Introduction
In ruminant nutrition, additives are currently used to modulate rumen fermentation and improve performance (Calsamiglia et al., Reference Calsamiglia, Ferret and Devant2002; Goiri et al., Reference Goiri, Oregui and Garcia-Rodriguez2010). Ionophores are the main class of additives used today, but their adoption has been reconsidered and restricted due to the potential effect on microbial resistance to antibiotics (Russell and Houlihan, Reference Russell and Houlihan2003).
At present, possible bacterial resistance when antibiotics are used as additives to improve animal performance has led to scientific and industrial interest in the use of natural additives in animal health (Gois et al., Reference Gois, Cairo, Cantarelli, Costa, Fontana, Allaman, Sbardella, de Carvalho and Costa2016). Chitosan is a non-toxic, biodegradable biopolymer that has drawn the attention of producers because of its potential application in veterinary medicine and food preservation, notably for its modulatory properties in bacterial, fungal and yeast development (Kong et al., Reference Kong, Chen, Xing and Park2010). However, the use of chitosan as an additive in animal feeding with a view to improving nitrogen (N) retention, feed efficiency and production performance in ruminants has not been well investigated.
Regarding the beneficial effects of additives in animal feeding, the antimicrobial activity of chitosan was demonstrated for the first time by Allan and Hadwiger (Reference Allan and Hadwiger1979) and, according to Tang et al. (Reference Tang, Zhang, Kieft, Ryan, Baker, Wiesmann and Rogelj2010), it has a broad range of action against gram-positive and gram-negative bacteria at minimal inhibitory doses. Vishu Kumar et al. (Reference Vishu Kumar, Varadaraj, Gowda and Tharanathan2005) reported that chitosan's mechanism of action is similar to that of ionophores, as many studies have shown that gram-positive bacteria are more susceptible to chitosan than gram-negative bacteria.
Some researchers, such as Araújo et al. (Reference Araújo, Venturelli, Santos, Gardinal, Cônsolo, Calomeni, Freitas, Barletta, Gandra, Paiva and Rennó2015) and Vendramini et al. (Reference Vendramini, Takiya, Silva, Zanferari, Rentas, Bertoni, Consentini, Gardinal, Acedo and Rennó2016) among others, used chitosan in the feeding of rumen-fistulated Nellore steers and dairy cows, respectively, and observed differences in total apparent digestibility, with better energy utilization efficiency. In a study with sheep, Goiri et al. (Reference Goiri, Oregui and Garcia-Rodriguez2010) found that chitosan supplied at 136 mg/kg body weight (BW) provided ruminal fermentation for more efficient energy pathways without reducing the apparent digestibility of organic matter. Despite this promising result, there are currently few studies evaluating the effects of chitosan in vivo on small ruminants, especially in feedlot lambs raised for meat production.
Under the hypothesis that the alteration in ruminal fermentation promoted by chitosan results in improved apparent digestibility of dry matter (DM) and nutrients and a consequent increase in production performance and carcass characteristics, the present study was carried out to evaluate the effect of different levels of chitosan on the intake of nutritional components and digestibility, weight gain, microbial synthesis, carcass morphometrics and characteristics of feedlot lambs.
Materials and methods
Location, animals and housing
The experiment took place at the Experimental Farm of São Gonçalo dos Campos, belonging to the School of Veterinary Medicine and Animal Science, Federal University of UFBA, from May to November 2016.
The animals were kept on a feedlot regime for 90 days, after a period of 15 days of adaptation to facilities, diets and daily handling. During this stage, they received Tifton-85 grass hay ad libitum as roughage feed. After the acclimation period, the experimental phase was started, consisting of three consecutive 30-day periods for the collection of samples and data for evaluation of intake and digestibility of DM and nutrients, N balance, animal performance, carcass characteristics and morphometric measurements.
