INTRODUCTION
Delirium is an acute, fluctuating disturbance of consciousness, arousal, cognition, and perception (American Psychiatric Association, 1994; Stagno et al., Reference Stagno, Gibson and Breitbart2004; Boettger & Breitbart, Reference Boettger and Breitbart2005). It is common in the medically ill (Siddiqi et al., Reference Siddiqi, House and Holmes2006) and is the most prevalent neuropsychiatric complication in palliative care settings and among individuals with advanced cancer (Centeno et al., Reference Centeno, Sanz and Bruera2004; Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006; Agar, Reference Agar, Currow and Plummer2008; Breitbart & Alici, Reference Breitbart and Alici2008; Leonard et al., Reference Leonard, Agar and Mason2008). Despite the prevalence in palliative care, delirium is often under-recognized and misdiagnosed leading to lengthier hospital stays; escalating healthcare costs; and increased mortality, morbidity and human suffering (Breitbart et al., Reference Breitbart, Gibson and Tremblay2002; Stagno et al., Reference Stagno, Gibson and Breitbart2004; Siddiqi et al., Reference Siddiqi, House and Holmes2006; Spiller & Keen, 2006; Irwin et al., Reference Irwin, Rao and Bower2008). This article reviews the prevalence, assessment, and treatment of delirium in the palliative care patient, with emphasis on the assessment and management of delirium resulting from hepatic encephalopathy (HE).
A number of different terms are used to describe delirium including confusion, organic brain syndrome, cognitive impairment, and altered mental state. The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM IV) (1994) identifies three core features of delirium regardless of etiology: 1) disturbance of consciousness with reduced clarity of awareness and reduced ability to sustain or shift attention; 2) cognitive impairments such as memory deficits and orientation, language, and perceptual disturbances; and 3) an acute onset, usually hours to days, with a fluctuating course.
Additional characteristics are identified if the delirium is believed to be the consequence of a general medical condition, substance intoxication or withdrawal, or multiple etiologies (American Psychiatric Association, 1994, pp. 84–86). Delirium as a result of multiple etiologies includes both the medical condition and/or medication side effects. In the context of advanced disease, delirium is most often associated with multiple etiologies and can be described as “overdetermined.” Careful assessment of risk factors and prevailing etiology will help shape and direct the plan of care. Some of the medical factors contributing to changes in mental status near the end of life include infection, brain metastasis, HE, electrolyte imbalance, and hypoxemia. Psychosocial contributors such as pain, depression, emotional stress, and difficulties seeing and hearing are also important to consider. Some of the medications that are commonly used at the end of life and are associated with mental status changes include opioids, corticosteroids, metoclopramide, benzodiazepines, tricyclic antidepressants, and scopalamine (Barnes et al., Reference Barnes, Kite and Kumar2010; Casarett & Inouye, Reference Casarett and Inouye2001; Fleishman et al., Reference Fleishman, Lesko, Breitbart, Breitbart and Holland1993).
SUBTYPES OF DELIRIUM
Stagno et al. (Reference Stagno, Gibson and Breitbart2004) describe historical distinctions between delirium and acute confusion based on varying levels of psychomotor activity. Acute confusion or “torpor” was associated with disorientation and hypoactivity. The more agitated, hyperactive variant was described as “delirium.” Eventually, the two concepts came to be associated with the single phenomenon, delirium. By combining changes in arousal with changes in motor activity, the term “hypoalert-hypoactive” was distinguished from “hyperalert-hyperactive” and these were regarded as two distinct subtypes of delirium (Stagno et al., Reference Stagno, Gibson and Breitbart2004).
Contemporary phenomenology identifies three subtypes of delirium: hyperactive, hypoactive, and the mixed form (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006; McLeod, 2006). There is little consensus regarding definitions for these subtypes, and different authors use the terms in different ways. This factor impacts significantly on the early detection, assessment, diagnosis, and treatment planning for delirium (Macleod, 2006; Breitbart & Alici, Reference Breitbart and Alici2008; Leonard et al., Reference Leonard, Agar and Mason2008).
The most frequently encountered subtype is the mixed form, occurring ~52% of the time in patients diagnosed with delirium. As the name implies, the mixed form includes features of both hyperactive and hypoactive delirium. Often the agitated, hyperactive periods are recognized whereas the more withdrawn, hypoactive features are missed or perceived to indicate an improvement (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006). These assumptions delay or preclude appropriate therapy and for this reason, the mixed type of delirium is believed to have the worst prognosis (Stagno et al., Reference Stagno, Gibson and Breitbart2004).
The least prevalent but most commonly recognized subtype is hyperactive delirium. It occurs in ~15% of the patients diagnosed with delirium with estimates increasing up to 46% in the palliative care setting. The characteristics of hyperactive delirium include agitation, anxiety, combativeness, and possible hallucinations (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006; Breitbart & Alici, Reference Breitbart and Alici2008).
