Introduction
Most lichenological studies exclusively consider epiphytic lichens that grow on the first 2 m from the ground, the height reachable without climbing or logging the trees. Often, lichens are recorded only on tree trunks, sometimes in the limited area of sample grids. The clear advantages of such methods are the substantially better feasibility of fieldwork and higher comparability of trees sampled. At the same time, much of the information concerning species diversity on trees studied remains unavailable. This missing information might be useful for estimating the lichen species richness of forest sites, also for purposes of conservation and bioindication. For example, Fritz (Reference Fritz2009) has cautioned that surveying only the lowest 2 m in old beech forests can lead to the underestimation of the number of lichen species with conservation concern and their population sizes. Marmor et al. (Reference Marmor, Tõrra and Randlane2010) found that lichen composition in the upper canopy of spruce and pine forests is a far more informative dust pollution indicator than species composition in the lower canopy.
The great variety of different microhabitats on trees, from root cavities to treetop twigs, provides suitable conditions for lichen species with various ecological requirements. Many epiphytic lichen species are associated with specific substrata, such as trunks or branches (e.g. Holien Reference Holien1997; Caruso & Thor Reference Caruso and Thor2007; Lie et al. Reference Lie, Arup, Grytnes and Ohlson2009). Lichen communities also change on trees with height from the ground. Although there are relatively few studies dealing with the diversity of canopy lichens, the existing studies have clearly proved that the occurrence and biomass of many lichen species change vertically on trees, and some species are more abundant in the mid or upper canopy (e.g. McCune Reference McCune1993; McCune et al. Reference McCune, Rosentreter, Ponzetti and Shaw2000; Rosso et al. Reference Rosso, McCune and Rambo2000; Campbell & Coxson Reference Campbell and Coxson2001; Ellyson & Sillet Reference Ellyson and Sillet2003). According to Williams & Sillett (Reference Williams and Sillett2007), many epiphytic species show distinct distribution patterns across the height strata and substratum types in redwood canopies. It can be derived that the total diversity of lichens on trees can be covered only if all substrata and height ranges are studied.
Picea abies (L.) H. Karst. (hereafter ‘spruce’) and Pinus sylvestris L. (hereafter ‘pine’) are the most common tree species in boreal Europe. So far lichen communities have been studied in spruce canopies (e.g. Kermit & Gauslaa Reference Kermit and Gauslaa2001; Caruso & Thor Reference Caruso and Thor2007; Lie et al. Reference Lie, Arup, Grytnes and Ohlson2009) where lichens have been sampled mostly at selected heights rather than across the whole height gradient; and only macrolichens have been studied in pine canopies (Marmor et al. Reference Marmor, Tõrra, Saag and Randlane2011). In the present work the entire lichen biota is surveyed from the bottom to the top of the tree on all available substrata on spruce and pine. Sampling lichens in height ranges provides information about the vertical changes in lichen species richness on tree trunks and branches, and also about the occurrence of individual species. The main aims are to find out 1) how large the differences in lichen species richness are between the lowermost 2 m near the ground and the whole trees, that is how many species are missed if only the first 2 m are studied, and 2) how does the occurrence of individual lichen species change with height in the canopy, that is, which species are missed if only the lowermost 2 m are studied. We hypothesize that a high proportion of lichen species growing on trees remains unrecorded if only the lowermost metres of trees are sampled.
Materials and Methods
Study area
The study was carried out in Estonia, located in Northern Europe on the eastern shores of the Baltic Sea. The mean annual temperature is c. 5°C (monthly mean varies from −6°C to 16°C), and the mean precipitation is c. 630 mm (EMHI). About half of the territory of the country is covered with forests. Estonian forests belong to the hemiboreal subzone of the boreal forest zone, lying in the transitional area where the southern taiga forest subzone changes into the spruce-hardwood subzone (Ahti et al. Reference Ahti, Hämet-Ahti and Jalas1968; Laasimer & Masing Reference Laasimer, Masing and Raukas1995). Pine, spruce and birch (Betula pendula) are the most abundant tree species in the area.
Our sample plots were located in the coniferous forests in southern Estonia. Lichens were studied in three plots: 1) 58·1113°N, 27·0314°E; 2) 58·1134°N, 26·9965°E; 3) 57·6957°N, 27·3622°E (Fig. 1). Spruce and pine were the main tree species in all sites; Vaccinium myrtillus, V. vitis-idaea and Oxalis acetosella dominated the undergrowth. The sites were located in forest-agricultural landscape and were surrounded by variously-aged forests from young growths to mature stands. Sample trees were chosen inside the forest stand in order to reduce the effect of sidelight. All sample plots were located in planned clear-cut stands where trees were going to be cut down anyway.

