Introduction
Information on the age and growth of fish is essential to adequately manage the exploitation of fish stocks (Summerfelt & Hall, Reference Summerfelt and Hall1987; Hilborn & Walters, Reference Hilborn and Walters1992; Methot & Wetzel, Reference Methot and Wetzel2013; Kai, Reference Kai2016). By defining the age structure of a fish population rates of key processes such as growth, recruitment and natural mortality can be estimated with statistical and/or mathematical modelling (Summerfelt & Hall, Reference Summerfelt and Hall1987; Francis, Reference Francis2016; Thorson & Minte, Reference Thorson and Minte-Vera2016). Hence, age-based parameters are paramount for any stock assessment model that considers stock structure by age and the monitoring of the age structure over time (Summerfelt & Hall, Reference Summerfelt and Hall1987; Catalano et al., Reference Catalano, Matthew and Allen2010; Francis, Reference Francis2016). Age and growth information is also useful for unstructured models, such as data-limited or meta-analytic models, and to estimate longevity through empirical methods (Canales & Leal, Reference Canales and Leal2009; Kousteni & Megalofonou, Reference Kousteni and Megalofonou2015).
The cardinalfish Epigonus crassicaudus de Buen, 1959 belongs to the family Epigonidae, a poorly studied group of perciform fishes (Okamoto & Motomura, Reference Okamoto and Motomura2011; Okamoto et al., Reference Okamoto, Motomura and Asahida2011). Adult cardinalfish inhabit deep waters on the continental slope and water in close proximity to seamount features where they can form dense spawning and feeding aggregations. The juveniles of this group commonly occur in the pelagic zone near the continental shelf at depths of 230–410 m (Parin, Reference Parin, Parin and Novikov1987). Epigonus crassicaudus is endemic to Chile, and the distribution extends the length of the coastline. Based on commercial fisheries data, they are found at the highest abundance between 29°00′S and 42°50′S (Leal et al., Reference Leal, Contreras and Oyarzun2009) in depths ranging from 100 to 550 m (Wiff et al., Reference Wiff, Quiroz and Tascheri2005). Cardinalfish landings in Chile have been recorded since 1992, when 579 tons were reported as by-catch of the Chilean hake (Merluccius gayi) and shrimp (Heterocarpus reedi) fisheries. Reported landings of cardinalfish peaked at 5792 tons in 2000, but landing dropped dramatically thereafter and in 2009, only 185 tons were recorded. Today, the fishery is closed, and the fisheries legislation (Subpesca, 2016) allows only for by-catches of 2 and 12 tons for the artisanal and commercial fleets, respectively.
To date the age determination for this species has been somewhat controversial. Previous studies using whole otoliths produced zone count readings which resulted in maximum age being estimated as 15 years (Gálvez et al., Reference Gálvez, Rebolledo, Pino, Cubillos, Sepúlveda and Rojas2000; Cubillos et al., Reference Cubillos, Aguayo, Neira, Sanhueza and Castillo-Jordán2009; Contreras-Reyes & Arellano-Valle, Reference Contreras-Reyes and Arellano-Valle2013). In contrast, Ojeda et al. (Reference Ojeda, Wiff, Labrín and Contreras2010), by reading transverse or cross-sections of polished and burnt otoliths, reported a maximum age of 54 years. The marked increase in concavity of the otolith morphology as otoliths grow, could explain the differences between these methods with the transverse section method more clearly highlighting the growth zones and the detail of the sequence of the assumed annuli from the nucleus to the otolith edge. The maximum age estimated by Ojeda et al. (Reference Ojeda, Wiff, Labrín and Contreras2010) was similar to that estimated in other species in the genus, such as Epigonus telescopus (Tracey, Reference Tracey1993; Tracey et al., Reference Tracey, George and Gilbert2000; Andrews & Tracey, Reference Andrews and Tracey2007; Vieira et al., Reference Vieira, Figueiredo, Figueiredo and Menezes2013), with these methods subsequently validated from the New Zealand region by Tracey et al. (Reference Tracey, Andrews, Horn and Neil2017).