The current study involved 60 Santa Inês lambs with an average BW of 21 ± 2.2 kg, at 4–5 months of age. The animals were dewormed, vaccinated against rabies and clostridial diseases, supplemented (ADE vitamin complex) and then identified. Lambs were distributed at random in a completely randomized design and housed in individual, covered stalls with the suspended slatted floor with an individual area of 1 m2. The stalls were equipped with drinkers and troughs.
Experimental diets and handling
Diets were formulated to be isonitrogenous and isoenergetic, following the recommendations of the National Research Council (NRC, 2007) to provide the nutrients required by lambs for an estimated daily weight gain of 200 g (Table 1). The experimental diets were composed as follows: (1) no chitosan addition; (2) inclusion of 136 mg chitosan/kg BW; and (3) inclusion of 272 mg chitosan/kg BW. The feed was weighed on a digital scale, and its provision was adjusted so that orts accounted for approximately 100 g/kg of the offered DM. Chitosan was placed over the concentrate upon delivery of the total diet in order to visually ensure consumption of the additive in its entirety under animal production conditions, as described by Mingoti et al. (Reference Mingoti, Freitas, Gandra, Gardinal, Calomeni, Barletta, Vendramini, Paiva and Rennó2016). During the entire experimental period, samples of ingredients and diets were collected, quartered and packed in identified plastic bags that were stored in a freezer at ‒20 °C for later chemical analysis.
Table 1. Centesimal and chemical composition of the control-based diet used for feedlot lambs

*Chitosan added to control-based diet, at the time of supply to the animals, according to each experimental treatment: 136 or 272 mg/kg BW.
a Provides per kg of product: calcium – 120 g; phosphorus – 87 g; sodium – 147 g; sulphur – 18 g; copper – 590 mg; cobalt – 40 mg; chromium – 20 mg; iron – 1.800 mg; iodine – 80 mg; manganese – 1.300 mg; selenium – 15 mg; zinc – 3.800 mg; molybdenum – 300 mg; fluorine (max) – 870 mg; Phosphorus (P) solubility in 2% (min) citric acid – 95%. DM = dry matter; FM = fresh matter; NDFap = neutral detergent fibre corrected for ash and protein.
b Estimated using the NRC (2001) model.
Lambs received the diets twice daily (at 09.00 and 16.00 h) as a complete mixture with a roughage : concentrate ratio of 50:50. The Tifton-85 grass (Cynodon dactylon) hay used as roughage was chopped to particles of approximately 5 cm. The concentrate consisted of ground maize, soybean meal, urea, a specific mineral supplement for sheep and cottonseed.
Chemical analysis
After thawing, samples of roughage, concentrate, orts and faeces were pre-dried in a forced-air oven at 55 °C for 72 h. Next, they were ground through Wiley-type knife mills with a 1-mm sieve and stored in plastic bottles with lids and labelled, ready for laboratory analyses.
Dry matter (method 967.03), ash (method 967.03), crude protein (CP; method 981.10), and ether extract (EE; method 920.29) contents of samples of feeds and orts were determined according to the methodology described in AOAC (1990). Neutral detergent fibre (NDF) and acid detergent fibre (ADF) contents were obtained following Van Soest et al. (Reference Van Soest, Robertson and Lewis1991), while the neutral detergent insoluble protein (NDIP) contents were determined according to Licitra et al. (Reference Licitra, Hernandez and Van Soest1996). Lignin was determined according to method 973.18 (AOAC, 2002), in which the ADF residue was treated with 72% sulphuric acid.
Total digestible nutrients (TDN) were obtained based on the apparent digestibility (d) of the nutritional components, using the following equation proposed by the NRC (2001):
Digestible energy (DE) and metabolizable energy (ME) were calculated using the following equations, as recommended by the NRC (2001):
Feed intake and digestibility
To determine the intake of nutritional components, orts were collected and weighed daily. Intake was determined by subtracting the amount of each nutrient in the orts from the total quantity of that nutrient present in the feed supplied.