Fig. 1. Location of sample plots.
Field methods
Fieldwork was carried out in autumn 2008. Five spruce and five pine trees were cut down for the study in every site, making a total of 30 trees. The age of trees was measured by counting the annual rings on tree stumps. For studying the vertical gradient of lichens, all trees were divided into height ranges, first range extending from the ground up to 2 m (the height reachable without additional equipment or logging) and all the following ranges being 4 m long. The treetops which were ≤2 m long were included in the previous height range, and treetops which were >2 m long in the next height range. The attachment point was used for defining the height range of branches. Lichen presence or absence was recorded separately in every height range. All lichen species growing within a height range on tree trunks, branches or twigs were recorded at site or collected for later identification. Specimens collected were identified using a microscope and spot tests. Thin-layer chromatography with solvent A (Orange et al. Reference Orange, James and White2001) was used for identifying secondary compounds in sterile crusts and some Usnea specimens.
Statistical analysis
Software applications STATISTICA 7 and PC-ORD 5 were used for the statistical analysis. Data were analyzed separately according to the tree species. A t-test was used for comparing the total lichen species richness on trees with species richness on the lowest 2 m near the ground. Pearson correlation analysis was used for finding out whether the number of lichen species on the first 2 m of trees is correlated with the number of species on entire trees. Spearman's correlation coefficient was calculated for describing the vertical changes in lichen species richness separately for tree trunk and branches; when calculating the mean number of lichen species on branches the height ranges with no branches were included in analyses with 0 species.
Indicator Species Analysis with Monte Carlo test of significance was used for finding out the preferred height range(s) of lichen species, jointly for trunks and branches. In addition, detrended correspondence analysis (DCA; axes rescaled, no down-weighting of rare species or any prior data transformations) was used for describing lichen species composition in the height ranges. Infrequent lichen species that were recorded only on 1–2 trees were left out from the Indicator Species Analysis and DCA. Fisher exact test (one-tailed) was used to determine the trunk-branches preferences of lichen species.
Results
Tree characteristics
Lichens were recorded on 15 spruces and 15 pines. Height of spruces varied from 22–35 m with an average of 29 m, and height of pines from 26–36 m with an average of 32 m. Age of spruces varied from 100–147, average age 123 years, and age of pines varied from 100–149, average 125 years. Depending on tree height, 6–9 height ranges were separated for trees studied.
Lichen communities
A total of 96 lichen species were found on the trees studied: 86 on spruces and 69 on pines (Table 1). The total number of lichen species found on the lowermost 2 m near the ground was 49 on spruces and 33 on pines. Eleven species were restricted to the lowest height range on spruces and five on pines; the most frequent being Cladonia cenotea. Thirty-seven lichen species were recorded only higher than 2 m on spruces and 36 on pines, the most frequent being Buellia griseovirens, Lecanora pulicaris, L. symmicta, Lecidella subviridis, Melanelixia subaurifera, Melanohalea exasperatula, Micarea denigrata, Ochrolechia arborea, and Trapeliopsis flexuosa. One species, Hypogymnia physodes, was found on all trees studied in nearly all height ranges.
Table 1. The frequency (percentage of occurrences) of lichen species at heights ≤2 m and >2 m; the statistically significant substratum preferences of species (according to Fisher test) have been added: T – trunk, B – branches

* Nomenclature follows Randlane et al. Reference Randlane, Saag and Suija2011
The mean number of lichen species on entire spruces was 41 and on pines 34, whereas the mean number of species on the lowermost 2 m was 14 in spruces and 12 in pines (Fig. 2). There was a significant correlation between the total lichen species richness on a tree and species richness on its lowest 2 m in the case of pines (r=0·80; n=15; P=0·0003); in spruces the correlation was insignificant (r=0·22; n=15; P=0·43). Comparing vertical changes in lichen species richness between trunk and branches, a clear difference was found in the case of pines. Mean number of species on pine trunks decreased significantly with height (Rs=−0·75; n=126; P≪0·001), whereas the number of species on branches increased (Rs=0·76; n=126; P≪0·001; Fig. 3A & B). There was no significant vertical change in lichen species richness on spruce trunks; the number of species on spruce branches was highest in mid canopy (Fig. 3C & D).