The uncertainty associated with reading annuli could trigger significant errors in the catch-at-age matrix. In the same way, the validation of the first annulus is a key aspect because it provides a baseline of the reading pattern and without a correctly defined starting point, age determinations will be consistently biased (Campana, Reference Campana2001). In this regard, counting otolith micro-increments is considered the most reliable method for validating the first annulus in teleost fishes (Geffen, Reference Geffen1982; Waldron, Reference Waldron1994; Campana, Reference Campana2001). To help fill in this gap in knowledge, the present study aimed to validate the time of formation of the first annulus and then fit a growth model for the E. crassicaudus. Such an approach will improve the fit of data for smaller fish providing a more accurate estimation of growth parameters.
Materials and methods
Samples
The sample area was located between 33°00′S and 41°16′S along the South-east Pacific coast of Chile. A total of 3632 fish were collected by bottom trawl commercial fishing vessels (2012–2015) at depths between 100 and 500 m, as part of a monitoring programme of the demersal fishery carried out by the Chilean Fishery Research Institute (Instituto de Fomento Pesquero (IFOP)). Biological sampling consisted of recording the fork length (FL), total weight, gonad weight and sex for each fish. Sagittal otoliths were extracted, dried and stored in paper envelopes and carefully labelled to ensure traceability.
Validation of the first annulus
To validate the time of formation of the first annulus, the right sagittae of 20 juveniles (9–12 cm FL) were mounted in epoxy resin on glass slides and then polished in the sagittal position for examination of primary increments using the slide-glass-embed-method (SGEM; Plaza et al., Reference Plaza, Honda, Sakaji and Nashida2005). Because of a slight concavity of the sagittae, serial polishing was used to obtain a complete sequence of primary micro-increments. After obtaining a thin section, micro-increments were counted from primordium using a digital image system composed of a light microscope (Carl Zeiss, Axio model), a digital camera (Canon EOS T5i) and Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA). All counting and measurements of primary microincrements were performed twice for each otolith by two independent readers using the ‘manual tag’ and ‘caliper’ tools available in Image-Pro Plus at magnifications of 400× and 1000×. In all cases, multiple photographs were taken and combined to obtain a complete sequence of micro-increments from the core to the otolith edge before beginning the translucent zone.
Annual growth estimation
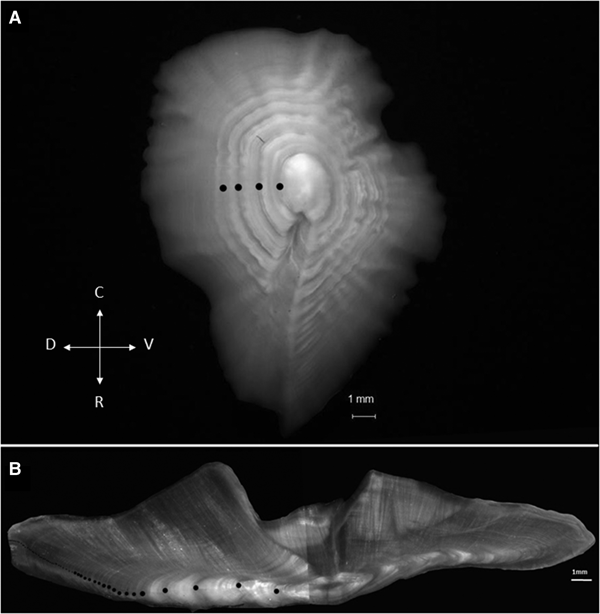
Age determination was assessed using transversal sections of the right sagittal otoliths, which were prepared according to the method described by Ojeda et al. (Reference Ojeda, Wiff, Labrín and Contreras2010). The otolith sections were read and measured with reflected light under a stereoscopic microscope at 20× magnification. Then, the radius of the first seven formed annuli were measured from the core to the post-rostrum of sagittae. The first annulus is formed at a distance of 1.5 ± 0.15 mm from the core, and the seventh annulus is located ~5.5 ± 0.45 mm from the core. After this region, it is only possible to count the annuli because they become very narrow (Figure 1). Then, the macrostructure and microstructure of the first opaque and translucent zones were compared in these areas to identify the first annulus.

