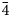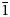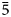Introduction
Sulfate minerals with transition and/or alkali metals are represented typically by hydrated species (Hawthorne et al., Reference Hawthorne, Krivovichev, Burns, Alpers, Jambor and Nordstrom2000). Anhydrous sulfate minerals with transition metal cations are mainly restricted to active fumaroles with strongly oxidising environments (Vergasova and Filatov, Reference Vergasova and Filatov2012; Balić-Žunić, et al., Reference Balić-Žunić, Garavelli, Jakobsson, Jonasson, Katerinopoulos, Kyriakopoulos and Acquafredda2016; Siidra et al., Reference Siidra, Nazarchuk, Zaitsev, Lukina, Avdontseva, Vergasova, Vlasenko, Filatov, Turner and Karpov2017; Pekov et al., Reference Pekov, Zubkova, Agakhanov, Pushcharovsky, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Britvin2018; Siidra et al., Reference Siidra, Borisov, Lukina, Depmeier, Platonova, Colmont and Nekrasova2019a). Our mineralogical investigations of the fumaroles of Tolbachik volcano over the last few years have revealed a number of new anhydrous sulfate mineral species: markhininite TlBi(SO4)2 (Siidra et al., Reference Siidra, Vergasova, Krivovichev, Kretser, Zaitsev and Filatov2014); puninite Na2Cu3O(SO4)3 (Siidra et al., Reference Siidra, Nazarchuk, Zaitsev, Lukina, Avdontseva, Vergasova, Vlasenko, Filatov, Turner and Karpov2017); hermannjahnite CuZn(SO4)2 (Siidra et al. Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018b); saranchinaite NaCu(SO4)2 (Siidra et al., Reference Siidra, Lukina, Nazarchuk, Depmeier, Bubnova, Agakhanov, Avdontseva, Filatov and Kovrugin2018a; Kovrugin et al., Reference Kovrugin, Nekrasova, Siidra, Mentré, Masquelier, Stefanovich and Colmont2019); belousovite KZn(SO4)Cl (Siidra et al., Reference Siidra, Nazarchuk, Lukina, Zaitsev and Shilovskikh2018c); itelmenite Na2CuMg2(SO4)4 (Nazarchuk et al., Reference Nazarchuk, Siidra, Agakhanov, Lukina, Avdontseva and Karpov2018); koryakite NaKMg2Al2(SO4)6 (Siidra et al., Reference Siidra, Nazarchuk, Zaitsev and Vlasenko2019b) and glikinite Zn3O(SO4)2 (Nazarchuk et al., Reference Nazarchuk, Siidra, Nekrasova, Borisov and Shilovskikh2019).
Herein we report on the chemical composition, structure and properties of majzlanite (Cyrillic: майзланит), K2Na(ZnNa)Ca(SO4)4. Majzlanite is named in honour of Prof Dr Juraj Majzlan (b. 1973). Juraj Majzlan works in the Institute of Geosciences, Friedrich-Schiller-University, Jena. In addition to other many achievements and contributions in the fields of mineralogy and crystallography, Juraj Majzlan has made significant contribution to the mineralogy of sulfates (see e.g. Majzlan et al., Reference Majzlan, Grevel, Kiefer and Johnson2017; Zittlau et al., Reference Zittlau, Shi, Boerio-Goates, Woodfield and Majzlan2013; Grevel et al., Reference Grevel, Majzlan, Benisek, Dachs, Steiger, Fortes and Marler2012).
Both the mineral and the mineral name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2018-016, Siidra et al., Reference Siidra, Nazarchuk, Zaitsev and Shilovskikh2018d). Type material is deposited at the Mineralogical Museum, St. Petersburg State University, St. Petersburg, Russia (catalogue no. 1/19690).
Occurrence and association
Majzlanite occurs as a product of fumarolic activity. It was found in June 2016, in the Yadovitaya fumarole, Second scoria cone, Northern Breakthrough (North Breach), Great Fissure eruption, Tolbachik volcano, Kamchatka, Russia. The Second Scoria Cone is located ~18 km SSW of the active shield volcano Ploskiy Tolbachik (Fedotov and Markhinin, Reference Fedotov and Markhinin1983). Majzlanite is associated closely with langbeinite (Fig. 1), thénardite (Fig. 2) and euchlorine. The temperature of gases at the sampling location was ~300°C. All the samples recovered were packed immediately and isolated to avoid any contact with the external atmosphere.