Fig. 2. Number of lichen species on the first 2 m from the ground (A) and the total number of lichen species (B) on pines (t=−12·8; n=15; P≪0·001) and spruces (t=−19·7; n=15; P≪0·001).

Fig. 3. The mean lichen species richness (±0·95 confidence interval) in the height ranges on trunks and branches. A & B, pine; C & D, spruce.
According to the results of DCA, height from the ground had a major impact on lichen species composition; correlation (r 2) between DCA axis 1 and height was 0·73 in spruces and 0·90 in pines (Figs 4 & 5). Indicator Species Analysis revealed that many species preferred some specific height range(s). Some species were associated with the lowest height range, whereas several species occurred only in mid or upper canopy (Fig. 6). New species not present in previous height ranges were discovered with increasing height until the treetops (Fig. 7).

Fig. 4. DCA joint-plot of lichen species in the height ranges on spruces (correlation between axis 1 and height: r 2=0·73). For abbreviations of lichen species names see Table 1.

Fig. 5. DCA joint-plot of lichen species in the height ranges on pines (correlation between axis 1 and height: r 2=0·90). For abbreviations of lichen species names see Table 1.

Fig. 6. The vertical changes in lichen species composition on pines (A) and spruces (B), according to the results of Indicator Species Analysis (statistically significant indicator values have been marked with dark grey, and other ≥10 indicator values with light grey). For abbreviations of lichen species names see Table 1.
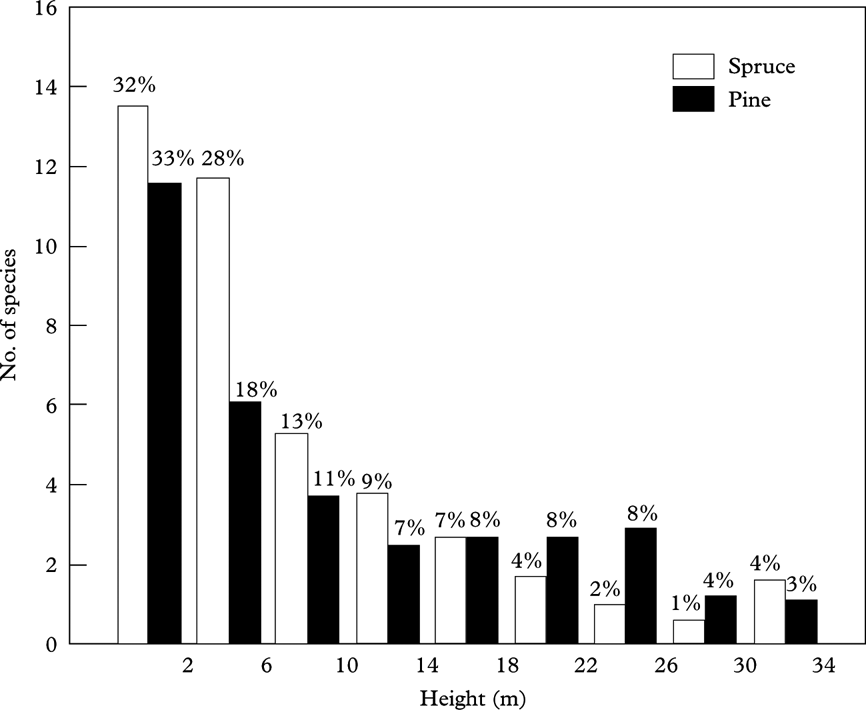
Fig. 7. The addition of new lichen species, absent in previous height ranges, with height from the ground: mean number of such species in every height range and percentage of mean species richness on whole trees.
One species, Lecanora farinaria, was recorded for the first time for Estonia during this study. It was found higher than 2 m (up to 22 m) on spruce branches in all three sample plots, on six trees altogether. Six of the lichen species recorded, Fellhanera subtilis, Lecanora albellula, Lecidea hypopta, L. leprarioides, Micarea nitschkeana, and Rinodina efflorescens, are rather rare in Estonia (in up to 10 localities so far, according to local species databases eSamba and eElurikkus). Five species, Evernia mesomorpha, Usnea barbata, U. fulvoreagens, U. substerilis, and U. wasmuthii, are nationally red-listed (Randlane et al. Reference Randlane, Jüriado, Suija, Lõhmus and Leppik2008). All red-listed and three of the rather rare species (F. subtilis, M. nitschkeana, R. efflorescens) were recorded exclusively more than 2 m from the ground; most of these were growing on spruce branches.