Fig. 1. Sagittal otolith of E. crassicaudus from the South-east Pacific off Chile. (A) Left whole otolith of a specimen of 37 cm FL and 55 years old, where the black dots indicate the first four annuli; D: dorsal; R: rostrum; V: ventral; C: caudal. (B) Right sectioned otolith of the same specimen, where the black dots indicate the narrow annuli through the ventral zone.
Two independent readers examined the ageing precision, and the results were compared using the index average per cent error (IAPE) and coefficient of variation (CV) (Beamish & Fournier, Reference Beamish and Fournier1981; Chang, Reference Chang1982). These metrics were complemented by use of the age-bias plot method (Campana et al., Reference Campana, Annad and McMillan1995):
 $$\hbox{IAPE} = \displaystyle{{100} \over N}\mathop \sum \limits_{J = 1}^N \left[ {\displaystyle{1 \over R}\mathop \sum \limits_{i = 1}^R \displaystyle{{\vert {X_{ij} - X_j} \vert } \over {X_j}}} \right]$$
$$\hbox{IAPE} = \displaystyle{{100} \over N}\mathop \sum \limits_{J = 1}^N \left[ {\displaystyle{1 \over R}\mathop \sum \limits_{i = 1}^R \displaystyle{{\vert {X_{ij} - X_j} \vert } \over {X_j}}} \right]$$where N is the number of fish aged, X ij is the ith annuli count of the jth fish, and X j is the median annuli count for the fish.
The growth parameters were estimated by fitting the von Bertalanffy growth curve (von Bertalanffy, Reference Von Bertalanffy1938) to the length-at-age data at capture as follows:
where FL = average fork length at age t, L ∞ = asymptotic length, k = growth coefficient and t 0 = hypothetical age at zero length.
Due to lack of small fish (ages 1–5), a second growth fitting combined length-at-age data at capture (age >5 years old) with back-calculated length-at-age data (age <6 years old) based on the linear relationship between the otolith radius and fish length in accordance with the body proportional hypothesis (BPH) proposed by Whitney & Carlander (Reference Whitney and Carlander1956) and revised by Francis (Reference Francis1990) as follows:
where FL is the fork length at the time of capture (cm), L i is the fork length (cm) when the annulus i was formed, and a and b are the intercept and slope of the linear regression, respectively. R is the otolith radius at capture (mm), and r i is the otolith radius at annulus i.
Through back-calculation procedures several previous lengths for each fish were determined. Hence, to avoid the use of auto-correlated data, a single value of back-calculated length was chosen for each fish, using a randomization process. With this procedure, the data used could be considered independent. The two series of data were fitted using maximum likelihood estimation with Gaussian distributed errors, through the non-linear routine ‘nls’ available in the statistical software package R (Ihaka & Gentleman, Reference Ihaka and Gentleman1996).
Results
Validation of first annulus
The patterns of primary micro-increments of E. crassicaudus from juveniles (9–12 cm FL) were very distinctive and had high resolution over the caudal area from the primordium until the beginning of the first translucent zone. The number of daily micro-increments formed in the central opaque area ranged from 240 to 287 days (μ = 256 ± 4d), reaching a mean radius close to 1300 ± 154 µm (1063–1625 µm). This area was followed by a translucent zone (TZ) that completed the annulus, which showed no presence of resolvable micro-increments. However, in some otoliths, some areas with the presence of visible increments were observed, which had thicknesses of ~1.08 ± 0.37 µm (range 0.7–2.0 µm) (Figure 2). The TZ was characterized by the absence of distinctive primary micro-increments, consistent with that observed in the central opaque zone, rather than micro-increments that appeared to be merged. The TZ had a width measured through the caudal axis of ~124 ± 9.5 µm (range 112.0–141.0 µm).

Fig. 2. Sagittal otolith from a 9 cm FL E. crassicaudus juvenile caught in the South-east Pacific off Chile. (A) Right whole otolith showing the first translucent zone (TZ) (black dot) and the reading track in dashed line. (B) Section of the left polished otolith showing the sequence of microstructure of the opaque zone (OZ) and TZ. (C) Close up of the transitional area from OZ (1395 µm) to TZ (123 µm), after which the sequence of micro-increments is interrupted.