Fig. 1. Bluish-white transparent crystals of majzlanite (marked by a white arrow) in association with langbeinite in the voids of basaltic scoria.

Fig. 2. Back-scattered electron image. Majzlanite rim (light grey) on K-bearing thénardite(?) (dark grey). White areas within majzlanite and spotted areas within K-bearing thénardite (?) are surface damage by an electron beam.
Physical properties
Majzlanite occurs in the voids of volcanic scoria (Fig. 1) associated closely with langbeinite as irregular grains up to 50 μm × 50 μm × 80 μm. Majzlanite is grey with a bluish tint (Fig. 1), has a white streak and vitreous lustre. The mineral is brittle with uneven fracture. Cleavage or parting was not observed. Hardness corresponds to 2–3 on the Mohs’ scale. The density could not be measured due to the lack of suitable sample, but was calculated as 2.961 g cm–3 using structural data and the empirical formula. No fluorescence was detected.
Optical properties could not be measured because of thin intergrowths of majzlanite with K-bearing thénardite(?) (Fig. 2). Majzlanite is soluble in warm H2O.
Chemical composition
One grain, 21 μm × 13 μm in size (Fig. 2) with majzlanite as a rim on K-bearing thénardite(?), was analysed by energy-dispersive spectrometry using a Hitachi S-3400N scanning electron microscope equipped with an Oxford Instruments X-Max 20 Energy Dispersive Spectrometer. The electron-beam accelerating voltage was 20 kV and the current 1.8 nA. A defocused beam (up to 4 μm spot size) was used and the X-ray acquisition time was 30 s. The mineral is unstable under the electron beam and back-scattered electron images show strong damage of the mineral surface (Fig. 2). Analytical data are given in Table 1.
Table 1. Analytical data (wt.%) for majzlanite.

*Mean of three analyses.
S.D. – standard deviation
The empirical formula calculated on the basis of 16 O atoms per formula unit is K1.99Na1.93Zn0.84Ca0.77Cu0.33Mg0.16(S3.94Al0.06Si0.01)O16 and the simplified formula is K2Na(Zn,Na,Cu,Mg)Σ2(Ca,Na)(SO4)4.
The ideal formula, taking into account structural data and charge balance is K2Na(ZnNa)Ca(SO4)4, which requires K2O 15.34, Na2O 10.10, ZnO 13.26, CaO 9.13, SO3 52.17, total 100.00 wt.%.
X-ray crystallography
Experiment
Powder X-ray studies were done using a Rigaku R-Axis Rapid II diffractometer with a cylindrical image-plate detector, with CoKα radiation. For the powder-diffraction study, a Gandolfi-like motion on the φ and ω axes was used to randomise the sample and observed d values and intensities were derived by osc2xrd software (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). The powder data are presented in Table 2. Unit-cell parameters refined from the powder data are: a = 15.9775(9) Å, b = 9.5196(5), c = 9.1216(4) Å, β = 94.921(4)° and V = 1382.29(8) Å3.
Table 2. Powder X-ray diffraction data (d in Å) for majzlanite.