Discussion
In the present work we have recorded all lichen species on entire trees, allowing us to not only arrive at conclusions concerning the height preferences of individual species, but also the vertical changes in total lichen species richness. According to the results, the vertical trends in the number of lichen species recorded in a height range differ between tree trunk and branches, and also between the tree species. In the case of spruces, there were no significant vertical changes in the number of lichen species growing on the trunk; species richness on branches was highest in mid canopy (Fig. 3C & D). In pines, the number of lichen species on trunks was highest up to c. 10 m from the ground, and decreased significantly in upper parts of the trunk (Fig. 3A & B). This change can be associated with the changes in pine bark structure, as the smooth peeling bark in upper parts of the trunk provides no firm substratum for lichens. The number of lichen species on pine branches was highest in the upper canopy (Fig. 3); longer branches usually begin much higher in pines compared to spruces.
The comparison between the number of lichen species on the lowermost 2 m and on whole trees (Fig. 2) demonstrates that studying lichens only on the first 2 m of tree trunk and branches leads to a significant underestimation of total lichen species richness on trees. New lichen species that were not present in previous height ranges were added with height until the treetops (Fig. 7). On average, only about one third of the lichen species on a tree were present in the lowermost 2 m near the ground in the case of both spruces and pines, which means that if surveying only the lowest 2 m, two thirds of a tree's lichen species remain unrecorded. However, on the scale of a forest stand, an increase in the number of sample trees is likely to decrease the number of unrecorded species, whereas the frequency of many species still remains biased.
In pines, the number of lichen species on the lowermost 2 m was strongly correlated with the number of lichen species on entire trees. Therefore, species richness on the lowest 2 m of trees could be used for finding the potentially most species-rich pine trees in a forest site. In the case of spruces, such a correlation was insignificant indicating that old spruces may support diverse lichen communities even if species richness on the first 2 m near the ground is not impressive. However, many lichen species that were absent in the lowest 2 m of spruces, including several species growing on branches, were already present in the next height range extending from 2 to 6 m; and on average 60% of the whole lichen species diversity on spruces was recorded in the height range from ground up to 6 m (Fig. 7). This means that climbing the trees or using adjusted equipment (e.g. blade on the top of a high stick or telescopic bar) to sample lichens in that relatively easy to reach height range could add much to the species list of spruces. In the case of pines, on average 51% of the lichen species recorded on trees was present already up to 6 m from the ground, whereas relatively more new species compared to spruces were added in the upper canopy (Fig. 7).
Earlier studies have proved that the occurrence of many lichen species changes vertically on trees and some species are mostly growing higher than 2 m (e.g. McCune et al. Reference McCune, Rosentreter, Ponzetti and Shaw2000; Rosso et al. Reference Rosso, McCune and Rambo2000; Ellyson & Sillet Reference Ellyson and Sillet2003; Williams & Sillett Reference Williams and Sillett2007; Marmor et al. Reference Marmor, Tõrra and Randlane2010). Lichen species composition also changed vertically according to our results, height from the ground being strongly correlated with DCA axis 1 in both tree species studied. The lowest height range did not overlap with other ranges in DCA joint-plots for either spruce or pine (Figs 4 & 5). The higher vertical zonation of lichen communities was more pronounced for pine than for spruce, however. This result corresponds with the findings of Jarman & Kantvilas (Reference Jarman and Kantvilas1995), who pointed out that there was only a small overlap in cryptogam communities between basal and canopy parts of Huon pine in Tasmania. Figure 6 demonstrates which relatively frequent lichen species could be associated with some specific height ranges on spruce and pine.
There were several species, for example Chanotheca stemonea, Cladonia cenotea and C. norvegica, which preferred to grow in the lowest height range. Some of these have also been associated with trunk bases by Holien (Reference Holien1997). Many lichens, such as Bryoria fuscescens, Lecanora pulicaris, Melanohalea exasperatula and Tuckermannopsis chlorophylla, that were not found or were infrequent on the first 2 m of our sampled trees, are generally very common in Estonia and can also be found in low height ranges. Some of them are already known from previous studies to be more common higher in spruce canopy. Kermit & Gauslaa (Reference Kermit and Gauslaa2001) have recorded a high frequency of M. exasperatula in spruce treetops in Norway; Bryoria spp. have been found by Gauslaa et al. (Reference Gauslaa, Lie and Ohlson2008) to be more abundant at heights greater than 2 m.