Differentiating hyperactive delirium from agitation, anxiety, and/or an underlying dementing illness is critically important. Agitation is neither a necessary nor sufficient feature of hyperactive delirium. It may be associated with fecal impaction; urinary retention; unrelieved pain; or symptoms associated with panic, mania, or medication toxicity (Breitbart & Alici, Reference Breitbart and Alici2008). In a prospective analysis of 100 patient records subsequent to the diagnosis of delirium, Boettger and colleagues (Reference Boettger, Passik and Breitbart2009) identified 18 patients with both delirium and dementia. Whereas there were no differences between the two groups with regard to hallucinations, delusions, sleep–wake disturbances, or psychomotor activity, the disturbances of consciousness and cognitive impairment were significantly more severe in the delirious patients with an underlying dementia.
Hypoactive delirium is the third subtype, and is characterized by lethargy, somnolence, and withdrawal. It is estimated to occur slightly more frequently than hyperactive delirium; however, there is a consensus in the literature that it is grossly underdetected and misdiagnosed, and therefore untreated or mistreated (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006; Siddiqi et al., Reference Siddiqi, House and Holmes2006; Spiller & Keene, 2006; Stagno et al., Reference Stagno, Gibson and Breitbart2004; Leonard et al., Reference Leonard, Agar and Mason2008). This is particularly grievous given the increased likelihood of reversibility with early detection (Centeno et al., Reference Centeno, Sanz and Bruera2004), and the documented distress to patients and their caregivers both in and out of the hospital (Breitbart et al., Reference Breitbart, Gibson and Tremblay2002; Namba et al., Reference Namba, Morita and Imura2007; Irwin et al., Reference Irwin, Rao and Bower2008; Stagno et al., 2008).
Spiller and Keen (2006) describe a 29% prevalence of delirium for 100 acute admissions to a palliative care unit. Most of those patients (86%) were diagnosed with hypoactive delirium. In a 48 hour point prevalence study involving eight specialist palliative care units, the incidence of delirium was 29.4%, 78% of these cases being the hypoactive subtype of delirium. These figures suggest a much higher than previously reported incidence and may reflect increased attention to subtle signs of assessment and a more discerning approach to the symptoms of lethargy, withdrawal, depression, and fatigue.
In the same two-part study, Spiller and Keen (2006) documented a high correlation between ratings on a depression screening instrument and the severity of delirium. In the context of advanced disease, the symptoms of fatigue, lethargy, and diminished performance are most often ascribed to the underlying disease process suggesting that both depression and hypoactive delirium are undetected and therefore untreated.
Kuebler and colleagues (Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006) warn that “cognitive disorders in the medically ill interface between medicine and psychiatry and [are] all too often owned by neither (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006, p. 402).” This interface of symptoms and comorbidities may also suggest common pathways and shared physiological processes (Leonard et al., Reference Leonard, Agar and Mason2008).
ASSESSMENT AND DIAGNOSIS
The clinical diagnosis of delirium is based on bedside observations and the evaluation of key features (Cesarett & Inouye, Reference Casarett and Inouye2001). Table 1 identifies not only the clinical features of delirium, it also suggests specific questions and differential considerations for assessment and treatment planning (Breitbart & Alici, Reference Breitbart and Alici2008).
Table 1. Clinical features of delirium and bedside clinical examinationa

a Based on clinical experience assessing the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition. Text Revision) components of delirium.
bClock Drawing Test primarily assesses the severity of cognitive impairment. Despite its frequent use in the clinical setting, it has low utility in differentiating delirium from dementia when used alone. Reprinted with permission, Breitbart and Alici (Reference Breitbart and Alici2008, p. 2900).
An accurate baseline assessment is fundamental, particularly for patients in palliative care, with severe medical illness; advanced age; and/or taking multiple medications such as sedative-hypnotics, anticholinergics, corticosteroids, and opioids (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006). Nurses are well positioned to make observations over time and to recognize the subtle changes associated with hypoactive delirium. Kuebler et al. (Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006) assert that the failure to recognize delirium, especially the hypoactive subtype, happens because assessments are cognitively loaded on orientation, rather than being a “multifocal appraisal including memory, attention and perception” (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006, p. 404). The current authors would also suggest that it is a failure to appreciate the differential diagnostic possibilities of behavior in general, and changes in mental status in particular. Table 2 illustrates some of the most common differential considerations when diagnosing delirium (Centeno et al., Reference Centeno, Sanz and Bruera2004).
Table 2. Differential diagnosis of delirium

Reprinted with permission, Centano et al., 2004, p. 189.
A multitude of delirium assessment scales have been identified. They differ depending upon the purported use, which is either to screen, evaluate symptom severity, or establish the diagnosis of delirium (Casarett & Inouye, Reference Casarett and Inouye2001; Schuurmans et al., Reference Schuurmans and Shortridge-Baggett2003). Adamis and colleagues (Reference Adamis, Sharma and Whelan2010) reviewed and reported the psychometric properties for > 24 delirium instruments, and they conclude that although more research is needed, a small number of scales currently demonstrate robust levels of validity and reliability including the Confusion Assessment Method (CAM), the Delirium Rating Scale (DRS) and its revision (DRS-R-98), the Memorial Delirium Assessment Scale (MDAS), and the Neelon-Champagne Confusion Assessment Scale (NEECHAM).
