The post-operative mortality rates for neonates with congenital cardiac disease undergoing surgical intervention has reduced significantly in recent years.Reference Gillespie, Kuijpers and Van Rossem1–Reference Walker, Holland, Winlaw, Sherwood and Badawi3 Survival to hospital discharge and beyond, for even the most complex and challenging anomalies, is possible because of many factors, including prenatal detection and diagnosis, innovations in surgical and interventional techniques, and improved perioperative care. Nevertheless, with improved overall survival come new challenges as these infants grow and develop. Somatic growth patterns, cognitive and neurodevelopmental performance in pre-school- and early school-aged children, as well as quality of life are considered important markers for success, as is survival to hospital discharge.Reference Davis, Davis and Cotman4 Infants are now more likely to undergo surgical or catheter-based interventions at a younger age than in the past, often within the first few days or weeks of life. Earlier intervention and exposure to various perioperative techniques and care have contributed to improved survival rates, but may also adversely impact future developmental processes and outcomes.Reference Bellinger, Wypij and duPlessis5–Reference Uzark, Lincoln and Lamberti10 A neonate with congenital cardiac disease may require lengthy in-hospital convalescence, well beyond the neonatal period, at a time during which critical milestones are usually achieved. Particularly, the task of successfully mastering the coordination of oral feeding and/or breastfeeding may be interrupted or completely disrupted.Reference Medoff-Cooper and Irving11 In the longer term, this may impact somatic growth, as well as cognitive and socio-emotional growth.Reference Davis, Davis and Cotman4, Reference Imms12 The ability to achieve oral feeding independence may be a marker of future neurodevelopmental outcome in general.Reference Mizuno and Ueda13, Reference Tsai, Chen and Lin14 With this in mind, the aim of this study was to describe the characteristics of oral feeding outcomes in a population of neonates and infants with a variety of congenital cardiac defects, most of whom had undergone surgical repair or palliation before 1 month of age. We examined obstacles to achieving oral feeding success, defined as not requiring any supplemental mode of nutritional delivery such as gastrostomy or nasogastric tube, and identified factors that may predict failure or success.
Materials and methods
The Institutional Review Board approved this retrospective study. Data were obtained via a systematic review of the electronic medical records for a period of 1 year, between January and December, 2006. The admission database and the cardiovascular surgical database of the Children's Hospital Cardiac Intensive Care Unit were used to identify neonates greater than 31 weeks of gestation and less than 1 month of age having undergone cardiovascular surgical interventions.
Data were obtained by the hospital's Quality and Patient Safety Department analysts from each patient's electronic medical record using built-in clinical flow sheets.
Variables included the amount taken by mouth with a bottle for each feeding both as an episode and as an amount, breastfeeding as a time interval only coded as yes/no, and requirement of a feeding tube at time of discharge was also coded as yes/no.
Analysis for oral feeding amount
We performed primary statistical analysis using hierarchical linear modelling (HLM 6.0; Raudenbush, Bryk and Congdon, 2006), with additional descriptive and correlational analysis using Statistical Products and Service Solutions (SPSS 16.0; Morgan, Griego and Gloeckner, 2001). Using multilevel modelling, we completed analyses assessing an oral feeding amount. Multilevel modelling allows for measurement of individual changes impacting the amount of oral feeding at one feeding episode, typically every 3 hours, appreciating these individual changes over the course of the hospital stay. Specifically, the amount taken at one specific oral feeding episode was entered at the first level of the model and stable demographic or clinical characteristics were entered at the second level. The predictor variables were entered at the first level of the model if they were different at each oral feeding episode, for example, the amount of feeding gavaged, or at the second level if there were stable infant characteristics. Scores on the primary dependent variable – feeding amount – were positively skewed because of a large number of “zero” values that were used to indicate that there was not an oral feeding; however, transformation to improve normality did not change the results, and therefore the untransformed data are presented.
Analysis for discharge home with a feeding tube
With SPSS 16.0 and using ANOVA, we analysed data indicating that a neonate had a feeding tube at time of discharge from the hospital to home – coded yes/no – using analysis of variance to compare the characteristics of patients discharged with a tube versus those discharged without a tube.