The digestibility coefficients of DM, CP, EE, NDF and non-fibrous carbohydrates (NFC) were calculated as the ratio between the consumed amount of each nutrient and its respective faecal excretion and expressed as g/100 g.
Chitosan was placed over the concentrate at a concentration of 150 mg/kg BW, twice daily, before feeding. The chitosan used in the present experiment had the following technical specifications: deacetylation level – 863 g/kg, apparent density – 0.33 mg/ml; pH – 7.9, viscosity <200 cPs, ash – 14 g/kg, and DM – 883 g/kg (Polymar Indústria, Comércio Importação e Exportação LTDA from Fortaleza, Ceará, Brazil).
Animal performance
Lamb performance was calculated after weighing the animals individually at the start of the experiment and again every 24 days. Lambs were always weighed in the morning, after a fasting period of approximately 16 h, to determine average daily gain (ADG).
Slaughter procedures and carcass characteristics
To evaluate the qualitative and quantitative characteristics of the carcass, the animals were deprived of solid feed for 16 h and weighed to determine their final BW. On the next day, they were transferred to a commercial slaughterhouse where they were slaughtered according to the current norms stated in the Normative Instruction of the Ministry of Agriculture and Food Supply established by Secretariat of Agricultural and Livestock Defense (Brasil, 2000).
Lambs were stunned by electronarcosis then slaughtered by exsanguination via sectioning of the jugular and carotid vessels; the blood was collected and weighed. Subsequently, animals were skinned and eviscerated, the gastrointestinal tract weighed, and the head (atlanto-occipital joint section) and feet (carpal and tarsometatarsal joints section) removed and weighed. The bladder and gall bladder were emptied and carcasses washed to determine the empty BW, followed by dressing and weighing to determine the hot carcass weight (HCW) (Cezar and Sousa, Reference Cezar and Sousa2007). Next, the carcasses were chilled in a cold room at ±4 °C for 24 h, where they were hung by the common calcaneal tendon, before being weighed to determine the cold carcass weight.
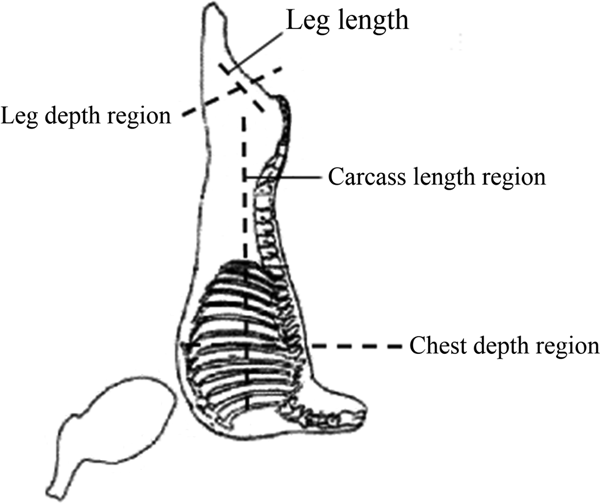
After the 24-h chilling period, the following carcass morphometric measurements were determined, following Cezar and Sousa (Reference Cezar and Sousa2007): carcass length (maximum distance between the anterior edge of the symphysis pubis bone and the anterior edge of the first rib at its midpoint); leg length (distance between the perineum and the anterior edge of the tarsometatarsal joint surface); leg depth (greatest distance between the proximal and distal edges of the leg); and chest depth (maximum distance between the sternum and the back of the carcass at the sixth thoracic vertebra). The representation of carcass morphometric measurements is presented in Fig. 1.

Fig. 1. Points of carcass morphometric measurements in lambs fed diets containing different levels of chitosan.