The CAM is a screening instrument initially validated on the DSM IIIR diagnostic criteria but now more closely aligned with DSM-IV-TR which is recognized as the “gold standard” (Breitbart & Alici, Reference Breitbart and Alici2008; Adamis, et al., Reference Adamis, Sharma and Whelan2010; Barnes et al., Reference Barnes, Kite and Kumar2010; Breitbart & Alici, Reference Breitbart and Alici2008; Ryan et al., Reference Ryan, Leonard and Guerin2009). It was intended for use by multidisciplinary clinicians, and when compared across groups, Wei et al. (Reference Wei, Fearing and Inouye2008) report an overall sensitivity of 94% and a specificity of 89% (confidence intervals were 91–97% and 85–94% respectively). The CAM has been translated into 10 languages and is widely regarded as an excellent diagnostic tool for delirium (Casarett & Inouye, Reference Casarett and Inouye2001; Schuurmans et al., Reference Schuurmans, Deschamps and Markham2003a,Reference Schuurmans and Shortridge-Baggettb; Adamis et al., Reference Adamis, Sharma and Whelan2010).
The evaluation of symptom severity may best be approached by using the DRS or DRS-R-98, or the MDAS. The DRS-R-98 was designed to address shortcomings in the original DRS, including the ability to distinguish between the hypoactive and hyperactive subtypes of delirium. It was intended for use by psychiatric clinicians and includes 16 items, 13 of which assess severity, and 3 that are specific to diagnosis (Casarett & Inouye, Reference Casarett and Inouye2001; Adamis et al., Reference Adamis, Sharma and Whelan2010).
The MDAS is based on the DSM-IV diagnostic criteria and was originally designed for repeated assessments over time of cancer patients receiving intravenous opioid therapy. It has established validity and reliability in palliative care settings with a sensitivity of 97% and a specificity of 95% at a cutoff score of 7.
The NEECHAM is a delirium screening tool designed by nurses to assist in rapid bedside assessment. It has three subscales, which include all of the elements of the CAM as well as physiological parameters such as vital sign stability, oxygen saturation, and urinary continence (Schreier, Reference Schreier2010). Some researchers argue that the physiological measures do not contribute to the evaluation of symptom severity and may actually be more discriminating with regard to an acute confused state (Adamis et al., Reference Adamis, Sharma and Whelan2010), and Schreier (Reference Schreier2010) cites evidence of difficulty extrapolating from the scale to the medical record. Adamis et al. (Reference Adamis, Sharma and Whelan2010) describe it as well liked and easy to use, with multiple translations available.
Delirium may occur as a result of infection; dehydration; metabolic factors including impaired renal or hepatic function; psychosocial factors including depression, unrelieved pain, and emotional stress; or toxicities associated with medications and the synergistic risk of polypharmacy (Cesarett & Inouye, 2001; Centeno et al., Reference Centeno, Sanz and Bruera2004; Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006; Alici-Evcimen & Breitbart, Reference Alici-Evcimen and Breitbart2008). For the palliative care patient, there are likely to be several contributing factors acting in tandem. It is crucial to review the physical examination findings, laboratory tests, and all medications to evaluate potentially reversible causes as soon as possible.
The physical examination will include vital signs with baseline comparisons; neurological assessment and evaluation of possible infection, organ failure, urinary retention, constipation, or obstruction. It is essential to evaluate whether or not the patient is actively dying or manifesting a terminal delirium. This knowledge, combined with an appreciation of individual goals of care and patient/family preferences will influence the extent to which aggressive diagnostic measures are undertaken. Patients and family need information as well as education to make an evaluation of benefit versus burden concerning both diagnostic tests and interventions. Consider, for example, delirium associated with dehydration. Whereas an intravenous line may be cumbersome, some patients would choose this intervention if the improved hydration was associated with enhanced cognitive function and perceived quality of life.
Laboratory tests and radiological examinations may be utilized to evaluate hepatorenal function; electrolyte disturbances; and the possibility of infection, disease progression or obstruction. Clinical markers for metabolic-nutritional factors include: a body mass index (BMI) <20; weight loss >5 kg or 10%; albumin <3.5 g/dL; and lymphocyte count <1000/μL. Metabolic-toxic factors contributing to delirium would include a diagnosis of liver or renal failure; albumin <3.0 g/dL; or creatinine >2.0 mg/dL; dehydration with a blood urea nitrogen/creatinine ratio >20; and hypoxia with oxygen saturation <91 or hemoglobin <9.0 g/dL. Clearly, many of these markers are present in patients with advanced disease and those receiving palliative care. Warranting therefore, a high index of suspicion for delirium in these populations (Bond & Neelon, Reference Bond and Neelon2008; Harris, Reference Harris2007).