Results
We identified 56 neonates with congenital cardiac disease who required surgical intervention and survived. Of them, 38 were male (69%) and 18 were female (31%). Most neonates (n = 42) were above 35 weeks of gestation and 14 were below 35 weeks. Mean weight at admission was 3.09 kg with a range from 1.700 to 4.800 kg. In all, 13 infants (23%) had associated genetic syndromes. Pre-operative median Aristotle score was 8.59, with a range between 3 and 15 (Table 1). The anatomic diagnoses for the 56 neonates were heterogeneous, and classified as cyanotic and right-sided obstructive lesions, left-sided obstructive lesions including hypoplastic left heart syndrome, and left-to-right shunt lesions (Table 2). A total of 17 infants presented with univentricular physiology and underwent a palliative procedure, and 39 infants exhibited biventricular physiology and underwent a complete surgical repair.
Table 1 Characteristics of patients.

CICU = Cardiac Intensive Care Unit; CPB = circulatory bypass; DHCA = deep hypothermic circulatory arrest; TEE = trans-oesophageal echocardiogram
*Data are expressed as n and %; mean and minimum-maximum or standard deviation
Table 2 Distribution of cardiac defects of survivors (n = 56).
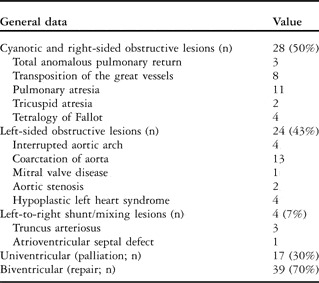
The median age at which infants were admitted to the Cardiac Intensive Care Unit was 1.9 days. The median time from birth to undergoing surgery was 8.4 days. Of the 56 infants, 29 (52%) were fed orally in our hospital before surgery. Of those, 12 were breastfed and the remaining were fed with a bottle, regardless of maternal preference to breastfeed. A total of 25 neonates (45%) required pre-operative mechanical ventilation for more than 24 hours before surgery, rendering oral feeding impossible. These infants received nutritional support via nasogastric feeds and parenteral nutrition. Owing to concerns of mesenteric compromise, two other infants were exclusively supported with parenteral nutrition. The majority of the infants – 42 out of 56 or 75% – were surgically treated within the first week of life and almost half required mechanical ventilation leading up to the surgery, and thus there was little or no opportunity for either breastfeeding or bottle feeding during this time. After surgery and through subsequent extubation, the time from surgery to first oral feeding attempt was a mean of 12 hours with a range from 0.13 to 120 hours.
A total of 22 neonates had surgery under cardiopulmonary bypass; of those, 10 (16%) underwent deep hypothermic circulatory arrest with a mean duration of 38.8 minutes. The mean cross-clamp time (n = 22) was 1.2 hours and the mean bypass time (n = 22) was 3.2 hours. The mean length of hospital stay was 32.15 days with a range from 3.81 to 160 and a median of 21.45 after removing one outlier of 278.18 days.
Predictors of oral feeding amount
Table 3 shows results for prediction of the amount taken orally at each feeding episode. Not surprisingly, infants who took less nutrition by gavage achieved more nutrition orally, and those who took less nutrition orally had a longer hospital stay. Aristotle scores did not predict the amount of oral feeding. Cross-clamp time, but not bypass or circulatory arrest time, predicted the amount of nutrition infants were able to take orally at each feeding attempt over the course of the hospitalisation. Other predictors of a greater volume of oral feeding at each feeding attempt were the amount taken orally at the first feeding, and a shorter time from surgery to the first oral feeding. Predictors of less oral feeding volume at each feeding attempt included the presence of a genetic syndrome, a longer duration of any type of ventilator support, or involvement of occupational therapy. Infants who achieved greater oral volumes and received more of their feedings orally throughout their hospitalisation weighed less at the time of hospital discharge.
Table 3 Predictors of greater amount taken orally at each feeding attempt.

TEE = trans-oesophageal echocardiogram
*Significant predictor variable
Predictors of discharge with a feeding tube
Of the neonates discharged home, 24 (43%) required an in-dwelling feeding tube because they were unable to achieve sufficient growth. Of those infants, 13 (41%) had a genetic syndrome. From the total population, 17 infants went home on a combination of an in-dwelling feeding tube and oral feedings with a bottle, six infants (10.7%) required placement of a gastrostomy tube, and one required a jejunostomy tube. Of these six infants, four were syndromic. Table 4 summarises the secondary outcome variables, which reflected the inability to receive full volumes orally, and therefore needing a feeding tube on discharge home. Similar to the oral feeding amount, discharge with a feeding tube was predicted by greater time from surgery to the first oral feeding, longer time requiring ventilator support, the involvement of an occupational therapy consult, and the presence of a genetic syndrome. Occupational therapy in this population entails specialists trained to diagnose feeding pathology, in terms of anatomic and developmental obstacles. These specialists work with the infant, providers, and family to achieve successful oral feeding. Cross-clamp time did not predict being discharged with a feeding tube; however, circulatory arrest time did. Discharge with a feeding tube was also predicted by greater total amount of nutrition received via gavage, longer length of stay, and less volume taken orally at the first oral feeding episode. Similar to the findings for oral amount, being discharged with a feeding tube was associated with greater weight at the time of hospital discharge. Uni- or biventricular physiology was not a significant predictor of the need for a discharge with a feeding tube (p = 0.116).