The carcass was assessed subjectively for conformation, fatness and visceral fat content according to the categories and scores described by Cezar and Sousa (Reference Cezar and Sousa2007). Conformation was determined with emphasis on anatomical regions (leg, rump, loin, shoulder and their muscle planes) using the following scores: 1 (poor) to 5 (excellent). Fatness and visceral fat were measured with emphasis on the thickness and distribution of the fat planes relative to the skeleton into degrees of fatness from 1 (too lean) to 5 (too fat).
After the cold carcasses were weighed, they were divided lengthwise. The two halves of the carcasses were separated and the following commercial cuts obtained: neck (separated from the carcass by an oblique section at its lower extremity between the last cervical and first thoracic vertebrae, thus comprising the seven cervical vertebrae); shoulder (obtained by disarticulating the tissues joining the scapula and the humerus to the thoracic region formed by the first six thoracic vertebrae and the upper portion of the first six ribs); ribs (cut comprising the 13 thoracic vertebrae, with corresponding ribs and sternum); loin (taken perpendicularly to the spine, between the 13th thoracic-first lumbar vertebra and the last lumbar-first sacral vertebra); and leg (separated from the carcass at its upper extremity between the seventh lumbar vertebra and the first sacral vertebra, through the flank section).
The five commercial cuts described above were weighed individually as they were extracted from the carcasses and the weights summed to determine the reconstituted cold-half-carcass weight, as proposed by Cezar and Sousa (Reference Cezar and Sousa2007).
Nitrogen balance and microbial protein synthesis
The N content in samples of consumed material, faeces and urine was measured according to Method 981.10 described by AOAC (1990). Nitrogen retention (Retained N, grams/day) was determined by the following formula:
Urine samples were collected on the 78th, 80th and 82nd days of the experiment, approximately 4 h after the morning feed supply. Urine collection was performed on each of the animals used in the digestibility trial during spontaneous urination, using plastic jars. After each collection, samples were filtered through gauze and a 10-ml aliquot was separated for dilution in 40 ml 0.036 N sulphuric acid solution (Valadares et al., Reference Valadares, Broderick, Valadares Filho and Clayton1999). Urine samples were used for the quantification of urinary concentrations of urea, creatinine, total N, allantoin, uric acid, xanthine and hypoxanthine. Concentrations of creatinine, uric acid and urea in the urine were detected using commercial kits (Bioclin, Sergipe, Brazil).
The daily excretion of creatinine (mg/kg BW) was determined by multiplying the creatinine concentration by the urinary volume of each lamb divided by average BW, as shown below:
where DEC is daily excretion of creatinine (mg/l) (total collection), TCC is total concentration of creatinine (mg/l), UV is urinary volume (l) and BW is animal body weight (kg).
Each animal was considered to excrete 17.05 mg creatinine per kilogram of BW. Based on the creatinine concentration in the spot urine sample, the daily excreted volume was calculated as shown below:
Analyses of allantoin in urine were performed by the colorimetric method, as described by Chen and Gomes (Reference Chen and Gomes1992). The conversion of urea into urea N was achieved by multiplying the obtained values by a factor of 0.4667.
The total excretion of purine derivatives was calculated as the sum of the quantities of allantoin, uric acid, xanthine and hypoxanthine present in the urine, expressed as mmol/d. The absorbed purines (X, mmol/day) were estimated from the excretion of purine derivatives (Y, mmol/day), using the following equation proposed by Chen and Gomes (Reference Chen and Gomes1992) for sheep:
where 0.84 is the absorption efficiency of exogenous purine, 0.150 LW0.75 corresponds to the endogenous excretion of purine derivatives and e‒0.25X is the rate of substitution of the de novo synthesis for exogenous purines.
The ruminal synthesis of protein (g micP/day) was calculated as a function of the absorbed purines (X, mmol/d) using the equation below, as described by Chen and Gomes (Reference Chen and Gomes1992):
where 70 is the purine N content (mg N/mmol), 0.83 is the digestibility of the microbial purines and 0.116 is the purine N : total bacterial N ratio.