Drugs are the most frequent cause of delirium, and a review of medications should include prescribed and over-the-counter medications, and herbal or dietary supplements, as well as any illicit drug use (Alici-Evcimen & Breitbart, Reference Alici-Evcimen and Breitbart2008). It is important to evaluate not only each individual medication but also the likelihood of synergistic augmentation of effect and risk of toxicity. Anticholinergics, anticonvulsants, anti-Parkinson agents, corticosteroids, sedatives, alcohol, opioids, and illicit drugs are particularly important to monitor. Sedating over-the-counter preparations and homeopathic agents such as black cohosh, valerian, kava, or St. John's wort are most often taken for difficulty sleeping, restlessness, anxiety, or depression (Ody, Reference Ody1993; Schultz et al., Reference Schultz, Hänsel and Tyler1998).
Opioids and their metabolites may contribute to delirium, but it is essential to carefully discern all potential risk factors before making a decision to change a patient's pain management regimen. In the context of good pain relief, a 10–25% reduction in total daily dosing of the current opioid may improve symptoms of delirium. An opioid rotation, using equianalgesic dosing and ~25–50% reduction for incomplete cross-tolerance may also provide benefit. The reduction when switching to methadone is 75–90% (American Pain Society, 2003; Fine & Portenoy, Reference Fine and Portenoy2007; Fine et al., Reference Fine and Portenoy2009), and consultation with a professional experienced in methadone dosing is recommended. If the patient's pain is not well controlled, an opioid rotation will allow an equianalgesic reduction in opioid dosing. This rotation and reduction is designed to improve drug- or dose-related toxicities without compromising analgesic benefit. In the context of compromised or failing renal function, it is advisable to discontinue or avoid using morphine because of the risk of metabolite accumulation (morphine-6-glucuronide and morphine-3-glucuronide) and subsequent neurotoxicity. Hydromorphone and fentanyl are better alternatives. (Pasero & McCaffrey, Reference Pasero and McCaffery2011).
MANAGEMENT OF DELIRIUM: NONPHARMACOLOGICAL
The management of delirium includes pharmacological and nonpharmacological approaches that emphasize prevention, early detection, and the comprehensive assessment of contributing factors. The nonpharmacological management is aimed at reducing the symptom burden associated with cognitive impairment, sleep disturbances, sensory impairment, and dehydration. Providers are encouraged to respect the patients’ subjective world and to coordinate care according to changes in consciousness levels during the day (Namba et al., Reference Namba, Morita and Imura2007, pp. 592–593).
Protocols aimed at promoting orientation include calendars, clocks, name boards, and therapeutic activities such as reviewing current events, playing word games or participating in life review discussions (Casarett & Inouye, Reference Casarett and Inouye2001). Families have expressed the need for support in managing feelings of guilt, helplessness, and exhaustion in coping with delirium (Namba et al., Reference Namba, Morita and Imura2007).
Provision of a restful environment with monitoring of excessive noise and intrusive smells can help address sleep disturbances, and the use of patient-preferred music can provide a calming, familiar environment. There is a growing body of evidence supporting the soothing and comforting use of scented oils in combination with massage (Kuebler et al., Reference Kuebler, Heidrich, Vena, Ferrell and Coyle2006)
MANAGEMENT OF DELIRIUM: PHARMACOLOGICAL
The pharmacological management of delirium is often aimed at reducing perceptual disturbances or agitation, and antipsychotics are the most frequently used class of drugs. Despite the dearth of double-blind, randomized, placebo-controlled trials (Leonard et al., Reference Leonard, Agar and Mason2008), haloperidol has been prescribed and studied extensively, and is considered to be the gold standard in the management of delirium (Alici-Evcimen & Breitbart, Reference Alici-Evcimen and Breitbart2008; Jackson & Lipman, Reference Jackson and Lipman2004; Vella-Brincat & Macleod, Reference Vella-Brincat and Macleod2004).
Boettger & Breitbart (Reference Boettger and Breitbart2005) reviewed the empirical literature on the use of atypical antipsychotics in the management of delirium. Despite the limited studies to date, the authors suggest growing support for the use of risperidone, olanzapine, and quetiapine, with the caveat of limited efficacy using olanzepine for hypoactive delirium in the elderly. Namba and colleagues (Reference Namba, Morita and Imura2007) point out the need for both support and education as families experience ambivalent emotions surrounding the use of psychotropic medication.
In a randomized, double-blind comparison trial with 244 AIDS patients, Breitbart and colleagues (Reference Breitbart, Marotta and Platt1996) examined the efficacy and side effect profiles for haloperidol, chlorpromazine, and lorazepam. Low-dose neuroleptic therapy was associated with both benefit and low side effect profile. However, all patients on the lorazepam arm of the study experienced treatment-limiting adverse effects without any benefit. “The authors became sufficiently concerned with the adverse effects to terminate that arm of the protocol early” (Breitbart et al., Reference Breitbart, Marotta and Platt1996, p. 231). It has been suggested that benzodiazepines only be used for the management of delirium associated with withdrawal from alcohol or sedative drugs (Centeno et al., Reference Centeno, Sanz and Bruera2004).
