricle offers excellent long-lasting haemodynamic performance.
In conclusion, tricuspid valve replacement could be considered as the first option in small infants with congenitally corrected transposition of the great arteries having significant tricuspid regurgitation. The top-hat method is a useful technique for the small annular ring.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care.