Table 4 Predictors of whether patient was discharged with a feeding tube.

TEE = trans-oesophageal echocardiogram
*Significant predictor variable
In all, 29 neonates (51%) also had an occupational therapist or lactation therapist involved to support oral feeding development; however, determination of who received therapy and the timing of when the therapy began varied. Of the 13 neonates who were syndromic, 11 were involved in occupational therapy. The presence of such specialised support did not improve infants’ ability to fully orally feed at the time of discharge. Occupational therapy support was significantly related to worse outcomes in terms of both oral amount and the likelihood of being discharged with a feeding tube. Among infants who went home with a feeding tube (n = 24), 19 (80%) had assistance from occupational therapy, six (25%) had assistance from lactation therapy, and five (21%) received both services. Lactation services were provided to a total of 13 infants (23%), of whom seven (54%) went home without a tube.
Discussion
The results of this study highlight the formidable task faced by neonates and infants with congenital cardiac disease in achieving oral feeding success after cardiac surgery. None of the infants in this study were discharged home exclusively breastfeeding, and almost half required in-dwelling tubes to provide nutritional support despite the use of a feeding guideline for initiation and advancement of enteral nutrition.Reference Braudis, Curley and Beaupre15 Inability to achieve oral feeding success may portend neurodevelopmental obstacles and delays in this population, as well as affect growth, cognitive and socio-emotional development, and parental confidence and behaviour.Reference Davis, Davis and Cotman4, Reference Imms16–Reference Schmid, Schreier, Meyer and Wolke19 The timing of extrauterine physiologic milestone maturation has been well described in the premature infant population, that is, 24–36 weeks.Reference Bakewell-Sachs, Medoff-Cooper, Escobar, Silber and Lorch20 The question that arises is whether term infants, as it happens with premature babies, who undergo early complex cardiac surgery are vulnerable to disruption of the physiologic maturation of the feeding milestone. A large number of the infants in this study were born before 40 weeks of gestation, and studies are looking at the impact of being born before 40 weeks on physiologic regulation.Reference Harrison21–Reference Barros, Mitsuhiro, Chalem, Laranjeira and Guinsburg25 Although this requires further investigation, our study strongly suggests that surgical repair and subsequent hospitalisation for early-to-late preterm infants, following the latter concept, may influence oral feeding outcomes.
We found that intraoperative parameters such as cross-clamp time and time of deep hypothermic circulatory arrest negatively influenced development of the ability to orally feed. A measure of surgical complexity, the Aristotle score, was not found to be a predictor of the success or failure of oral feeding. Very importantly, this study documented that the longer the time after surgery to initiation of oral feeds the worse the oral feeding outcomes. This is certainly a complex issue as the time to initiation of oral feeds after cardiac surgery is likely multifactorial. It is interesting to note that although not all of the patients were exposed to cross-clamping or deep hypothermic circulatory arrest, both of which have been shown to negatively influence neurodevelopment, this study identified that within a population of infants with congenital cardiac disease other factors play an important part in predicting oral feeding outcomes.
As expected, mechanical ventilation or other types of non-invasive respiratory support negatively influenced the ability to successfully feed orally. As with many programmes, our approach is for early extubation of these infants, often to non-invasive positive pressure modes such as Continuous Positive Airway Pressure. The benefits of non-invasive ventilation over an endotracheal tube are significant; however, improving the chances of achieving oral feeds is not one of them. Our policy is to not orally feed while on Continuous Positive Airway Pressure support, and thus oral feeding skills remain underdeveloped.
The fact that none of the infants discharged in this study were solely dependent on breastfeeding is of concern. This might have been related to the patient's acuity and post-operative conditions, but may also be due to our practices that have since then radically changed. We believe that we should further encourage mother–infant skin-to-skin contact and direct breastfeeding as early as feasible. We noticed in this study that convalescence from surgical intervention and limited contact with the mother affect oral feeding. An overall focus on supporting the oral feeding experience would be positive for both the infant and the parent, and the health-care provider can do this by explaining the principles of neurobehavioral development, pointing out the infant's strengths, needs, and cues, and by praising the parents for their observations and interactions based on the individual cues of the infant.