Age determination
The annual age was determined in 2831 otoliths with high resolution, with length and age ranges of 11–40 cm in FL and from 2–67 years (females, N = 1873), 9–40 cm FL and from 4–65 years (males, N = 879) and 9–20 cm FL and from 1–8 years (unknown sex, N = 79). The modal lengths and ages were 34 cm FL and 8 years old, respectively. The age determination from different readers showed a high level of reproducibility (IAPE = 6.2%, CV = 8.7%). The von Bertalanffy growth equation fitted well to both types of data, i.e. the length-at-age data at the time of capture and the combined data, where back-calculation lengths for the first 5 years of life were added. However, the parameters from the combined data showed a higher R 2 than those derived from only the cross-sectional length-at-age data. The back-calculation was reliable because the relationship between fork length (cm) and the otolith radius (mm) was significantly linear (F = 17,282.24; P < 0.001; FL = 6.21 + 2.79 × OR), where 86% of the variability in the otolith size was accounted for by fish length. The asymptotic length (L ∞) and growth coefficient (k) did not show significant differences when longitudinal and cross-sectional data were compared; however, the t 0 showed an enhanced estimate when the back-calculated lengths for the first years were combined with the length-at-age data at the capture date (Table 1). There were no significant differences in von Bertalanffy growth parameters between sexes regardless of the kind of length-at-age data (P = 0.12). This result indicated that the growth of this species could be modelled using combined data from both sexes. The results of the fits showed different von Bertalanffy growth curves for combined sexes, before and after 8 years of age because the back-calculation-length used for juvenile fish improved the growth estimation of fish under this age. The comparison with growth parameters of cardinalfish estimated by previous authors showed that the present study obtained a t 0 closer to the origin of the age-length relationship when the ageing analysis estimated a long lifespan (Table 2).
Table 1. Von Bertalanffy growth parameters estimated for cardinalfish off the South-east Pacific Chilean coast

The results correspond to a fit using length-at-age at capture when it was combined with the back-calculated-length data for juvenile fish under 6 years old. SE: standard error; logLike: negative log-likelihood; L ∞: asymptotic length; k: growth coefficient; t 0: the age at which the fish has zero length; R 2: determination coefficient.
Table 2. Comparison of the von Bertalanffy growth parameters of E. crassicaudus combined by sex reported by the present and previous studies off Chile

N: sampling size; t max: maximum age found in this study; SE: standard error; L ∞: asymptotic length; k: growth coefficient; t 0: the age at which the fish has zero length.
Discussion
The reading of micro-increments allowed the validation of the first annulus in juveniles of cardinalfish through a clear identification of the first fast-growing opaque zone (OZ), which encompassed a mean age of 8.5 months, after which a clear translucent zone (TZ) was formed. Hence, only 3.5 months are needed to complete the first year of life (annulus) in this species. The mean width of the first TZ formed after the nuclear OZ corresponded to 121 µm, and the limit of the resolution of the light microscope was ~0.2 µm with the best available objective lenses. Hence, it is reasonable to infer the width of the first TZ of this species is enough to contain ~496 micro-increments under this resolution.
The microstructural features of the first annulus in some teleost fishes have been reported by some previous authors (e.g. Panella, Reference Panella, Rhoades and Lutz1980; Taubert & Tranquilli, Reference Taubert and Tranquilli1982; Victor & Brothers, Reference Victor and Brothers1982; Waldron & Armstrong Reference Waldron and Armstrong1989; Waldron, Reference Waldron1994; Wright et al., Reference Wright, Woodroffe, Gibb and Gordon2002). These studies have demonstrated that a true annulus is characterized by a marked discontinuity in the pattern of micro-increments in an immediately adjacent opaque zone, followed by the appearance of very thin or unresolvable micro-increments (TZ) and then by a new OZ with a clear sequence of micro-increments. Consequently, TZ are formed in a low growth period that could sometimes include a complete pause in the growth sequence. In addition, it is well known that the TZ is associated with a period of slow growth, which in some species may be associated with the winter period but in other cases matches the summer season, and even this can be mediated by the effect of the reproductive process that demands energy as well. However, it can be inferred for the first year that the onset of formation of the first TZ may be variable depending on the duration of the spawning season of the species and the season of the year in which it occurs. However, at the micro-structural level, in most cases, the annuli begin to form before 365 days, particularly when the micro-increments in the translucent zone become unresolvable, as has been reported in a number of studies (e.g. Radtke et al., Reference Radtke, Fine and Bell1985; Waldron, Reference Waldron1994; Waldron & Kerstan, Reference Waldron and Kerstan2001; Beckman & Calfee, Reference Beckman and Calfee2014). Consequently, this seems to be true for cardinalfish, where the first annulus was correctly identified in the current study and where the mean radius (1.5 ± 0.15 mm) of the first annulus is similar to other species of the same genus, as reported for E. telescopus by Vieira et al. (Reference Vieira, Figueiredo, Figueiredo and Menezes2013).