Data analysis
The dependent variables were subjected to analysis of variance in a completely randomized design, using the PROC MIXED procedure of SAS software version 9.0 (Statistical Analysis System – SAS Institute Inc., Cary, NC, USA), according to the following model:
where Y ij is the observed value of the dependent variable, μ is the overall mean, T i is te fixed effect of treatment i (i = 1 to 3), and e ij is the random error.
Orthogonal contrasts were used to evaluate linear or quadratic effects of chitosan inclusion levels on the dependent variable. In the case of significance of the orthogonal contrast, the regression model was fitted using PROC REG (SAS software 9.0). The significance level was set at P < 0.05 of probability to type I error for all analyses performed.
Results
Chitosan inclusion in the diet had no effect on the intakes of DM (in kg, g/kg BW), NDF (kg and g/kg BW), EE, OM or NFC of the feedlot lambs (Table 2), However, CP (P = 0.036), ME (P = 0.002) intake and the digestibility coefficients of DM and nutritional components (OM, CP, EE, NDF, NFC and ME) responded quadratically to the addition of chitosan (P < 0.05).
Table 2. Effect of diet with the addition of chitosan on the intake and digestibility of feedlot lambs

a Standard error of the mean.
b % digestibility/100.
c The metabolizable energy (ME) was calculated following the NRC recommendations (2001), using the equations: DE = TDN × 0.04409 (MJ/kg) and ME = DE × 0.082.
*Significant at P < 0.05.
BW, body weight; NDFap, neutral detergent fibre corrected for ash and protein.
Nitrogen content in faeces and urine was not influenced by the diets with or without the addition of chitosan (Table 3). However, ingested and retained N and microbial protein synthesis (g micP/d and g micP/kg ME) showed a quadratic effect in response to chitosan levels (P < 0.05). The animal performance, carcass characteristics and morphometric measurements of carcasses were not influenced by the addition of chitosan to the diets (Tables 4 and 5, respectively).
Table 3. Nitrogen (N) balance and microbial synthesis in lambs fed diets with varying chitosan levels

a Standard error of the mean.
BW, body weight; micP, microbial protein; TDN, total digestible nutrients.
*Significant at P < 0.05.
Table 4. Performance and carcass characteristics of lambs fed diets with varying chitosan levels

a Standard error of the mean.
BW, body weight; DP, carcass dressing percentage (CCW/slaughter weight × 100).
*Significant at P < 0.05.
Table 5. Morphometric measurements of the carcass of feedlot lambs fed diets with varying chitosan levels

a Standard error of the mean.
BW, body weight.
*Significant at P < 0.05.
There was no difference in ADG, feed conversion, or feed efficiency of the animals as a function of the chitosan levels in the diet (Table 4). Hot and cold carcass weights and carcass dressing percentage were also not influenced by the experimental diets. Likewise, the yields of the cuts (percentages of tail, neck shoulder, ribs, loin and leg) relative to the carcass were not influenced by dietary addition of chitosan.
The addition of chitosan to lamb diets did not affect the following carcass morphometric variables: internal and external carcass length, chest and rump width, chest depth, and chest and rump circumference. Chitosan addition also did not affect carcass conformation, fatness or visceral fat content (Table 5).
Discussion
Chitosan was added to animal diets, aiming to improve the utilization of dietary energy by increasing propionate production, which would consequently lead to a simultaneous reduction of methane production (Goiri et al., Reference Goiri, Oregui and Garcia-Rodriguez2010). However, no reduction in ruminal deamination or increase in the amount of short-chain fatty acids was observed due to the addition of chitosan. In the present study, the intakes of DM and NDF (kg and g/kg BW) were not influenced by the addition of chitosan to the diet of feedlot lambs.