Both neuroleptics and benzodiazepines may exacerbate symptoms of delirium associated with metabolic disturbances. This factor combined with the development of new and novel therapies makes a differential appraisal of contributing factors essential for effective treatment planning. The remainder of this article focuses on the pathogenesis, assessment, diagnosis, and management of hypoactive delirium in the patient with HE.
HE AS A CAUSE OF DELIRIUM
HE is a serious complication of acute and chronic liver disease that encompasses a continuum of neuropsychiatric abnormalities (Prakash & Mullen, Reference Prakash and Mullen2010). HE is one of the principal manifestations of chronic liver disease, and a cardinal feature of acute liver failure. It occurs in 60–80% of patients with cirrhosis (Bajaj, Reference Bajaj2010) and should be considered when evaluating the onset of dementia in this population. HE may present as overt HE, which is easily detected by clinical evaluation, or as minimal HE, which is only detectable with psychometric testing (Sundaram & Shaikh, Reference Sundaram and Shaikh2009; Prakash & Mullen, Reference Prakash and Mullen2010).
HE is multifocal, involving cognitive, affective/emotional, behavioral, and bioregulatory domains (Ferenci et al., Reference Ferenci, Lockwood and Mullen2002). Symptoms may range from subtle changes in mental status and personality to deep coma (Ferenci et al., Reference Ferenci, Lockwood and Mullen2002; Cash et al., Reference Cash, McConville and McDermott2010). The impact on patients and families can be significant. Even with milder forms of HE, subtle cognitive changes may affect learning and memory, the ability to work, the ability to drive safely, sleep patterns, and other disruptions that impair quality of life for patients and families. (Bajaj et al., Reference Bajaj, Saeian and Schubert2009, Reference Bajaj2010; Foster et al., Reference Foster, Lin and Turck2010). Appropriate diagnosis and treatment of HE are essential to improve quality of life, decrease the recurrence of HE, and reduce the need for HE-related hospitalization (Riordan & Williams, Reference Riordan and Williams2010). The rising prevalence of hepatitis C increases the number of people at risk for developing cirrhosis and HE (Ferenci, Reference Ferenci2010). HE must be considered when caring for patients with liver dysfunction and mental status changes or delirium. It is important to note that the standards of care and best practices for managing delirium in advanced illness are distinctly different than those recommended to treat HE. The prevention, early identification, and prompt treatment of HE are integral components of palliative care throughout the trajectory of illnesses related to chronic liver disease.
Pathogenesis and Causes of HE
The exact pathophysiological mechanisms of HE are not clearly understood (Cordoba & Minguez Reference Cordoba and Minguez2008; Cash et al., Reference Cash, McConville and McDermott2010; Wolf, Reference Wolf2011). Patients with liver disease, portosystemic shunting, or the surgical placement of portosystemic shunts (TIPS; used in people with cirrhosis or transplants) are at risk for developing HE.
HE is caused by accumulation in the brain of toxic nitrogenous substances from the gut that are normally metabolized and excreted by the liver (Cordoba & Minguez, Reference Cordoba and Minguez2008; Foster et al., Reference Foster, Lin and Turck2010). These toxic substances affect brain function by altering neurotransmissions that affect consciousness and behavior (Blei & Cordoba, Reference Blei and Cordoba2001).
The pathogenesis of HE is thought to be multifocal, with the accumulation of ammonia in the brain caused by the liver's inability to convert ammonia to urea being one of the prime factors (Wright & Jalan, Reference Wright and Jalan2007; Cordoba & Minguez, Reference Cordoba and Minguez2008; Foster et al., Reference Foster, Lin and Turck2010; Prakash & Mullen, Reference Prakash and Mullen2010). However, ~10% of patients with HE have normal plasma ammonia levels (Wolf, Reference Wolf2011). Normally, ammonia is generated in the gut from nitrogenous components in the diet, deamination of glutamine, and breakdown of urea by urease present in gut flora (Cordoba & Minguez, Reference Cordoba and Minguez2008). In liver disease, there is a decreased number of functioning hepatocytes to detoxify ammonia, and portosystemic shunting may divert ammonia-containing blood away from the liver into the systemic circulation where ammonia accumulates (Wolf, Reference Wolf2011). Ammonia then crosses the blood–brain barrier and is absorbed and metabolized by astrocyctes, which make up ~30% of cerebral cortex (Cash et al., Reference Cash, McConville and McDermott2010; Wolf, Reference Wolf2011).