A transparent prismatic crystal fragment of majzlanite was mounted on a thin glass fibre for X-ray diffraction analysis using a Bruker APEX II DUO X-ray diffractometer with a micro-focus X-ray tube operated with MoKα radiation at 50 kV and 40 mA. The data were integrated and corrected for absorption using a multi-scan type model implemented in the Bruker programs APEX and SADABS (Bruker-AXS, 2014). More than a hemisphere of X-ray diffraction data was collected. The structure was solved by direct methods and was refined using SHELXL software (Sheldrick, Reference Sheldrick2015). All of the atoms were refined anisotropically. Details of data collection and structure refinement are provided in Table 3. Bond-valence sums, fractional coordinates and atom-displacement parameters are provided in Table 4 and selected interatomic distances in Table 5. All bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991). Taking into account multiple cation substitutions, the bond-valence sums are in good agreement with the expected oxidation states. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Crystallographic data and refinement parameters for majzlanite.
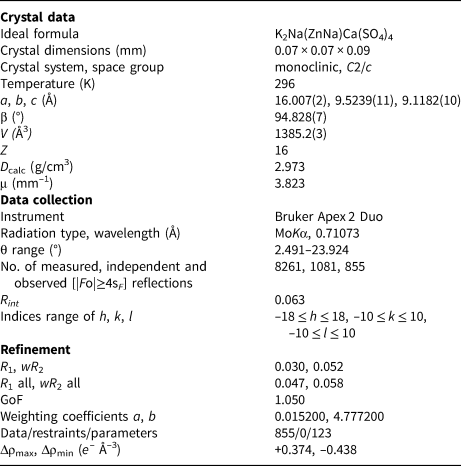
Table 4. Bond-valence sums, coordinates, anisotropic and isotropic displacement parameters (Å2) of atoms in majzlanite.

*Zn0.621(4)Na0.379(4) where Zn = Zn0.44 + Cu0.18 and Na = Mg0.08 + Na0.30; **Ca0.758(11)Na0.242(11);*** S0.98Al0.015Si0.005. Bond-valence sums (BVS) for mixed sites were calculated using parameters for Zn–O and Ca–O bonds from Brese and O'Keeffe (Reference Brese and O'Keeffe1991).
Table 5. Selected interatomic distances (Å) in majzlanite.

Crystal structure
Cation coordination
The crystal structure of majzlanite contains one symmetrically independent K site, one Zn, one Na, one Ca and two S cation sites. The K site is coordinated by 11 O atoms, forming the irregular polyhedron shown in Fig. 3. The Na forms NaO6 octahedra. During the process of crystal structure refinement, it was found that K and Na sites are occupied exclusively by K+ and Na+ cations, respectively (Table 4).

Fig. 3. Coordination environments of atoms in majzlanite.
The Zn site has a distorted octahedral environment and mixed occupancy by Zn2+, Na+, Cu2+ and Mg2+ (Table 4). As the X-ray scattering power of Na and Mg atoms is indistinguishable, Na and Mg were regarded as one group with one scattering factor. Na and Mg atoms were summed to form Na* in the atom fraction. For the same reason, Zn2+ and Cu2+ were assumed to constitute Zn*. Replacement of Zn2+ by Na+ is unusual. Previously the similar substitution was found in synthetic alluaudite-related material, Na(Na0.6Zn0.4)Zn2(H0.6AsO4)(AsO3OH)2 (Ðorðević et al., Reference Ðorðević, Wittwer and Krivovichev2015) and in paseroite (Mills et al., Reference Mills, Bindi, Cadoni, Kampf, Ciriotti and Ferraris2012).
The Ca site coordination environments can be described as a strongly distorted CaO6 octahedron. Refinement of the occupancy of the Ca site reveals the presence of minor Na+ (Table 4).
Two S sites with the site-scattering values are compatible with occupancy by S6+ and tetrahedrally coordinated by O anions. SO4 groups exhibit the usual tetrahedral distances and angles. The <S–O> distances demonstrate similar values of ~1.46 Å and ~1.47 Å, which are in a good agreement with the <S–O> distance of 1.473 Å reported for sulfate minerals by Hawthorne et al. (Reference Hawthorne, Krivovichev, Burns, Alpers, Jambor and Nordstrom2000). The minor amount of Al3+ and Si4+ detected by microprobe in tetrahedral S sites plays an important role as a charge compensating agent. The content of Al3+ and Si4+ was fixed in agreement with the microprobe data reported above.
The following structural formula was obtained: K(Zn0.44Na0.30Cu0.18Mg0.08)(Na0.5)(Ca0.38Na0.12)(S0.98Al0.015Si0.005O4)2 which is in excellent agreement with that obtained by microprobe analysis.
Structure description
The topology of framework in majzlanite is complicated. NaO6 and CaO6 octahedra are connected to sulfate tetrahedra via common corners (Fig. 3). ZnO6 octahedra link to SO4 tetrahedra via both common corners and edges. Due to this fact, Zn–O5 and Zn–O8 bonds are elongated (Table 5) in the ZnO6 octahedron. The Zn–S2 distance in majzlanite is 2.8704(4) Å. Edge-sharing between sulfate tetrahedra and transition-metal octahedra is exceptionally rare. It has never been observed in zinc sulfates. The short Cu–S distance of 2.593 Å between CuO6 octahedron shared via common edges with SO4 group is observed in chlorothionite K2CuCl2(SO4) (Giacovazzo et al., Reference Giacovazzo, Scandale and Scordari1976). However, edge-sharing is common for NaO6 octahedra and SO4 tetrahedra (e.g. thénardite, Na2SO4: Hawthorne and Ferguson, Reference Hawthorne and Ferguson1975; tamarugite, NaAl(H2O)6(SO4)2: Mereiter, Reference Mereiter2013). The Na–S distance is 2.965 Å in tamarugite and 3.112 Å in thénardite.
The CaO6 octahedron shares two O6–O8 edges with two ZnO6 octahedra, forming trimers (Fig. 4a). These trimers link to each other via corner- and edge-sharing with sulfate tetrahedra, forming complex rod-like one-dimensional units elongated along the c axis (Fig. 4b). Additionally, the rods are decorated by NaO6 octahedra, which in turn share common edges with ZnO6 octahedra (Figs 4d,e). Cavities in the framework are occupied by K+ (Fig. 4f).