It is important to note that in previous studies using whole sagittal otolith readings, the estimated maximum age was ~15 years (e.g. Gálvez et al., Reference Gálvez, Rebolledo, Pino, Cubillos, Sepúlveda and Rojas2000; Cubillos et al., Reference Cubillos, Aguayo, Neira, Sanhueza and Castillo-Jordán2009). Conversely, in the current study, on average, 59 rings in older fish were identified, with a maximum age of 67 years, using transversal sections. This finding supports the hypothesis of a long lifespan previously proposed by Ojeda et al. (Reference Ojeda, Wiff, Labrín and Contreras2010) for this species and agrees with previous studies on the congeneric E. telescopus from New Zealand, where maximum ages of 104 years old have been reported (Tracey et al., Reference Tracey, George and Gilbert2000; Andrews & Tracey, Reference Andrews and Tracey2007). The results of the latter and present study support the hypothesis that members of this genus have a long lifespan. Despite the fact that most epigonids have been poorly studied, some of them have the potential to be subject to commercial exploitation. Give their potentially high longevity, precautionary management may be required to minimize severe impact from fisheries.
The length distribution of cardinalfish observed from commercial fishing did not represent the entire population structure, because smaller fish (<16 cm FL) do not appear to be vulnerable to capture in the fishing gear used. The lack of smaller fish affected the fitting of the von Bertalanffy growth model, resulting in an estimated negative t 0 too far from zero, due mainly to the lack of the ages 1–3 (Candy et al., Reference Candy, Constable, Lamb and Williams2007). This problem has not been solved satisfactorily by previous authors. Cubillos et al. (Reference Cubillos, Aguayo, Neira, Sanhueza and Castillo-Jordán2009), using a non-linear model with random effects (NLRE), estimated a positive value of t 0, although a higher ‘k’ and a shorter asymptotic length were estimated, due to the use of whole otoliths, triggering age underestimation and a longevity not exceeding 13 years. Contreras-Reyes & Arellano-Valle (Reference Contreras-Reyes and Arellano-Valle2013) using the data of Ojeda et al. (Reference Ojeda, Wiff, Labrín and Contreras2010) applied a robust and flexible statistical model to the age–length relationship, but again negative values of t 0 close to −3 were estimated. Furthermore, the growth curve did not fit well to the observed mean length of juveniles younger than 4 years old, which also affected the other parameters (k and L∞), due to the high correlation between them.
To resolve this problem, we suggested a novel method where age-length data at catch and back-calculated length of juvenile <12 cm were combined, to obtain reliable estimations of von Bertalanffy growth parameters. Undoubtedly, the back-calculation data are not independent; however, to avoid autocorrelation, only one back-calculated previous length-at-age, chosen by a randomization process, was included in the analysis. Hence, it is reasonable to propose that estimated parameters in the current study are more reliable than previous studies because the value of k and t 0, although negative, were closer to zero, estimating a mean fit closer to the real fork length of fish between 0 and 3 years old. Consequently, the incorporation of the back-calculation length of younger fish in the analysis solves the lack of data in the first age category 1–3 due to the selectivity of fishing gear. Such a problem generates bias in the estimation of growth parameters, which could impact on other estimations of life history indicators, such as natural mortality. Consequently, the estimation of accuracy Vb growth parameters, as reported in the present study, would be very valuable for developing management measures once this species had recovered again.
Acknowledgements
The authors would like to acknowledge the Stock Assessment Department and the Survey Management Department at IFOP for providing the necessary information for this study.