Astrocytes function to maintain the structural integrity of the central nervous system (CNS) and the blood–brain barrier, and reduce CNS ammonia levels by using ammonia to convert glutamate to glutamine (Foster et al., Reference Foster, Lin and Turck2010; Wolf, Reference Wolf2011). As glutamine accumulates with HE, the astrocytes swell and there is increased activity of γ-aminobutryic acid (GABA). Some degree of cerebral edema is present in all grades of HE (Prakash & Mullen, Reference Prakash and Mullen2010). Astrocyte swelling can be further exacerbated by inflammatory mediators, hyponatremia and benzodiazepines (Cordoba & Minguez, Reference Cordoba and Minguez2008). Abnormalities in glutamine and catecholamine pathways and manganese are also implicated in the development of HE (Blei & Cordoba, Reference Blei and Cordoba2001; Cordoba & Minguez, Reference Cordoba and Minguez2008). Upregulation or downregulation of transport proteins occur in some liver diseases as well (Wolf, Reference Wolf2011).
Inflammation and infection are associated with brain disturbances related to HE (Wright & Jalan, Reference Wright and Jalan2007; Cordoba & Minguez, Reference Cordoba and Minguez2008). Precipitants of HE include acute liver failure, renal failure, gastrointestinal bleeding, infection, constipation, medications that act on the CNS, diuretic therapy, nonadherence to therapy for HE, and, infrequently, dietary protein overload (Wright & Jalan, Reference Wright and Jalan2007; Wolf, Reference Wolf2011). Most people with chronic liver disease have at least one and often more than one precipitant of HE (Cash et al., Reference Cash, McConville and McDermott2010). Early identification and treatment of precipitating factors are essential to the treatment of HE.
Classification and Grading of HE
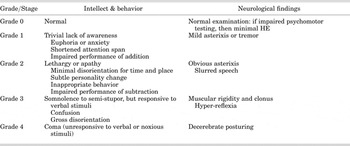
The Working Group on Hepatic Encephalopathy differentiates types of HE according to presentation and etiology. HE Type A is associated with acute liver failure. HE associated with portosystemic bypass with no intrinsic hepatocellular disease is classified as Type B. HE that is associated with cirrhosis and portal hypertension or portal systemic shunts is classified as Type C and further divided into episodic, persistent, or minimal HE. The West Haven criteria shown in Table 3 are a grading system to establish the severity of HE and are based on changes in consciousness, intellectual function, and behavior (Ferenci et al., Reference Ferenci, Lockwood and Mullen2002). The West Haven Criteria is based on subjective assessments of behavior, intellectual function, alteration of consciousness, and neuromuscular function (Prakash & Mullen, Reference Prakash and Mullen2010). Limitations of this system include the highly subjective and elusive nature of symptoms of HE in stages 1 and 2, a lack of specific definitions, and an intuitive approach to grading often used by providers (Cordoba & Minguez, Reference Cordoba and Minguez2008; Hassanein et al., Reference Hassanein, Blei and Hilsabeck2009). The Glasgow Coma Scale measures response to eye opening, verbal behavior, and motor responsiveness, and may be useful in patients with grades 3 and 4 HE. The Hepatic Encephalopathy Scaling Algorithm (HESA) includes well-defined criteria and combines these criteria with psychometric tests (Cordoba & Minguez, Reference Cordoba and Minguez2008). The Clinical Hepatic Encephalopathy Staging Scale (CHESS) is a linear scale that scores HE from 0 (normal mental status) to 9 (deep coma). CHESS is a simple scale that correlates with the New Haven Criteria and Glasgow Coma Scale (Ortiz et al., Reference Ortiz, Cordoba and Doval2007). The CHESS evaluates the patient's orientation, alertness, ability to respond to commands, and ability to talk.
Table 3. West Haven criteria for grading of mental status in hepatic encephalopathy (HE)

Assessment and Diagnosis of HE
Early diagnosis and treatment of HE is essential. However, HE is a diagnosis of exclusion, made only after ruling out all other causes of brain disorders (Ferenci et al., Reference Ferenci, Lockwood and Mullen2002). Other causes of encephalopathy including intracranial lesions or infections, metabolic imbalances, toxic encephalopathy from alcohol or drugs, organic brain syndrome, and postseizure encephalopathy must be ruled out (Ferenci et al., Reference Ferenci, Lockwood and Mullen2002; Sunaram & Shaikh, 2009; Wolf, Reference Wolf2011). The diagnosis of HE also requires the presence of liver dysfunction or a portosystemic shunt (Cash et al., Reference Cash, McConville and McDermott2010).
The signs and symptoms of HE comprise a wide variety of physical, psychiatric, behavioral, emotional, and neurological problems depending upon the severity of the disease. Changes in consciousness range from subtle mental clouding and mild delirium to stupor and coma (Riordan & Williams, Reference Riordan and Williams2010). Inverted sleep patterns, personality changes, or impaired intellect are common. Because signs and symptoms are often subtle in early stages of HE, clinicians must be vigilant when assessing individuals experiencing changes in mental status or behavior and consider hepatic encephalopathy as a possible contributing factor in persons with liver dysfunction and delirium.