Fig. 4. (a) Trimeric units forming (b) rod like arrangements with (c) SO4 tetrahedra in the structure of majzlanite. CaO6 = pink; ZnO6 = sky blue; and SO4 = yellow. Channels are filled by K (green balls) and Na (blue balls) cations. (d, e, f) NaO6 polyhedra (orange) are added. See the text for details.
Final remarks
The framework in majzlanite is unique among minerals and synthetic phases. Anhydrous sulfate compounds containing Zn, Na, K and Ca are unknown. Moreover, anhydrous Zn sulfates are exceptionally rare. Only three anhydrous Zn sulfates are known to date: hermannjahnite CuZn(SO4)2 (Siidra et al., Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018b), glikinite Zn3O(SO4)2 (Nazarchuk et al., Reference Nazarchuk, Siidra, Nekrasova, Borisov and Shilovskikh2019) and belousovite KZn(SO4)Cl (Siidra et al., Reference Siidra, Nazarchuk, Lukina, Zaitsev and Shilovskikh2018c). Majzlanite is distantly chemically related to itelmenite Na2CuMg2(SO4)4 (Nazarchuk et al., Reference Nazarchuk, Siidra, Agakhanov, Lukina, Avdontseva and Karpov2018). The occurrence of zinkosite ZnSO4 is doubtful to date (Wildner and Giester, Reference Wildner and Giester1988). However, given the widespread occurrence of chalcocyanite CuSO4 in exhalative mineral assemblages (Siidra et al., Reference Siidra, Nazarchuk, Agakhanov, Lukina, Zaitsev, Turner, Filatov, Pekov, Karpov and Yapaskurt2018b), zinkosite could also be found in Tolbachik fumaroles as a reliable mineral species. Queitite Pb4Zn2(SO4)(Si2O7)(SiO4) (Hess and Keller, Reference Hess and Keller1980) also contains sulfate anions, however the crystal structure is based on zinc-silicate layers with lead sulfate in the interlayer. Properties of queitite, such as poor solubility, are controlled mostly by silicate anions strongly bonded to Zn2+ cations and SO4 groups bonded with Pb2+. A typical characteristic of all high-temperature fumarolic zinc sulfates listed above is their high solubility.
Multiple cation substitutions were also reported recently in the fumarolic sulfate philoxenite (K,Na,Pb)4(Na,Ca)2(Mg,Cu)3(Fe0.53+Al0.5)(SO4)8 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Britvin, Turchkova, Sidorov and Pushcharovsky2016). The structural architecture of philoxenite is completely different from majzlanite.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.68
Acknowledgements
We are grateful to two anonymous reviewers and Peter Leverett for valuable comments. This work was financially supported by the Russian Science Foundation, grant no. 16-17-10085. Technical support by the SPbSU X-ray Diffraction and Geomodel Resource Centres is gratefully acknowledged.