Other interventions that may be offered include providing parents with education on optimal infant behaviour that shows a readiness for feedings.Reference Harrison21, Reference Walker24 Feeding may be scheduled in such a manner as to support the infant's emerging regulation of the sleep–wake cycle. Promoting proper positioning, minimising interruptions, and limiting unnecessary intrusive examinations are other measures that may be undertaken by the care team. The environment in which the feeding may occur may be manipulated to enhance a quiet, restful, and engaging feeding experience, even in the intensive care arena. Skin-to-skin holding opportunities can be offered as soon as the infant is medically stable in order to support bonding and breastfeeding. The behaviour and achievement of quiet alert states of these infants may be different and hunger cues more subtle than those expected from a typical, healthy infant. The practice of moving feeding from a focus on volume to a focus on experience may also be in order.
It has been the practice at this institution to discharge infants with congenital cardiac disease using a nasogastric tube, and in some instances a gastrotomy or jejunostomy tube, if the infant is unable to meet the caloric needs sufficient to show growth, or if there is associated morbidity of the respiratory or otorhinolaryngological sphere. We recognise that either of these interventions may interfere with the development of oral motor feeding skills and may contribute to gastro-oesophageal reflux. We also recognise that these adjunct therapies are not without risks and may present major practical and emotional problems to families.
In this study, infants who received more of their feeds orally weighed less at the time of discharge, and infants who received more nutrition with an in-dwelling feeding tube showed greater weight gain at the time of discharge. The significance of this finding in this retrospective study is uncertain, but provocative. Perhaps, neonates should be encouraged to orally take whatever amount they can and have the remainder of their nutritional needs met with an in-dwelling device, rather than be pushed to ingest greater amounts on their own at the expense of a catabolic state. In contrast, caregivers may wish to consider accepting a lower volume of intake and less weight gain in order to allow all forms of oral feeding and avoid tube placement. Discharge home with a nasogastric tube to be managed by the family is not without risks, nor is subjecting these often-fragile infants to a surgical procedure such as a gastrostomy tube placement with or without a Nissen fundoplication. Nevertheless, this alternative may raise some concerns in convalescing neonates who orally feed and remain significantly catabolic with a negative energetic debt. Further investigation to answer the dilemma between the potential advantages and disadvantages of tube feedings – better nutrition and weight gain versus gastro-oesophageal reflux and underdevelopment of oral feeding skills – is required.
Last but not least, further studies are needed to elucidate the importance of the occupational and feeding therapists in the management of these neonates.Reference Imms12 These specialists no doubt have a role in the perioperative care of infants with congenital cardiac disease; however, it seemed surprising that their involvement was associated with worse oral feeding outcomes. Perhaps one explanation is that an occupational or swallow consult was ordered in those cases in which particularly severe feeding difficulties had already been observed, and among a cohort of patients who were more ill, less robust, and had a worse feeding prognosis regardless of these interventions.
Limitations of this study include its retrospective design and results that are reflective of a single centre's experience and nutritional strategies. Long-term neurodevelopmental outcomes for this cohort of patients were not available.
Conclusion
Neonates with congenital cardiac disease face significant and complex obstacles to achieving oral feeding success. In our population, time from surgery to initiation of oral feeding, the amount of the first feeding after intervention, cross-clamp and circulatory arrest times, and the presence of a genetic syndrome were significant markers of oral feeding outcomes. Almost half of the neonates in this study required invasive means to supplement oral feeds upon discharge, and none of the infants discharged from the hospital were successfully solely breastfeeding. Over the last 3 years, we have radically modified our policies and currently encourage aggressive and early feeding of our critically ill neonatal cardiac patients; we endeavour to feed before surgery and as early as possible after surgery, implying that we also promote early extubation and proactive weaning from any other ventilator support; and we request a systematic evaluation by occupational and speech therapy and lactation consultants. A future close follow-up will document whether this practice positively impacts feeding outcomes. We have also become more meticulous in identifying comorbidity that might affect feeding, for example diagnosing unidentified syndromes, vocal cord dysfunction, cryco-pharyngeal in-coordination, or gastro-oesophageal reflux. Future prospective studies are necessary to clarify the significance of these findings in this vulnerable population, in order to identify long-term outcomes and define more efficient strategies to optimise early feeding outcomes.
Acknowledgements
The authors thank Jeannie Zuk, PhD, RN, for reviewing the manuscript.