Overt HE usually can be diagnosed based on clinical findings, but a diagnosis of minimal HE usually requires neuropsychological testing, because symptoms such as forgetfulness, mild confusion, irritability, or diminished executive function are difficult to detect clinically and are more likely to be evident on neuropsychological testing. Tests for minimal HE include the Psychometric HE Score (PHES), The Repeatable Battery for the Assessment of Neurological Status (RBANS), Inhibitory Control Test, the Cognitive Drug Research Ltd (CDR) Assessment System, the Critical Flicker Test, the Inhibitory Control test, and electroencephalography (Prakash & Mullen, Reference Prakash and Mullen2010). These tests measure areas such as visuospatial functioning, attention, processing speed, and response inhibition. Patients with mild HE may demonstrate impaired complex and sustained attention and delays in choice reaction time needed for safe driving (Wolf, Reference Wolf2011). These patients may have normal function on mental status testing, but show abnormal psychometric testing (Wolf, Reference Wolf2011). MRI and CT scans may be used to rule out other diagnoses.
As HE progresses to overt HE, sleep disturbances; marked irritability; tremor; changes in personality; and difficulties with coordination, thinking, and writing may be observed. Symptoms become more acute and include disorientation, decreasing levels of consciousness, lethargy, somnolence, asterixis, stupor, and coma (Sundaram & Shaikh, Reference Sundaram and Shaikh2009). Asterixis is the ability to maintain a position; to test for it, instruct the patient to hold his/her arms outstretched in front, in a dorsiflexion position, as if stopping traffic. Observe for repetitive flapping motion and the inability to maintain position. It can also be elicited by tongue protrusion, dorsiflexion of the foot or having the patient grasp the examiner's fingers (Sundaram & Shaikh, Reference Sundaram and Shaikh2009). An acute episode of HE may occur over a period of hours or days.
Treatment of HE
Many treatment options are available for overt HE, but no evidence currently supports treatment of minimal HE (Mullen et al., Reference Mullen, Ferenci and Bass2007; Prakash & Mullen, Reference Prakash and Mullen2010). The cornerstone of treatment of overt HE is supportive care. Table 4 lists supportive care strategies aimed at removing or treating all precipitating factors, reducing gut-derived nitrogenous products, and identifying patients requiring long-term care (Mullen et al., Reference Mullen, Ferenci and Bass2007; Cash et al., Reference Cash, McConville and McDermott2010). Most people show clinical signs of improvement of HE within 24–48 hours, although serum levels of ammonia may take longer to decline (Prakash & Mullen, Reference Prakash and Mullen2010). While ruling out other causes of mental status changes and identifying and treating precipitating causes of HE, all patients should receive empiric therapy to reduce the production and absorption of ammonia in the gut (Prakash & Mullen, Reference Prakash and Mullen2010).
Table 4. Supportive treatment of hepatic encephalopathy (HE)

From: Blei & Cordoba J, Reference Blei and Cordoba2001; Cash et al., Reference Cash, McConville and McDermott2010; Prakash & Mullen, Reference Prakash and Mullen2010; Plauth et al., Reference Plauth, Cabre and Riggio2006.
Nutritional Support
Skeletal muscle metabolizes ammonia in chronic liver disease and loss of muscle mass increases the amount of ammonia to the brain. Comprehensive nutritional assessment of metabolic, nutritional, and functional variables by a qualified provider is essential to develop an interdisciplinary plan of care for individuals with liver disease with or without HE. An evidence-based guideline published by the European Society for Parenteral and Enteral Nutrition (ESPEN) recommends that patients with cirrhosis be assessed with simple methods such as the Subjective Global Assessment (SGA) or anthropometry to identify patients at risk for undernutrition. The Guideline recommends an energy intake of 35–40 kcal/kgBW/day and a protein intake of at least 1.2g/kg of protein daily. Oral nutritional supplements of whole protein may be required if patients are unable to meet their nutritional requirements from normal food despite adequate individual counseling. Branch chain amino acid (BCAA)-enriched formula should be used in patients with HE (Plauth et al., Reference Plauth, Cabre and Riggio2006).
Nonabsorbable Disaccharides
Lactulose and lactilol (lactilol is not available in the United States) are currently considered first-line therapy for HE. These products have a laxative effect, reduce the pH of the colon, and interfere with the uptake of glutamine in the gut thereby reducing the synthesis and absorption of ammonia. Lactulose may be administered by mouth, through a nasogastric tube, or rectally. The usual maintenance oral dose of lactulose is 15–45 mL every 8–12 hours, titrated to induce 2–3 soft bowel movements daily (Blei & Cordoba, Reference Blei and Cordoba2001; Mullen et al., Reference Mullen, Ferenci and Bass2007). Patients often report abdominal bloating, flatulence, and difficulty taking lactulose orally because of its very sweet taste. Care must be taken to carefully titrate the dose in order to avoid extensive diarrhea, dehydration, hyponatremia, and worsening of HE.