Comparing our species list (Table 1) with previous data about lichens in Estonian coniferous forests (Jüriado et al. Reference Jüriado, Paal and Liira2003; Marmor et al. Reference Marmor, Tõrra, Saag and Randlane2011; eSamba), it became apparent that some species occurring rather frequently on spruce and/or pine higher than 2 m from the ground in the present study, have been rather rarely recorded on the lowest 2 m of these tree species in Estonia in general. These lichen species, such as Fuscidea pusilla, Lecanora albellula, Micarea denigrata, Ochrolechia arborea, and Scoliciosporum chlorococcum, seem to be more exclusively associated with higher height ranges in Estonian coniferous forests and are likely to remain systematically under-recorded if only the first 2 m are studied. At the same time, F. pusilla and S. chlorococcum are relatively frequent on spruce branches on the first 2 m in Norway (Lie et al. Reference Lie, Arup, Grytnes and Ohlson2009).
The higher height ranges also provided some very interesting lichen records. One of the recorded species, Lecanora farinaria, is new for Estonia. It was found on the branches of six spruce trees. In addition, we registered five locally red-listed lichen species (Evernia mesomorpha, Usnea barbata, U. fulvoreagens, U. substerilis, and U. wasmuthii), growing on spruce branches higher than 2 m; and six nationally rare or rather rare species, three of them (Fellhanera subtilis, Micarea nitschkeana, Rinodina efflorescens) exclusively higher than 2 m. The results indicate that some rare lichen species and species of conservation concern may be associated with higher height ranges in old coniferous forests (all our sample plots were located in relatively old forests as the age of sample trees was ≥100 years). It is well known that many lichen species prefer older trees (e.g. Ranius et al. Reference Ranius, Johansson, Berg and Niklasson2008; Fritz et al. Reference Fritz, Niklasson and Churski2009; Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2009) and old forests with long ecological continuity (e.g. Coppins & Coppins Reference Coppins and Coppins2002; Josefsson et al. Reference Josefsson, Hellberg and Östlund2005; Fritz et al. Reference Fritz, Gustafsson and Larsson2008; Marmor et al. Reference Marmor, Tõrra, Saag and Randlane2011), whereas there are only a few studies where such species have been looked for higher on the tree trunk and canopy. For example, Fritz (Reference Fritz2009) found that several red-listed lichen species in Sweden are growing higher than 2 m on aspen trunks.
Different factors affect the vertical distribution of lichens in forest canopies (Sillett & Antoine Reference Sillet, Antoine, Lowman and Rinker2004) and could be behind the absence or infrequency of some lichen species in the lowest 2 m of trees. Microclimatic variables change in forest canopies: light conditions improve, wind speed and amplitudes of temperature and humidity increase in the treetops (Gross Reference Gross1993); as they were not directly measured in the present study, the possible effects on lichen communities will not be discussed. Substratum related changes are also one of the major drivers behind the vertical changes in lichen communities as substratum availability, quality and age change with height in the canopy. As in previous studies (e.g. Holien Reference Holien1997; Caruso & Thor Reference Caruso and Thor2007; Lie et al. Reference Lie, Arup, Grytnes and Ohlson2009), many species were more frequent on branches (Table 1). For example, several Usnea species were recorded mainly on spruce branches; Physcia tenella was most frequent on spruce twigs in the upper canopy; Trapeliopsis flexuosa was associated with pine branches in mid canopy, growing mainly on deadwood branch stumps (Placynthiella icmalea and P. uliginosa were also found on deadwood). The presence of nitro/neutrophilic species, such as Physcia adscendens, P. tenella, and Xanthoria polycarpa, in treetops has been associated previously with the effects of air pollution (Marmor et al. Reference Marmor, Tõrra and Randlane2010) and nutrients delivered by birds (McCune et al. Reference McCune, Rosentreter, Ponzetti and Shaw2000).
The Estonian State Forest Management Centre is gratefully acknowledged for permitting us to carry out the present study in state forests. Special thanks go to Martin Tõrra for cutting down the trees. Many thanks also to Inga Jüriado and Ave Suija for their help in identifying some exemplars, and to Martin Kukwa for confirming the identification of the nationally new species. Ants Kaasik is thanked for the comments on statistical analyses, and Andres Saag for general comments on the study. Financial support was received from the Norwegian Financial Mechanisms and EEA Financial Mechanisms (grant EMP9); the Estonian Science Foundation (grants no JD173 and 9109); the Estonian Ministry of Education and Research (targeted financing no 0153) and from the European Union through the European Regional Development Fund (Center of Excellence FIBIR).