Chalupa (Reference Chalupa, Ruckebusch and Thivend1980) and Horton (Reference Horton1980) reported the ability of chitosan to reduce the production of branched-chain fatty acids (i.e. isobutyrate and isovalerate) from rumen deamination. Moreover, chitosan in the rumen environment has been shown to allow for greater permeability of epithelia and reduce in vitro pH, promoting improved digestibility (Benediktsdóttir et al., Reference Benediktsdóttir, Baldursson and Másson2014).
Mingoti et al. (Reference Mingoti, Freitas, Gandra, Gardinal, Calomeni, Barletta, Vendramini, Paiva and Rennó2016) suggested that the increased digestibility provided by chitosan addition is due to its ability to affect rumen microorganisms and digestive processes, acting mainly on Gram-positive bacteria. This phenomenon explains the improved digestibility of NDF and CP.
This information supports the current results for the digestibility coefficients of DM and nutritional components. The quadratic effects observed with the addition of chitosan levels to the diet are due to N dynamics for microbial protein synthesis (g micP/d and g micP/kg TDN). This, in turn, is explained by the better digestibility of TDN and greater use of the energy available in the rumen environment provided by the addition of 136 mg chitosan/kg BW in the diet.
Del Valle et al. (Reference Del Valle, de Paiva, de Jesus, de Almeida, Zanferari, Costa, Bueno and Rennó2017), Araújo et al. (Reference Araújo, Venturelli, Santos, Gardinal, Cônsolo, Calomeni, Freitas, Barletta, Gandra, Paiva and Rennó2015) and Mingoti et al. (Reference Mingoti, Freitas, Gandra, Gardinal, Calomeni, Barletta, Vendramini, Paiva and Rennó2016), however, did not detect an influence of chitosan levels on microbial protein synthesis. Microbial growth is maximized by synchronization between the availability of fermentable energy and rumen-degradable N (Russell et al., Reference Russell, O'Connor, Fox, Van Soest and Sniffen1992). In this way, microbial protein synthesis depends greatly upon the availability of carbohydrates and N in the rumen environment (Clark et al., Reference Clark, Klusmeyer and Cameron1992; NRC, 2001).
In the current study, the use of 272 mg chitosan/kg BW was shown to be less efficient in regulating N balance when compared with the other diets. Russell and Strobel (Reference Russell and Strobel1988) demonstrated the sensitivity to monensin sodium of two species of Gram-negative bacteria with high rumen ammonia production capacity, assuming similarity in ruminal fermentation between ionophores and chitosan (Calsamiglia et al., Reference Calsamiglia, Ferret and Devant2002; Goiri et al., Reference Goiri, Oregui and Garcia-Rodriguez2010). This finding can be explained by the ionic interaction between the amine group of chitosan and the positively charged bacterial surface that caused destabilization of membrane permeability, thereby affecting the energy production pathways (Raafat and Sahl, Reference Raafat and Sahl2009; Kong et al., Reference Kong, Chen, Xing and Park2010), which is related mainly to fluctuations in the rumen pH. Therefore, the addition of 272 mg chitosan/kg BW to the animal diets was understood to have affected the development of the respective bacteria.
The use of chitosan elevates the digestibility of CP, DM and NDF, in addition to improving N balance and microbial protein synthesis in feedlot lambs. However, the intake, carcass characteristics and morphometric characteristics of the carcasses of these animals are not influenced by the dietary use of chitosan.
In general, research with animals involving the use of chitosan is still in the early stages, considering the potentialities of its use. However, in the current study, the rumen action dynamics of this additive in the diet of feedlot lambs at the concentration of 136 mg/kg BW was found not to be useful.
Financial support
The authors thank the Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação of IFNMG - Salinas Campus for the PBQS fellowship grant.
Conflicts of interest
None.
Ethical standards
The current research was conducted in strict conformity with the Brazilian legislation on the use of animals, after approval by the Committee of Ethics in Animal Use of the School of Veterinary Medicine and Animal Science of the Federal University of Bahia (approval no. 16/2016).