Antibiotics
A variety of antibiotics have been used to treat HE. Neomycin is reported to be as effective as lactulose, but study results vary (Mullen et al., Reference Mullen, Ferenci and Bass2007). However, ototoxicity and nephrotoxicity remain significant adverse effects of neomycin therapy (Phongsamran et al., Reference Phongsamran, Kim and Abbott2010). Rifaximin, a semisynthetic, nonsystemic, gut-specific oral antibiotic, is United States Food and Drug Administration (FDA) approved to treat overt HE in patients ≥18 of age and has been shown to be effective in studies for both minimal HE and overt HE (Mullen & Prakash, Reference Mullen and Prakash2010). In a recent randomized, double-blind, placebo-controlled study, rifaximin maintained remission from HE more effectively than did placebo, and reduced the risks of hospitalization involving HE (Bass et al., Reference Bass, Mullen and Sanyal2010). Rifaximin is dosed at 550 mg twice daily by mouth. It is minimally systemically absorbed with few side effects and no reported drug–drug interactions (Mullen & Prakash, Reference Mullen and Prakash2010).
Follow-up Care
After treatment of an acute episode of HE, prevention of new episodes is an important part of discharge planning. Consider the following factors in patients with cirrhosis to reduce the risk of developing new episodes of HE: 1) Control of potential precipitating factors such as constipation, control of gastroesophageal varices, prophylaxis of spontaneous bacterial peritonitis, and cautious use of diuretics, based on each individual's history and current condition. 2) Use medications such as rifaximin and lactulose for preventive therapy. and 3) Refer appropriate candidates to a liver transplant center (Blei & Cordoba, Reference Blei and Cordoba2001). In addition, provide ongoing education regarding prevention, early identification, and treatment of HE, and emotional support to the patient and significant others. Consider palliative care and home health referrals for continuing supportive care.
TREATMENT CHOICE DIFFERENCES FOR DELIRIUM BASED ON CLINICAL SPECIALTY
The decision to use specific pharmacological interventions is not only dependent upon the assessed etiology, but also reflects the prescribing patterns of different medical specialties. Agar et al. (Reference Agar, Currow and Plummer2008) conducted a survey to evaluate the variability in managing delirium between medical oncologists, psychogeriatricians, palliative medicine specialists, and geriatric specialists. Using case scenarios, the authors determined that 85% of all specialists would order basic blood work. Medical oncologists evaluated oxygen saturation and head CT more often, psychogeriatricians relied on thyroid function tests, and palliative care specialists were less likely to order chest radiographs and urine cultures. The point of care was influential in the decision making, with palliative medicine specialists caring more often for patients at home. Medical oncologists were more likely to employ pre-emptive approaches using benzodiazepines as the preferred first line of pharmacotherapy. Palliative medicine specialists used significantly more neuroleptics in the management of hypoactive delirium.
In the context of liver disease, the differential management of hypoactive delirium requires discerning assessment. The use of neuroleptics and benzodiazepines may be associated with increased symptom burden and delays in using more appropriate therapies. Until recently, pharmacological therapies in the management of HE focused primarily on the reduction in ammonia production or facilitation of ammonia excretion (Bajaj, Reference Bajaj2010).
The use of nonabsorbable disaccharides such as lactulose has been extensively studied, with a lack of consensus surrounding efficacy. Phongsraman and colleagues (2010), asserted that “at the present time however, there is a lack of sufficient evidence to thoroughly refute the use of nonabsorbable disaccharides for the treatment of HE. For the palliative care patient, adherence issues may compromise the benefit and boost the burden of lactulose therapy. It is incumbent upon practitioners to consider all available treatment options with a goal of maximizing benefit.
CONCLUSIONS
Delirium is a debilitating neuropsychiatric complication that is highly prevalent in palliative care. It is multifactorial and may be related to infection, disease progression, metabolic state, or medication toxicity. There are three proposed subtypes of delirium with the hypoactive/ hypoalert variant being most often underdiagnosed and undertreated. The inadequate management of all types of delirium is associated with increased personal and family distress, lengthier hospital stays, and escalating healthcare costs.
Structured baseline and ongoing assessments are critical in identifying subtle changes in arousal and cognition; options to obtain consultation and referral are encouraged. There are a number of valid and reliable tools designed to assist in screening, symptom appraisal, grading levels of impairment, diagnosis, and treatment planning. Nurses are particularly well positioned to make bedside observations, to document changes over time, and to educate and support patients and their families. Searching for the etiology of delirium and developing individualized plans of care, consistent with patient goals, are key roles for palliative care providers from all disciplines.
These factors, combined with the new therapies used to manage HE, are expanding treatment options for patients along the trajectory of liver disease. At any given point, the goal is to preserve function, enhance quality of life, and reduce personal/collective suffering and distress.